INTRODUCTION
Group A streptococci (GAS) are one of the most common human pathogens that cause both invasive and non-invasive infections. GAS-associated diseases are more common in children than adults and clinical presentation ranges from pharyngitis and impetigo to invasive infections particularly acute rheumatic fever and acute glomerulonephritis [Reference Steer, Danchin and Carapetis1]. In the late 1980s concern about GAS disease was heightened in many countries, as outbreaks of invasive clinical infection were reported [Reference Efstratiou2]. Several factors have been considered to explain differences in disease frequency and severity, including changes in the virulence of the bacterium associated with expression of M protein and a group of exotoxins known as streptococcal superantigens (SAgs).
The M protein is an important virulence determinant in GAS which is determined by the emm gene. Currently, more than 170 emm types and 750 emm subtypes of GAS have been described [3]. Studies of GAS emm-typing distribution performed worldwide, have shown a differing distribution of emm types in different countries and regions [Reference McGregor and Spratt4–Reference Pruksakorn7]. SAgs are thought to contribute to the pathogenesis of severe GAS infections by virtue of their potent immunostimulatory activity and the distribution of profiles of genes encoding SAgs has been used as an additional epidemiological tool to explore the genomic heterogeneity and possible correlation between toxin gene content and disease type [Reference Rivera8].
Effective vaccines against GAS may be based on complex combinations of antigens, particularly type-specific M-protein components. In addition, virulence factors such as the SAgs may also be important for the design of potential vaccines. The formulations for these vaccines would require knowledge of the distribution of emm types and virulence factors among GAS isolates in a community. In some regions of China there are few studies available on emm types and virulence factors [Reference Ho9, Reference Jing10] and no large-scale study has been conducted in Chinese children. We therefore set out to document the emm types and Sag gene profiles in GAS isolates collected from paediatric patients during two periods separated by several years.
MATERIALS AND METHODS
Bacterial isolates
This survey involved 359 GAS isolates collected from children in China during two periods. In period A (1993–1994), 137 isolates comprised those from throat swabs (110), pus (16) and ear swabs (11); in period B (2005–2006) the collection of 222 isolates were from throat swabs (203), puss (10), vulvar secretions (5), pleural effusion (2), and blood (2). The clinical diagnoses of the patients during period A included pharyngitis (46), scarlet fever (54), impetigo (16), otitis media (11), nephritis (8), erysipelas (1), cellulitis (1). During period B, the clinical diagnoses were pharyngitis (44), scarlet fever (139), impetigo (10), erysipelas (9), bronchitis (6), colpitis (5), nephritis (2), urticaria (2), pleural effusion (2), sepsis (2) and psoriasis (1).
Specimens taken were inoculated onto 5% defibrinated sheep blood tryptone soy agar plates and incubated for 24 h at 37°C in 5% CO2. Preliminary identification of isolates as Streptococcus pyogenes was based on β-haemolysis on sheep blood agar, colony morphology and presence of group A antigen, which was confirmed by a Streptococcal grouping kit (Oxoid Ltd, UK). Chromosomal DNA was extracted from freshly grown isolates using a Chelex-based DNA extraction kit (Beijing SBS Genetech Co. Ltd, China).
emm typing
emm typing was performed according to the protocol of the Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/ncidod/biotech/strep/protocols.html). The 5′ end of the DNA sequences was compared to sequences listed in the CDC database (http://www.cdc.gov/ncidod/biotech/strep/strepblast.html).
Identification of SAg genes
PCR was used to detect the presence of eight SAg genes (speA, speC, speH, speI, speG, speJ, ssa, SMEZ). The PCR conditions were: denaturing for 1 min at 94°C, 30 cycles for 30 s at 94°C and annealing for 30 s at the temperature determined for each primer pair. Primers were as previously described [Reference Rivera8].
Statistical analysis
SPSS software, version 11.5 was used for all analyses (SPSS Inc., USA). In χ2 test a P value <0·05 was considered significant.
RESULTS
Distribution of emm types over the two sampling periods
A total of 24 emm types were identified among the 359 GAS isolates (Fig. 1). In period A, 24 emm types were detected, with the six most prevalent being emm3 (26·3%), emm1 (17·5%), emm4 (13·9%), emm12 (10·2%), st1815 (7·3%) and emm6 (6·6%); these types accounted for 81·8% of all the isolates. Similarly, in period B, nine emm types were identified, with emm12 (55·9%) and emm1 (39·6%) accounting for 95·5% of the isolates. Of four invasive isolates, two were from pleural effusion (of types emm1 and emm12) and two from sepsis cases both of which were type emm1. Over the two sampling periods emm1 (P<0·01) and emm12 (P<0·01) significantly increased, while emm3 (P<0·01) decreased in the second period. Types emm4, st1815 and emm6 which were prevalent in the first sample were not found in the second period.
