INTRODUCTION
The Health Protection Agency (HPA) is responsible for the national surveillance of communicable diseases in England and Wales. Local clinical microbiology laboratories routinely test diarrhoeal stool specimens from patients for the presence of Salmonella enterica and other gastrointestinal pathogens. Presumptive isolates of S. enterica are referred to the Salmonella Reference Unit (SRU) in the HPA Department of Gastrointestinal, Emerging and Zoonotic Infections (GEZI) for confirmation and characterization. Data on cases of salmonellosis confirmed by the SRU populate the national surveillance database.
There was a marked rise in laboratory-confirmed salmonellosis during the 1980s and 1990s. Reporting rose from <9000 cases in 1981, reaching a peak in 1997 with >30 000 cases recorded on the national surveillance database. Since then there has been a steady fall in the number of reported cases with around 10 000 being recorded in 2008 [1]. Non-typhoidal salmonellas are zoonoses and are usually transmitted through the consumption of contaminated foods. While the most common vehicles of infection include eggs, chicken, other meat and dairy products, outbreaks have been linked to a wide range of different types of food. The decline in indigenous salmonellosis that has been observed over recent years has been ascribed to a series of interventions designed to control the carriage of S. Enteritidis in the egg-laying flocks in the UK [Reference Cogan and Humphrey2]. It is known that Salmonella infection can also be acquired through animal contact, and reptiles are known to carry Salmonella in their intestinal tract [Reference Burnham3, Reference Pedersen4]. Reptiles have been linked to sporadic illness caused by certain less common serotypes including S. Arizonae, S. Diarizonae, S. Tennessee and S. Apapa [Reference Bertrand5, Reference Cooke6]. Little has been published on the association between S. Typhimurium and contact with reptiles, with the exception of an outbreak in the USA in 2005, where the source was traced to contaminated mice that were being used as food for pet snakes [Reference Fuller7, Reference Lee8].
In December 2008 a gradual but sustained increase of tetracycline-resistant S. Typhimurium definitive phage-type 191a (DT191a) in England and Wales was identified by specialists in the reference laboratory. This was a newly defined phage type [Reference Peters9]; hence there were no cases identified in any of the preceding years. The nature of this outbreak was unusual in that there was a slow but steady flow of cases (averaging around three per week). The average age of cases was 16 years and the median was 11 years (ranging from 4 months to 69 years). The majority of the cases were male (34/55). Cases were distributed throughout England. An investigation was launched to identify the source of infection.
METHODS
Microbiology
Phage-typing is an established method of detecting outbreaks of salmonellosis. All S. Typhimurium isolates referred to the SRU are phage-typed using the typing scheme for S. Typhimurium as described by Callow in 1959 [Reference Callow10] and extended by Anderson et al. in 1977 [Reference Anderson11]. Salmonella strains are also screened by the SRU for antimicrobial resistance using the methods described by Frost [Reference Frost12].
Epidemiological investigations
Hypothesis generation
In England it is customary for either local public health practitioners based in health protection units (HPUs) or local authority environmental health departments to collect exposure histories from laboratory-confirmed cases of salmonellosis. Following the identification of the upsurge in cases of S. Typhimurium DT191a, HPUs were approached by epidemiologists in GEZI and all exposure history questionnaires relating to recent cases were collected. Although these questionnaires varied between HPUs, elements relating to exposures were sufficiently standardized. This enabled the frequency of exposure to a range of variables to be estimated in order to generate hypotheses for the source of infection.
Analytical epidemiology
Two strategies were developed to test the hypotheses that had been generated.
(1) A case-control study using case nominated controls matched for age, gender and area of residence.
(2) A case-case study using laboratory-confirmed cases of S. Enteritidis matched for age, gender and area of residence as the comparison group.
It was decided that it would be possible to test the use of S. Enteritidis cases as a comparison group because the hypothesis-generating exercise did not identify either eggs or chicken as potential vehicles of infection. Recent epidemiological investigations have shown that eggs and chicken remain the principal vehicles of infection for S. Enteritidis in England.
For the analytical epidemiological investigations a case was defined as an individual resident in England and Wales who had:
• experienced an episode of gastrointestinal illness [i.e. diarrhoea (⩾3 loose stools in a 24-h period)] and from whom a faecal isolate of S. Typhimurium DT191a with resistance to tetracycline had been confirmed by the SRU after 1 January 2009 and until sufficient cases were recruited to satisfy the sample size requirements (13 February 2009);
• not travelled outside the UK in the 7 days prior to the date of onset of illness;
• not shared a household with an individual with any gastrointestinal illness preceding their own illness by <8 days.
