CHD continues to be the leading cause of death, both in the USA and worldwide, with dyslipidaemia being a major risk of acute cardiovascular events( Reference Roger, Go and Lloyd-Jones 1 ). Other intimately related risk factors, such as central adiposity, insulin resistance, glucose intolerance and hypertension, tend to co-manifest with dyslipidaemia, creating a dysfunctional state referred to as the metabolic syndrome that dramatically increases the risk of type 2 diabetes and CHD( Reference Prasad, Ryan and Celzo 2 ).
Several lines of evidence show that diet is one of the most manageable factors for preventing the metabolic syndrome and its progression in the human population( Reference Leão, de Moraes and de Carvalho 3 ).
The obese Zucker rat (OZR) is a valid experimental model for the human metabolic syndrome due to its similarities with the characteristics and progression of this condition in humans( Reference De Artiñano and Castro 4 ). OZR have impaired satiety reflex and chronic elevation in food intake, increased activity of the adipose lipoprotein lipase (LPL), defective catabolism of chylomicrons and overproduction of hepatic VLDL( Reference Frisbee and Delp 5 ). By the age of 8 weeks, they develop obesity and a profound dyslipidaemic state, with hypertriacylglycerolaemia and hypercholesterolaemia( Reference De Artiñano and Castro 4 ).
Wild blueberries (Vaccinium angustifolium) are commercially available berries containing high levels of antioxidant polyphenols, mostly anthocyanins( Reference Häkkinen and Törrönen 6 ).
Our previous studies have demonstrated the potential of wild blueberries to enhance vasorelaxation pathways and reduce vasoconstriction in Sprague–Dawley( Reference Norton, Kalea and Harris 7 ) and spontaneously hypertensive rats( Reference Kristo, Kalea and Schuschke 8 ).
Anthocyanins have been shown in vitro to affect the expression of genes involved in lipid metabolism, including fatty acid synthase, LPL and ATP-binding cassette transporter 1 (ABCA1)( Reference Wei, Wang and Yang 9 – Reference Xia, Hou and Zhu 11 ). Anthocyanins have also been shown to modulate the transcriptional activities of different nuclear receptors that control lipid homeostasis, including PPAR in rats and sterol regulatory element-binding proteins (SREBP) in mice( Reference Seymour, Singer and Kirakosyan 12 – Reference Seymour, Tanone and anes 14 ).
In the present study, we used the OZR model of the metabolic syndrome to study the effects of a wild blueberry-enriched diet on plasma lipid profile and the genetic expression of selected enzymes and transcription factors that play crucial roles in lipid and cholesterol homeostasis.
Experimental methods
Zucker rats
A total of twenty male OZR (fa/fa) and twenty male LZR (Fa/Fa) were purchased from Charles River Laboratories at 8 weeks of age and randomly assigned to either a control (C) or a wild blueberry-enriched (WB) diet for 8 weeks.
The rats were housed in individual mesh-bottomed cages in a room at 22°C and under a 12 h light–12 h dark cycle. Food consumption was recorded daily, and the rats were weighed weekly. The experimental protocol was approved by the University of Maine Institutional Animal Care and Use Committee.
Wild blueberries
Wild blueberries were provided as a composite (Wyman's), freeze-dried powder following standard procedures (FutureCeuticals), vacuum-packed in individual plastic bags, stored at − 20°C and used within 3 months.
The concentration of anthocyanins and profile of the WB powder were determined using liquid chromatography/MS as described previously( Reference Del Bo’, Ciappellano and Klimis-Zacas 15 ). Total phenolic content was determined spectrophotometrically using the Folin–Ciocalteu method( Reference Singleton, Orthofer and Lamuela-Ravent 16 ). Fibre content was determined using the AOAC International method 991.43( 17 ). Sugar content was determined using ultra-HPLC/MS, as described previously( Reference Vendrame, Guglielmetti and Riso 18 ).