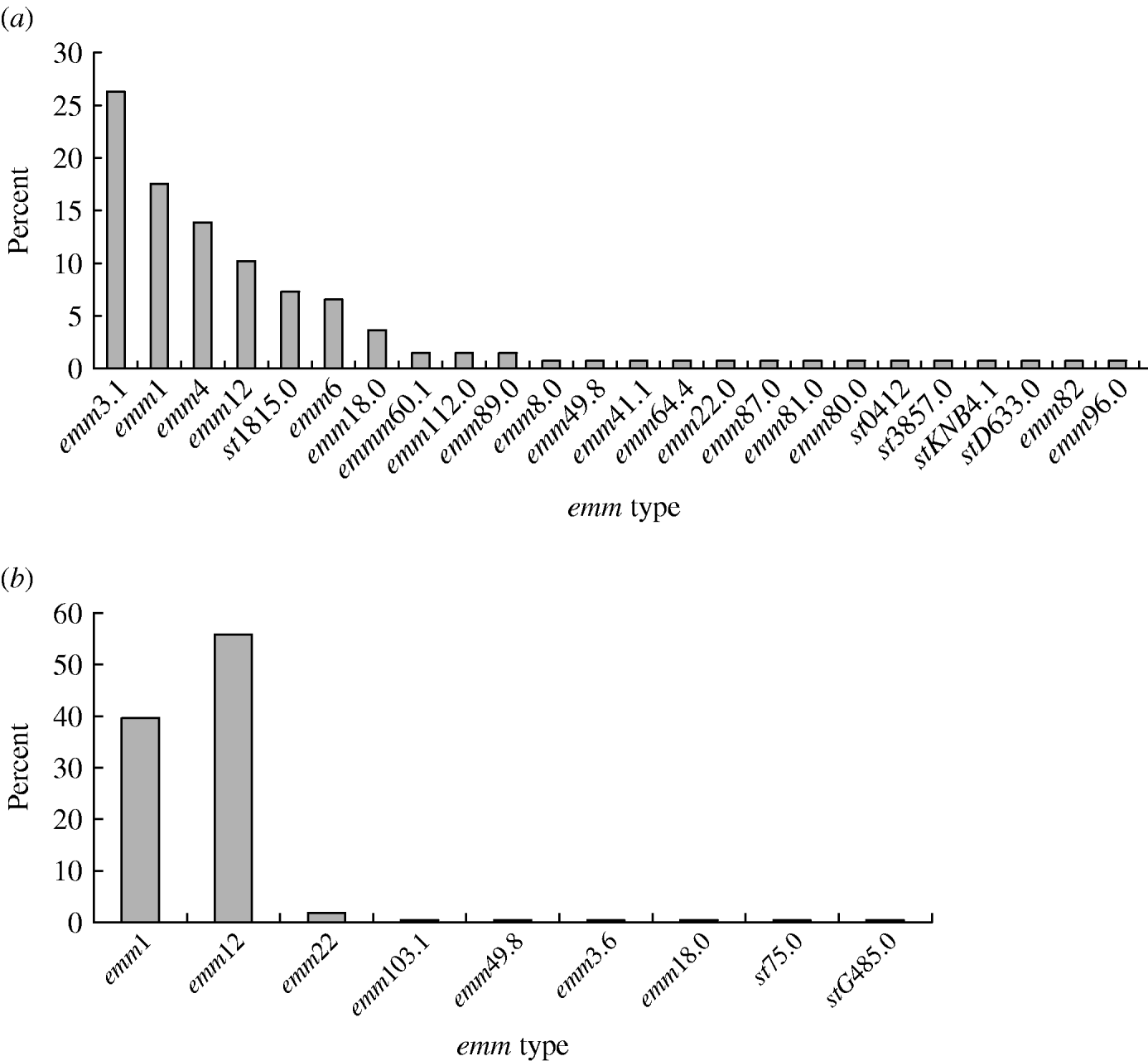
Fig. 1. Distribution of emm types among GAS strains isolated from patients during (a) period A (1993–1994), (b) period B (2005–2006).
Distribution of emm types among scarlet fever and pharyngitis isolates
In period A, 14 emm types were identified among the pharyngitis isolates; emm1, emm3 and emm12 alone constituted 21·7%, 21·8% and 13·0%, respectively. Ten types were represented among scarlet fever isolates of which emm1, emm3 and emm12, respectively, accounted for 14·8%, 44·4% and 7·4% of the isolates. By contrast, in period B, only four emm types were found among the pharyngitis isolates; emm1 and emm12 alone constituted 43·2% and 52·3% of the emm types, respectively. These types were also prevalent among scarlet fever isolates (emm1, 40·3%; emm12, 56·1%) where only five different types were identified. No significant difference was revealed in the emm-type distributions among scarlet fever and pharyngitis isolates (P>0·05) over the two sampling periods.