Asymptomatic controls were defined as individuals resident in England and Wales nominated by cases (or their carers) and who had:
• not experienced an episode of gastrointestinal illness in the 7 days before interview;
• not travelled outside the UK in the 7 days prior to the date of onset of illness;
• not shared a household with an individual with any gastrointestinal illness preceding their own illness by <8 days.
A comparator case was defined as an individual resident in England and Wales who had:
• experienced an episode of gastrointestinal illness and from whom a faecal isolate of S. Enteritidis had been confirmed by the SRU after 1 January 2009;
• not travelled outside the UK in the 7 days prior to the date of onset of illness;
• not shared a household with an individual with any gastrointestinal illness preceding their own illness by <8 days.
Standard structured case-control questionnaires were designed and administered to all subjects by telephone interview from GEZI. Questionnaires included a detailed section on reptiles and other animals that cases had exposure to inside or outside the home, ‘exposure’ to a reptile was defined as touching or feeding one, or staying in a house where a reptile was kept. The questionnaire also included questions relating to more common sources of infection, e.g. chicken, eggs, mince, salad and raw vegetables and trips abroad or within the UK. Parental permission was sought for cases aged <16 years for ethical reasons. Parents have the option of allowing a direct interview, although for very young cases, parents would answer on behalf of the case. All interviewers were fully briefed on the questionnaire and interviewing technique. Attempts to contact subjects were made up to three times at different times of the day or evening.
Sample size calculations suggested that at least 15 cases and controls would be needed for the study; however, we attempted to obtain 20 of each.
RESULTS
Microbiology
During the second half of 2008 phage-typing showed a number of S. Typhimurium isolates referred to the HPA SRU exhibited a hitherto undefined phage-typing pattern. This pattern was consistent and was observed in further isolates of S. Typhimurium, referred in 2008 and 2009. The pattern was therefore accorded definitive phage-type status – DT191a. Antimicrobial resistance screening of these S. Typhimurium DT191a isolates has shown them all to be resistant to tetracyclines but sensitive to all other antimicrobials in the test panel. As part of this investigation 17 DT191a isolates were fully serotyped according to the Kauffmann–White scheme [Reference Grimont and Weill13, Reference Bale14]. Two of these isolates had the typical diphasic antigenic structure for S. Typhimurium, i.e. 4,[Reference Bertrand5],12:i:1,2; the remaining 15 strains were found to be monophasic 4,[Reference Bertrand5],12:i:-.
Epidemiological investigations
Hypothesis generation
Eight of the ten questionnaires received from HPUs indicated that the case had been exposed to a reptile. Seven people kept snakes (mostly corn snakes), one of whom also had a lizard. The one remaining case kept geckos and bearded dragons. This level of exposure was considerably higher than those recorded in previous national outbreak investigations. Therefore the null hypothesis put forward for the study was that infection with S. Typhimurium DT191a was not associated with exposure to reptiles.
Analytical epidemiology
A total of 22 eligible cases were selected for interview, which were received in the laboratory between 1 January and the 13 February 2009. Interviews were completed for 21 of these cases. The median age was 16 years (range 2 months to 69 years), 12/21 cases were females. The most common symptoms in the cases were diarrhoea and fever (86% of cases questioned) and bloody stools (66%). This can be used as an indication of severity of disease, and compares with 26% of all non-typhoidal salmonellas (from surveillance in England and Wales).
Seven cases or parents of cases were asked to nominate asymptomatic controls according to the criteria described above. Only one case agreed to provide details of a suitable control, and an interview was conducted.
Forty-two cases of S. Enteritidis were selected for interview. Eleven were found to be ineligible because they had a history of recent foreign travel. Eleven were not contactable, one could not be interviewed owing to language difficulties and one refused consent. Therefore 18 eligible cases of S. Enteritidis were interviewed. The median age was 14 years (range 5 months to 70 years), 10/18 cases were female.
Statistical analyses
A comparison of age and gender between the cases of S. Typhimurium DT191a and S. Enteritidis showed no significant differences (P=0·92 and P=0·38, respectively).
Fourteen out of 21 cases interviewed indicated exposure to reptiles (as defined above) in the 3 days prior to onset of symptoms, either because one was kept in their home, or in one case, at university. None of the controls had been exposed to reptiles. Exact logistic regression was used to calculate matched odds ratios (mORs) for different exposures. Exposures with strong statistical evidence are presented in Table 1.