Diets
Diets were prepared from purified ingredients, stored at 4°C and used within 1 week. The C diet was prepared by mixing 691 g/kg of dextrose, 200 g/kg of egg-white solids, 60 g/kg of maize oil, 35 g/kg of mineral mix (AIN-93M), 10 g/kg of vitamin mix (AIN-93), 4 g/kg of dl-methionine and 0·002 g/kg of biotin. It provided approximately 71 % carbohydrate, 6 % fat, 17 % protein and 1687 kJ/100 g. The WB diet was of the same composition, except for the incorporation of an 8 % WB powder substituting for an equivalent amount of dextrose. It provided approximately 68 % carbohydrate, 6 % fat, 17 % protein, 1·5 % fibre, 0·12 % anthocyanin and 1645 kJ/100 g. On using the body surface area calculation( Reference Reagan-Shaw, Nihal and Ahmad 19 ), this amount would be equivalent to approximately two cups of fresh WB per d for an average human adult.
Tissue collection
At 16 weeks of age, after an overnight fast, the rats were anaesthetised with 95 % CO2–5 % O2 for approximately 2 min and exsanguinated by cardiac puncture. Blood was immediately collected for plasma separation and storage at − 80°C until further analysis. The liver and abdominal adipose tissue (AAT) were excised, snap-frozen in liquid N2 and stored at − 80°C for subsequent mRNA isolation.
Blood lipid profile
Plasma samples were analysed for TAG, total cholesterol (TC) and HDL-cholesterol concentrations. TAG and TC concentrations were measured using colorimetric kits (Cayman no. 10010303 and Cayman no. 10007640). HDL-cholesterol concentration was measured using a fluorometric kit (BioVision no. K613–100). Non-HDL-cholesterol (LDL+VLDL) concentration was calculated by subtracting HDL-cholesterol from TC.
Hepatic and AAT mRNA isolation and reverse transcription
Hepatic mRNA was isolated from frozen samples using the RNeasy Mini Kit (Qiagen no. 74 104), while ATT mRNA was isolated using the RNeasy Lipid Tissue Mini Kit (Qiagen no. 74 804). Absorbance at 260 and 280 nm was measured using UV-transparent cuvettes to estimate quantity and control for the quality of the isolated mRNA. The samples of mRNA were subsequently retro-transcribed to cDNA using the QuantiTect Reverse Transcription Kit (Qiagen no. 205313).
Real-time RT-PCR
The reverse transcription product was subjected to two-step, real-time, RT-PCR amplification on a quantitative PCR System (Bio-Rad CFX96) using SYBR Green master mix (SSoFast EvaGreen, Bio-Rad no. 172-5202; BioRad Laboratories) and commercially available rat-specific primer sequences (Qiagen) targeting the following genes: PPARα (Ppara, RefSeq NM013196); PPARγ (Pparg, RefSeq NM001145 366, NM001145 367, NM013124); sterol regulatory element-binding protein 1 (Srebf1, RefSeq XM001075 680, XM213329); fatty acid synthase (Fasn, RefSeq NM017332); LPL (Lpl, RefSeq NM012598); ABCA1 (Abca1, RefSeq NM178095). A standard curve was constructed for all the primers on a pooled sample of complementary DNA, and the efficiency was found to be in the 90–100 % range. For each primer and tissue, the analysis was performed in triplicate with a reaction volume of 20 μl per well (1·5 μl reverse transcription product, 10 μl SYBR Green mix, 2 μl primers and 6·5 μl RNase-free water). After enzyme activation (95°C for 30 s), forty-five amplification cycles were performed (denaturation at 95°C for 2 s and annealing/extension at 60°C for 5 s) followed by the construction of a melting curve (75–95°C) to ensure the specificity of amplification. The relative expression of the genes of interest was determined by the ΔΔCt method( Reference Livak and Schmittgen 20 ) relative to that of the housekeeping gene β-actin. The results are reported as fold variation compared with those of lean Zucker rats (LZR) on the C diet.
Statistical analysis
Results for each marker were analysed using a two-way ANOVA, with diet (WB v. C) and animal model (OZR and LZR) as independent factors. Significant main effects and interactions were further evaluated by means of Tukey's honestly significant difference post hoc test. Statistical analysis was performed using R statistical software version 2.15.1 (R Foundation for Statistical Computing). The results are expressed as means with their standard errors, and they were considered significant at P< 0·05.