Distribution of SAg genes
All eight SAg genes, speA (68·4%), spec (92·6%), speH (32·6%), speI (78·1%), speG (91·9%), speJ (28·4%), ssa (51·1%) and SMEZ (97%) were identified in isolates from the first sampling period. In period B, their respective frequencies were speA (46·8%), speC (98·6%), speH (63·5%), speI (76·8%), speG (96%), speJ (41·8%), ssa (92·5%) and SMEZ (100%). The incidence of ssa, speH and speJ genes (P<0·05) increased significantly over the two periods while speA decreased (P<0·05). Overall isolates from the first period carried six or more SAg genes (46·5%) compared with 78·4% in period B (P<0·05).
emm type and SAg gene profile
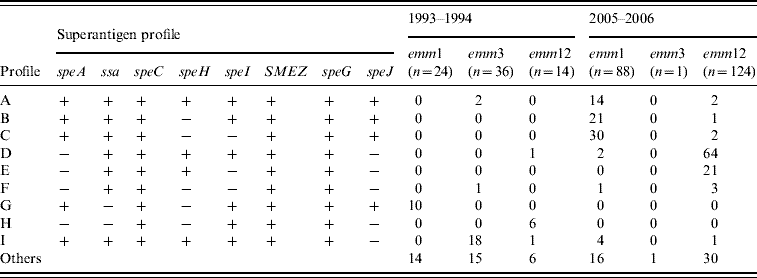
Table 1 shows the distribution of the erythrogenic toxin genes (speA and speC) in two common emm types. In period A, all emm1 isolates harboured speA but two-thirds carried speC genes (66·7%). This was reversed for emm12 isolates where speA was 35·7% and all were positive for speC genes. However, in the second sampling emm1 isolates harboured 94·3% and 97·7% of speA and speC, respectively, while emm12 isolates carried 8·87% and 100% of speA and speC. We also analysed the distribution of prevalent SAg gene profiles (Table 2), among the most common emm types (emm1, emm3, emm12). In period A, the profiles A and E combined accounted for only 7·8% of isolates while 41·6% of emm1 isolates were of profile G. Other common associations were emm12 and profile H (42·8%) and, emm3 and profile I (50%). This contrasted markedly with the second-sample isolates where profiles A, B and C combined accounted for 73·9% of emm1 isolates and profiles D and E combined represented 68·5% of emm12 isolates.
Table 1. Distribution of erythrogenic toxin genes (speA and speC) in GAS isolates between 1993–1994 (period A) and 2005–2006 (period B) in China

* P<0·05 by comparison of emm1 isolates between periods A and B.
** P<0·05 by comparison of emm12 isolates between periods A and B.
Table 2. The nine predominant superantigen gene profiles and their relationship with the prevalent M types among 359 GAS isolates from Chinese paediatric patients
SAg genes among scarlet fever and pharyngitis isolates
In period A, speA, speC, speH, speI, speG, speJ, ssa and SMEZ among the pharyngitis isolates alone constituted 67·4%, 89·1%, 34·8%, 76·1%, 86·9%, 26·1%, 47·8% and 89·1%, respectively, compared with the period B where they were 47·7%, 100%, 56·8%, 56·8%, 86·4%, 45·5%, 97·7% and 100%, respectively. For scarlet fever isolates the positive rates of speA, speC, speH, speI, speG, speJ, ssa and SMEZ genes were 75·9%, 88·9%, 48·1%, 79·6%, 92·6%, 31·5%, 62·9% and 87·0%, respectively, in period A, but 44·6%, 98·6%, 66·9%, 69·1%, 89·3%, 38·8%, 95·7% and 100%, respectively, in period B. No significant difference was revealed in the distribution of SAg genes among scarlet fever and pharyngitis isolates (P>0·05) for the same period.
DISCUSSION
The distribution of emm types in GAS isolates varies with geographical region and between and within individual countries. A survey in Italy spanning 11 years showed that the majority of isolates were of emm types 1, 4 and 12, but types 1 and 12 declined towards the end of the sampling period while types 3, 22 and 77 appeared [Reference Creti11]. Similar findings were reported from other countries [Reference Espinosa12–Reference Vlaminckx, van Pelt and Schellekens16]. The present study is the first to review a large number of GAS isolates in different parts of China over an extended period. We found the majority of isolates fell in emm types 1, 12 and 3, followed by types 4, 6 and st1815, with emm3, emm4, st1815 and emm6 declining over time. Meanwhile, emm1 and emm12 isolates appeared more recently and accounted for the most of the isolates at the end of the 12-year sampling period. During 2005–2006, GAS isolates became less diverse compared to 1993–1994 with 24 emm types decreasing to just nine emm types, indicating that a substantial change had occurred.