Table 1. Matched odds ratios and 95% confidence interval for exposures
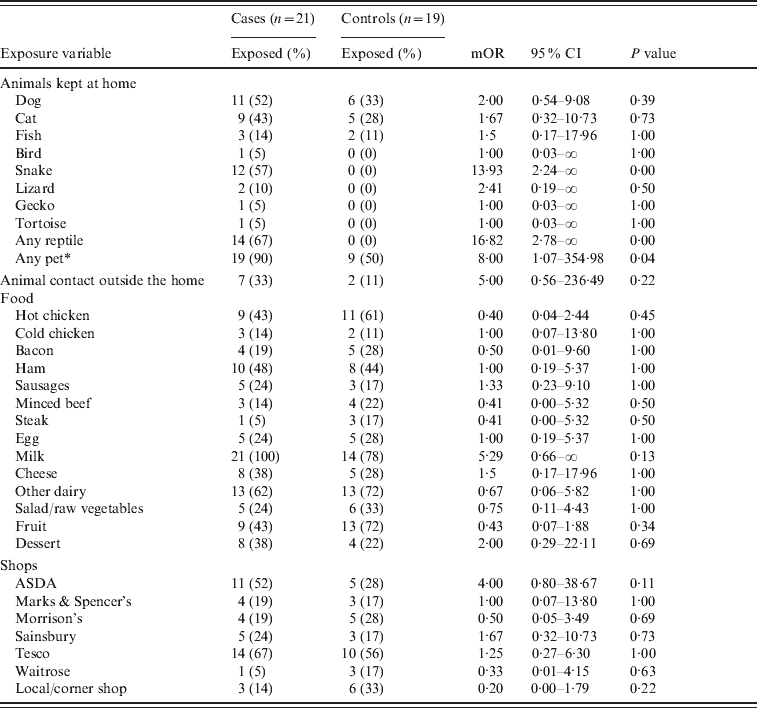
mOR, Matched odds ratio; CI, confidence interval.
* ‘Any pet’ includes all of the categories listed above as well as any other animal which is kept in the home.
The case-case study indicates that those with exposure to reptiles were nearly 17 times more likely to be ill than those who had no contact with reptiles, and snakes especially appear to be the most common reptile owned by cases. Reptiles kept included ten corn snakes, one rat snake, one bearded dragon and two where the species was unknown.
DISCUSSION
The case-case study showed a strong association between infection and exposure to pet reptiles, although the method of transmission remains unclear. Twelve of the 14 cases who kept reptiles reported that they had owned their pets for many years without problems (average 4·5 years, range: 9 months to 13 years). This suggests that transmission of S. Typhimurium DT191a could be related to the recent care and management of these reptiles. Of the 14 cases who reported ownership or contact with reptiles, 12 (86%) were found to have fed their pets on frozen mice.
Based on information collected from the questionnaires, a list of pet shops that had supplied the frozen mice was supplied to the Reptile and Exotic Pet Trade Association (REPTA) to determine the origins of their feed stock. Following up, REPTA arranged for the delivery of frozen mice samples representing all the major suppliers in England, to the Veterinary Laboratory Agency (VLA) for testing. Samples from a single supplier tested positive for S. Typhimurium DT191a, and these were traced back to a producer in the USA (see Fig. 1). Therefore, the most likely source of infection in this outbreak was frozen mice used as feed for pet reptiles, especially snakes.