Results
Food intake and animal weight
Average daily food intake was significantly higher in the OZR (30·1 (sem 2·6) g) than in the LZR (24·4 (sem 2·3) g, P< 0·05), but without statistically significant differences between the WB and C groups. In the OZR, both average weight gain during the experiment (270 (sem 46) g) and average body weight during the time of killing (583 (sem 72) g) were significantly higher than those in the LZR (168 (sem 17) and 383 (sem 20) g, respectively, P< 0·05), but without statistically significant differences between the WB and C groups throughout the feeding period.
Wild blueberry powder composition
The total anthocyanin content of the WB powder was 1·5 % (w/w), with malvidin-3-galactoside, peonidin-3-glucoside and delphinidin-3-glucoside being the most abundant anthocyanins. Total phenolic content (gallic acid equivalents) was 4·4 % (w/w). The most abundant individual phenolic compound was chlorogenic acid (510 mg/100 g). Total fibre content was 18 % (w/w) (15·2 % insoluble fibre and 2·8 % soluble fibre). Total sugar content was 68·4 % (w/w) (35·2 % glucose and 33·2 % fructose).
Blood lipid profile
Plasma TAG, TC and HDL-cholesterol concentrations were significantly higher in the OZR than in the LZR, independent of the diet (Table 1). Within the OZR, plasma TAG and TC concentrations were significantly lower in the WB group than in the control group, whereas no differences were observed for HDL-cholesterol concentration. Within the LZR, no statistically significant differences were observed for any of the above-mentioned parameters, although the trends were similar to those observed in the obese group.
Table 1 Fasting plasma concentrations of TAG and cholesterol in lean Zucker rats (LZR) and obese Zucker rats (OZR) following the consumption of a control (C) or a wild blueberry diet (WB)(Mean values and standard deviations, n 10)
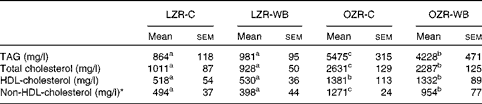
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Calculated by difference.
Hepatic gene expression
The expression of fatty acid synthase was dramatically higher in the OZR than in the LZR, independent of the diet (Fig. 1). The expression of SREBP-1 and PPARγ was also higher in the OZR, while that of ABCA1 and PPARα was not statistically different in the OZR and LZR. The expression of SREBP-1 and fatty acid synthase was significantly decreased in the OZR on the WB diet. Variations in the levels of other markers were not statistically significant.

Fig. 1 Relative expression of the genes related to lipid metabolism in the liver of lean Zucker rats (LZR) and obese Zucker rats (OZR): (A) PPARα; (B) PPARγ; (C) sterol regulatory element-binding protein 1; (D) fatty acid synthase; (E) ATP-binding cassette transporter A1. Values are means (n 10), with their standard errors represented by vertical bars. a,b,cMean values within a row with unlike letters were significantly different (P< 0·05). C, control diet; WB, wild blueberry diet.
Genetic expression in the abdominal adipose tissue
The expression on PPARα, PPARγ and ABCA1 was not statistically different in the LZR and OZR, independent of the diet (Fig. 2). The expression of fatty acid synthase, LPL and SREBP-1 was higher in the OZR than in the LZR. Following WB consumption, the expression of the transcription factors PPARα and PPARγ was increased in the AAT in both the OZR and LZR, while that of SREBP-1 was decreased. The expression of fatty acid synthase was significantly decreased in both the OZR and LZR, while that of LPL was increased in the LZR only. The expression of ABCA1 was increased in both the OZR and LZR.

Fig. 2 Relative expression of the genes related to lipid metabolism in the abdominal adipose tissue of lean Zucker rats (LZR) and obese Zucker rats (OZR): (A) PPARα; (B) PPARγ; (C) sterol regulatory element-binding protein 1; (D) fatty acid synthase; (E) lipoprotein lipase; (F) ATP-binding cassette transporter A1. Values are means (n 10), with their standard errors represented by vertical bars. a,b,c,dMean values within a row with unlike letters were significantly different (P< 0·05). C, control diet; WB, wild blueberry diet.
Discussion
The present study investigated the ability of a WB-enriched diet to improve parameters related to the pathogenesis of the metabolic syndrome, focusing, in particular, on blood lipid profile and expression of genes related to lipid metabolism in the liver and AAT. The experimental model used was the OZR, in which the development of the metabolic syndrome and its associated abnormalities is very similar to that observed in humans( Reference De Artiñano and Castro 4 ).