Seroepidemiological studies have shown that different emm types of S. pyogenes are associated with different diseases. Historically, emm types such as 1, 2, 3, 4, 12, 15, 49, 55, 56, 59, 60 and 61 have been associated with post-streptococcal glomerulonephritis, while types 5, 6, 18, 19 and 24 have been linked to rheumatic fever [Reference Bisno17–Reference Johnson, Stevens and Kaplan19]. We did not find a significant relationship between emm type and clinical origin of GAS, in accord with recent studies [Reference Rivera8, Reference Ho9, Reference Descheemaeker20], but it should be noted that there were relatively few representatives from invasive infections in the studied collection.
There are two reports in the literature on GAS emm-type distribution from regions of China. In Hong Kong, Ho et al. [Reference Ho9] found types 1, 4 and 12 to be among the most common and type 3 was totally absent from their survey of 1997–1998. Jing et al. [Reference Jing10] reported types 1 and 12 as the most common in China during 2003–2004 and also identified a broader range of types than found here in 1993–1994 which was the period of greatest diversity of types. However, this survey confirms the predominance of emm types 1 and 12 in the Chinese paediatric population resident in four large industrial cities in China. Moreover, it is clear that the pattern of emm-type distribution of GAS strains changes over time, and may be influenced by the sample population and/or different age groups. The results obtained here suggest that the currently available 26-valent M-protein based vaccine might provide >95% coverage for Chinese paediatric patients.
We found that the majority of GAS isolates collected from both periods carried around six or more SAg genes and there was a significant increase in the presence of ssa, speH and speJ genes over the 12-year period. The distribution of erythrogenic toxin genes speA and speC has been shown to be highly variable in different GAS populations. In a Polish study the speA gene was present, and speC absent, in emm1 isolates while all emm12 isolates lacked speA [Reference Szczypa5]. Similar results have been reported elsewhere [Reference Ekelund14, Reference Shulman21–Reference Vlaminckx23]. A survey from mainland China reported frequencies of 84% for speA and 44% for speC in emm1 isolates while 35% of emm12 isolates carried speA and all carried speC genes [Reference Jing10]. Associations between speA, speC and emm type were likewise apparent in our findings, especially for speA, whose frequency was similar to that found in previous local and international studies; however, the speC gene was more frequent in our emm12 strains than in other studies. This may be a particular feature of Chinese paediatric isolates.
The relationship of toxin gene profiles to emm types has been noted by several groups [Reference Szczypa5, Reference Ekelund14, Reference Descheemaeker20, Reference Ekelund22, Reference Vlaminckx23]. Here, nine predominant exotoxin gene profiles were identified, with some profiles associated with individual emm types, and these changed over the sampling period. Isolates with the same emm type commonly shared the same toxin gene profile and some strains of the same emm type had different toxin gene profiles in the two sampling periods. This suggests that the toxin gene profiles of GAS prevalent in Chinese children differ from those obtained by other studies [Reference Rivera8, Reference Ekelund14, Reference Tanna15, Reference Ekelund22]. Moreover, the distribution of SAg genes provides an additional epidemiological tool to explore genomic heterogeneity and the possible correlation between toxin gene content and disease type [Reference Rivera8, Reference Vlaminckx23]. Previous studies from Poland, The Netherlands, Denmark and Belgium [Reference Szczypa5, Reference Descheemaeker20, Reference Ekelund22, Reference Vlaminckx23] support the strong association between invasive GAS isolates with emm1 type and the presence of the speA gene. However, no significant difference in toxin gene profile was evident between the invasive and non-invasive isolates in other studies [Reference Rivera8, Reference Vlaminckx, van Pelt and Schellekens16]. It would have been of interest to explore associations of SAg genes with disease presentation but the lack of invasive isolates in this collection prevented this.
ACKNOWLEDGEMENTS
This work was supported by the Hi-technology Research and Development Program of the Ministry of Science and Technology, China (No. 2006AA02Z417). We thank Professor Pat Cleary of the Department of Microbiology Medical School, University of Minnesota, Patricia Ferrieri of the Department of Pediatrics, Division of Infectious Diseases, University of Minnesota Medical School, and Alexander Suvorov of the Department of Molecular Microbiology, Institute of Experimental Medicine, Russia, for their assistance in this project. This work was performed in the Department of Microbiology and Immunology of Beijing Children's Hospital. We thank all the staff of the department for their support, especially Lin Yuan.
DECLARATION OF INTEREST
None.