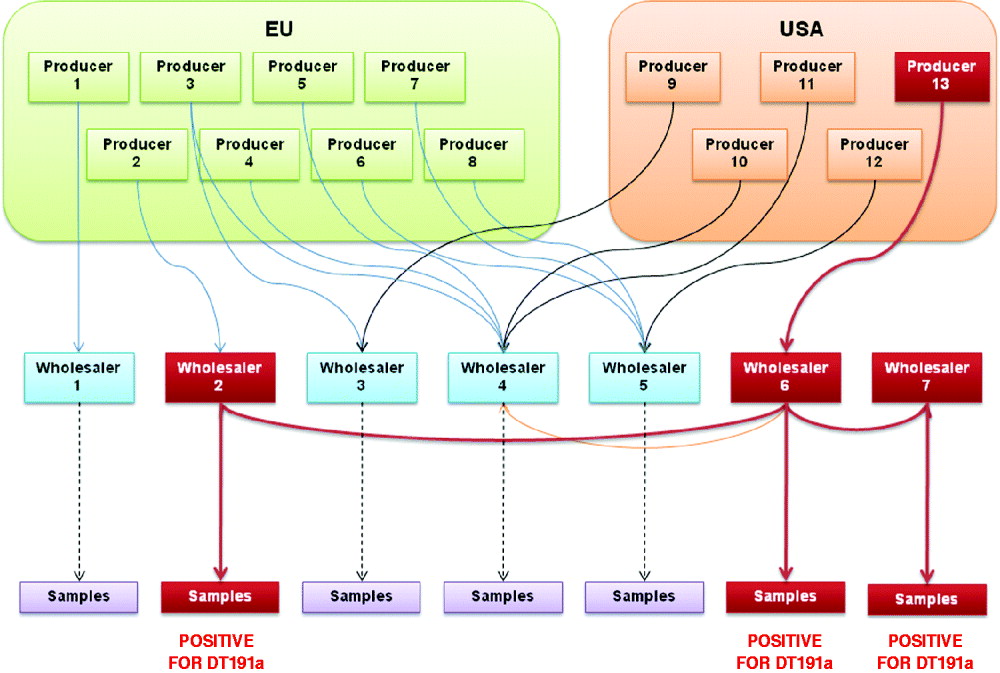
Fig. 1. Flowchart showing traceback of frozen mice from samples to producer.
Questions still remain concerning the route of transmission from contaminated mouse to case. It is not clear whether the cases became infected through handling the mice, or through handling the snakes that had in turn been infected via the mice? The questionnaire addressed whether cases had direct contact with the pet and whether the pet was allowed to roam freely in the house at any time, to which the responses were predominantly negative (only 3/14 cases with reptiles reported direct contact). Salmonella may have been transferred from the mouse or reptile to either the handler or a contaminated surface and then to the infant. Salmonella has been shown to survive in the environment for up to 4 days on a dry surface [Reference Kusumaningrum15], so it is possible for transmission to occur around the house via, for example, a contaminated door knob, tap, furnishings, or kitchen surfaces where frozen mice may have been left to thaw (see Fig. 2).
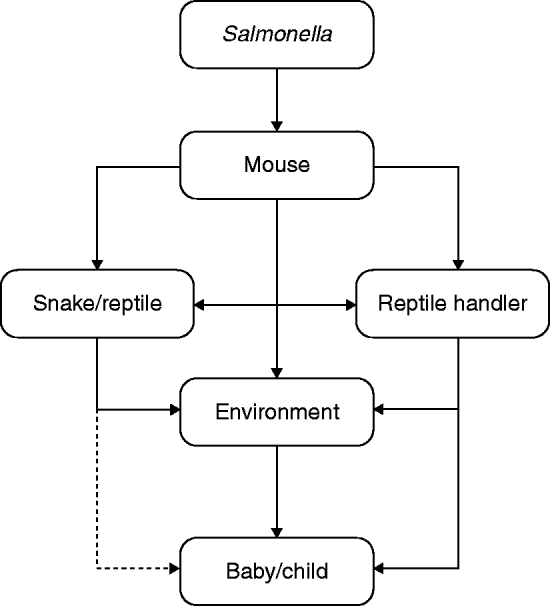
Fig. 2. Possible infection pathways.
Sales of mice from the implicated supplier were stopped in the UK while further enquires were made. On 13 August 2009, new import authorizations were issued, including strengthening of border controls, with random testing of consignments. As limited legislation existed to prevent further sales of these mice, all batches were clearly labelled with a health warning and handling instructions. Unfortunately, cases continued to be reported in the UK, and in January 2010 cases also began to emerge in the USA. On 24 July 2010, the company producing the mice for sale was closed by the American authorities, and we hope this will finally be an end to the outbreak in the UK.
Outbreaks in humans from contaminated animal feed are rare, contamination in animal by-products not intended for human consumption and covered by European Union regulation number 1774/2002 [16]. Unfortunately this does not currently cover frozen mice, but their inclusion is currently under discussion between the HPA and responsible government agencies.
The study also highlights the difficulties of conducting case-control studies when children are the principal population affected. Finding eligible childhood controls to interview is becoming increasingly difficult. Parents of infected infants were found to be willing to provide exposure histories for their own children for public health purposes. However, our interviewers found that many of those approached were reticent to provide contact details of friends and family members who could be invited to act as controls. The reasons given for this reticence were founded on increasing concerns regarding the disclosure of information on children to third parties. There is also anecdotal evidence that primary-care physicians are increasingly reluctant to provide name patient data for control selection to support public health investigations. In outbreaks where the age structure of the affected population broadly reflects that of the community it is still possible to select controls using a systematic dialling approach. However, as the use of land lines decrease there are concerns that this sampling frame will become less representative of the population as a whole.