The present study is the first to report on a significant TAG-lowering effect of WB in vivo. The overall improvement in blood lipid profile observed in the OZR following WB consumption with both plasma TAG and cholesterol concentrations being significantly decreased is consistent with previous animal studies reporting an association between blueberry consumption and lowered cholesterol and TAG concentrations. The consumption of 1, 2 and 4 % blueberry-supplemented diets for 8 weeks significantly reduced not only LDL-cholesterol concentration but also HDL-cholesterol concentration in pigs( Reference Kalt, Foote and Fillmore 21 ), while supplementation with a 2 % highbush blueberry powder for 90 d significantly reduced serum TAG concentration in OZR and LZR fed a high-fat diet( Reference Seymour, Tanone and anes 14 ).
In the present study, the decrease in TC concentration was accounted for by decreased LDL-cholesterol and/or VLDL-cholesterol concentrations, while no change was observed in HDL-cholesterol concentration, a significant finding considering the importance of maintaining or increasing HDL-cholesterol concentration with dietary interventions. Overall, the observed changes in lipid profiles following the consumption of the WB diet in the OZR appear to be markedly beneficial, considering that high plasma TC, non-HDL-cholesterol and TAG concentrations are implicated in the pathogenesis of atherosclerosis and recognised risk factors of CHD( Reference Roger, Go and Lloyd-Jones 1 ).
It must be noted that recent studies have not confirmed this effect in humans, possibly due to the high inter-individual variability. An intervention with 50 g freeze-dried highbush blueberries administered daily for 8 weeks, while showing a reduction in blood pressure and oxidised LDL concentration, did not lead to significant variations in lipid profiles in obese subjects with the metabolic syndrome( Reference Basu, Du and Leyva 22 ). Another interventional study on individuals at a risk of developing CVD has also failed to report any effects on lipid profiles after 6 weeks of consumption of a drink providing 25 g freeze-dried WB( Reference Riso, Klimis-Zacas and Del Bo’ 23 ), probably due to the already low baseline values. Purified anthocyanins (320 mg twice a day for 24 weeks) have been shown to significantly decrease LDL-cholesterol concentration and increase HDL-cholesterol concentration in hypercholesterolaemic individuals, without affecting TC and TAG concentrations( 24 ).
To better understand the metabolic effects of WB consumption, we focused on the genetic expression of selected enzymes and transcription factors that regulate lipid and cholesterol homeostasis.
Anthocyanins such as cyanidin-3-glucoside have been shown in vitro to regulate the expression and activity of key enzymes involved in TAG and cholesterol metabolism, including LPL( Reference Wei, Wang and Yang 9 ), fatty acid synthase( Reference Tsuda, Ueno and Kojo 10 ) and ABCA1( Reference Xia, Hou and Zhu 11 ), which could explain the observed effects of blueberry consumption on lipid profiles.
In the present study, the expression of LPL in the AAT was found to be almost threefold higher in the OZR than in the LZR. Following the consumption of the WB diet, the expression of LPL was significantly up-regulated in the LZR, reflecting the up-regulation of the expression of PPARα observed in the AAT. Indeed, the expression of LPL is under the transcriptional control of PPARα and promotes the release of fatty acids from circulating lipoproteins and their subsequent cellular uptake( Reference Wang and Eckel 25 ).
The expression of fatty acid synthase, which is under the transcriptional control of SREBP-1c( Reference Griffin and Sul 26 ), was more than twelvefold higher in the liver of the OZR and threefold higher in the AAT of the OZR than in those of the LZR. Following the consumption of the WB diet, the expression of fatty acid synthase was significantly decreased in the liver and AAT of the OZR and in the AAT of the LZR. Since fatty acid synthase promotes fatty acid synthesis and storage in the adipose tissue, it has been proposed as a possible target for the treatment of the metabolic syndrome( Reference Griffin and Sul 26 ). Its down-regulation by the WB diet could partly explain the TAG-lowering effect and is consistent with the down-regulation of the expression of SREBP-1 that we observed.
The expression of ABCA1 in the liver was similar in the OZR and LZR and was not affected by WB consumption. In the AAT, the WB diet significantly up-regulated the expression of ABCA1 in both the OZR and LZR. By increasing cholesterol efflux and apoA1-mediated reverse cholesterol transport( Reference Soumian, Albrecht and Davies 27 ), the up-regulation of the expression of ABCA1 could play a significant role in the cholesterol-lowering effect that we documented in the OZR following the consumption of the WB diet.
The expression of the above-mentioned enzymes and the balance between lipid anabolism and catabolism, in general, are regulated by the activity of transcription factors such as SREBP and PPAR. The modulation of these elements is the target of important lipid-lowering medications such as fibrates (PPARα agonists) and thiazolidinedione (PPARγ agonists), but it has also been reported to be associated with dietary bioactive compounds including anthocyanins( Reference Seymour, Singer and Kirakosyan 12 ).
SREBP-1 isoforms promote the synthesis and accumulation of TAG and cholesterol via the induction of multiple enzymes( Reference Horton, Goldstein and Brown 28 ). In mice fed an anthocyanin-rich purple-coloured ‘corn’ diet for 2 weeks in addition to either a standard or a high-fat diet, the expression of genes involved in fatty acid and TAG synthesis was down-regulated, including fatty acid synthase and SREBP-1 in the adipose tissue( Reference Tsuda, Horio and Uchida 13 ). In the present study, the expression of SREBP-1 was significantly higher in the OZR than in the LZR and was down-regulated in the OZR following WB consumption in both the liver and AAT. The down-regulation of the expression of SREBP-1 could partly explain the reduction in circulating TAG and cholesterol concentrations that was observed in the OZR following the consumption of the WB diet. The activation of PPARα also helps to explain such an effect on lipid profiles, since PPARα promotes lipid catabolism by up-regulating the expression of a variety of enzymes involved in fatty acid cellular uptake and β-oxidation, including LPL, acyl-CoA oxidase and acyl-CoA dehydrogenase( Reference Fruchart 29 ). The consumption of the WB diet significantly up-regulated the expression of PPARα in the AAT of both the LZR and OZR.
The expression of PPARγ in the AAT was similar in the LZR and OZR and was significantly up-regulated in both the groups following the consumption of the WB diet. PPARγ is primarily expressed in the adipose tissue and controls adipocyte differentiation and insulin sensitivity, by up-regulating the expression of GLUT as well as a variety of genes involved in insulin signalling( Reference Takano and Komuro 30 ). Similar effects were observed in the AAT and skeletal muscle of OZR fed a 2 % highbush blueberry diet for 13 weeks, resulting in increased PPARα and PPARγ activity and mRNA expression( Reference Seymour, Tanone and anes 14 ).
The dual PPAR agonistic effect observed on WB consumption is a positive outcome in the context of the metabolic syndrome, allowing to combine the lipid-lowering effects of PPARα up-regulation with the improved glucose tolerance associated with PPARγ activity.
Besides controlling lipid metabolism, the activation of both PPARα and PPARγ also has a strong anti-inflammatory effect, probably via the down-regulation of NF-κB activity( Reference Poynter and Daynes 31 ). Indeed, we have previously documented that a WB diet resulted in a significant attenuation of the expression of NF-κB in the liver of both LZR and OZR and decreased the levels of inflammation as measured by circulating concentrations and genetic expression of inflammatory markers including IL-6 and TNFα( Reference Vendrame, Daugherty and Kristo 32 ).
It should be noted that the present study evaluated the effects of the whole berry and at this point we are not able to determine the specific contribution of the polyphenols, fibre or other bioactive compounds of the berry to the observed effects.
In conclusion, 8-week WB consumption appears to have a positive impact on lipid profiles in dyslipidaemic OZR, with significant reductions in serum TAG and TC concentrations with the maintenance of beneficial HDL-cholesterol concentrations. Wild blueberry consumption also appears to positively affect the expression of key enzymes and transcription factors involved in lipid and cholesterol metabolism, thus reducing risk factors associated with the metabolic syndrome and its progression to type 2 diabetes and CHD.
Acknowledgements
The present study was supported by the Wild Blueberry Association of North America (WBANA) and Maine Agricultural and Forest Experiment Station (publication no. 3300).
S. V. and D. K.-Z. designed the research. S. V., A. D. and A. S. K. conducted the research. S. V. and D. K.-Z. wrote the paper. All authors read and approved the final manuscript. None of the authors has any conflicts of interest.