In agreement with previous studies [Reference McCarthy and Giesecke17], this investigation highlights the advantages of using a case-case study approach as an alternative to the conventional case-control study in circumstances where difficulties are encountered in the identification and enrolment of suitable controls. The use of cases of S. Enteritidis as a comparison group worked well in this study because the hypothesis generated pointed to a zoonotic exposure with no association with the common exposures expected of S. Enteritidis. The findings of the epidemiological study were validated by the subsequent detection of the outbreak strain in feeder mice. The identification of a suitable comparator group for case-case studies might prove more difficult where foodborne transmission of infection is suspected.
The median age of cases in the study was 5 years, with an average of 12 years. The predominantly young age groups involved may be indicative of a less virulent serotype causing infection in those with a challenged or weak immune system. Further evidence for this was supplied in one example, where both parents of the cases were tested and confirmed as having asymptomatic infections. This is not the first study to show that it is more likely to be the young who acquire Salmonella from a reptile-related source [Reference Bohme, Fruth and Rabsch18–Reference Mermin20]. In outbreaks where the mean age is low, it may be worth investigating the reptilian link alongside other sources such as baby food or infant formula.
It is unlikely that recall bias would have had an effect on the outcome of the study given that information on the ownership of reptiles is clear cut. However, recall may be a problem if a case has had indirect contact with a reptile outside the home, and this may explain those cases who indicated no contact with pet reptiles.
As of 20 August 2010, 420 cases of tetracycline-resistant S. Typhimurium DT191a have been reported. Cases continue to be reported at a rate of about four per week, which we hope will now finally begin to tail off. After the case-control study, 113 further cases were contacted and 87 (77%) reported known exposure to reptiles. During more recent follow-up of cases, when pressed on whether they had been in contact with a reptile of any kind, 93% of cases then confirmed that they had, even if they had answered ‘no’ to contact with pets outside the home. Additional exposures related to the use of frozen mice as a feed for pets include keepers of raptors, of which there were three reported after the case-control study. Potential alternate exposures may have included visiting friends without knowledge of reptile ownership, visiting zoos or visiting pet shops selling frozen mice.
Health Protection Scotland has also isolated the outbreak strain from about 50 cases, over 80% of which reported exposure to snakes. These cases have a similar age and gender distribution to the cases in England and Wales. In Scotland, the strain has also been isolated from a number of carnivorous mammals and birds kept in a zoo, and in corn snakes, the latter of which were associated with cases of infection. A similar association between corn snakes and frozen feeder mice has been noted.
Snakes and other reptiles, such as bearded dragons, are becoming more popular as pets. A recent report indicated that reptiles now outnumber dogs in terms of pets kept in UK households [Reference Copping21]. The UK has no official guidelines covering the risks of reptile handling/ownership in relation to Salmonella infection. In the USA, where it is estimated that 70 000 cases of Salmonella are attributable to reptiles every year, the CDC recommends that reptiles and amphibians should be kept out of households that include children aged <5 years or immunosuppressed persons. In households that do keep reptiles, they recommend thorough hand washing and preventing the reptile from roaming around the living areas of the house [22].
Raw frozen mice used as snake feed have been found to be a potential source of Salmonella and present a risk of contamination to those who handle them. Children aged <5 years appear to be particularly at risk of being infected with Salmonella from reptiles.
The cross-agency Human Animal Infections and Risk Surveillance (HAIRS) group considers risks of humans becoming ill with infections from animal contact. In association with HAIRS, The HPA, together with the Department of Health and Defra have produced a leaflet which provides the latest guidance on the Salmonella risks associated with keeping reptiles, highlighting that the chance of becoming infected can be significantly reduced by ensuring good hygiene whenever pets are handled or fed; this document is also available on the HPA's website.
ACKNOWLEDGEMENTS
The authors thank colleagues from the Gastrointestinal, Emerging and Zoonotic Infections Department, those in the Salmonella Reference Unit and those in the local and regional units who helped with this investigation. We also thank Lynda Browning from Health Protection Scotland for the information she provided.
DECLARATION OF INTEREST
None.





