Introduction
Tuberculosis (TB) is an infectious disease without widespread effective treatments available caused by Mycobacterium tuberculosis and can potentially involve any organ, affecting approximately a quarter of the world's population with more than 10 million new TB cases every year [Reference Singh1]. TB infections affect the lungs in the majority of patients, namely pulmonary tuberculosis (PTB), and also frequently occur in extrapulmonary sites, such as the intestine, meninges, bones, joints and lymph nodes, resulting in extrapulmonary tuberculosis (EPTB) [Reference Ferdous2, Reference Suárez3]. EPTB can exist alone, or coexist with PTB simultaneously [Reference Ohene4]. EPTB has various clinical presentations with head and neck TB as the most common presentation, posing a grave challenge to diagnosis because of its obscure paucibacillary nature and location [Reference Qian5, Reference Hayward6]. Thanks to rapid technological advancements in recent years, the currently used tests for rapid diagnosis have tremendously increased, such as molecular tests GeneXpert assay, liquid culture system (Bactec 960) and line probe assays [Reference Sharma, Mohan and Kohli7]. Clinical imaging examinations such as 18FDG-PET-CT and PET-MRI are critical to determine anatomical localisation and the extent of EPTB [Reference Gambhir8]. The standard medication for TB or EPTB is a 2-month administration of rifampicin, isoniazid, ethambutol and pyrazinamide followed by a 4-month administration of rifampicin and isoniazid [Reference Pascual-Pareja9].
A recent study reports EPTB in 33.4% of hospitalised TB patients in Beijing Chest Hospital, Beijing, China from January 2008 to December 2017 [Reference Pang10]. Moreover, a large-scale multicentre observation study comprehensively analyses demographic characteristics and prevalence of EPTB in inpatients covering 21 hospitals of 15 provinces in China [Reference Kang11]. However, there is a lack of studies on differential epidemiological characteristics of EPTB patients with or without PTB in China.
An EPTB registration and reporting system has been established in Shanghai, China, since mid-1990s. All newly diagnosed EPTB cases in medical institutions at different levels are reported to TB prevention and control institutes, which regularly conduct random checks on these EPTB cases and further report them to Shanghai Municipal Center for Disease Control. Shanghai Pulmonary Hospital Affiliated to Tongji University School of Medicine is the Shanghai Clinical Research Center for Infectious Diseases (TB), and its newly admitted TB cases account for 60–70% of all TB cases in Shanghai every year. Therefore, it is the most important medical institution for the diagnosis and management of TB in Shanghai. We conducted a retrospective study on all EPTB cases admitted in this hospital from January 2015 to December 2020, and comparatively analysed epidemiological characteristics of EPTB patients with or without PTB. This study would help reflect epidemiological characteristics of EPTB cases, improve clinical awareness of EPTB and provide helpful guidance on the formulation of treatment regimens.
Methods
Study design
We retrospectively studied all EPTB cases recorded in outpatient and inpatient registers of Shanghai Pulmonary Hospital affiliated to Tongji University School of Medicine from January 2015 to December 2020. The study was approved by the Ethnical committee of Shanghai Pulmonary Hospital affiliated to Tongji University School of Medicine. Obtaining written informed consent from each participant preceded this study.
Definitions of TB and diabetes mellitus (DM)
According to previous studies [Reference Di Tanna12, Reference Rodriguez-Takeuchi, Renjifo and Medina13], clinical diagnosis of PTB and EPTB was made based on the following terms: 1) typical clinical symptoms of TB such as low-grade fever in the afternoon, night sweats, fatigue and weight loss; 2) positive interferon-gamma release assays (IGRAs) result; 3) positive bacteriological test results of lesion tissue including acid-fast bacilli and positive culture result (Bectec960), or positive GeneXpert test result [Reference Kohli14]; 4) typical pathological manifestations including the presence of epithelioid cells, Langhans giant cells and caseous necrosis; 5) supportive imaging findings; 6) systemic and local anti-tuberculosis chemotherapy was effective.
DM [Reference Pippitt, Li and Gurgle15, Reference Kerner and Brückel16] was diagnosed based on typical clinical symptoms (polyuria, polydipsia and unexplained weight loss) and random plasma glucose ⩾ 11.1 mmol/l or fasting plasma glucose ⩾ 7.0 mmol/l (venous whole blood glucose ⩾ 6.1 mmol/l) or 2-h plasma glucose ⩾ 11.1 mmol/l in oral glucose tolerance test (OGTT). The death indicated all-cause mortality, including any death while still on treatment. Co-morbidities indicated other disorders or illnesses except EPTB or PTB in the same patient, including DM, psychological disorder, pneumoconiosis and others.
Statistical analysis
Statistical analysis was performed using SPSS (IBM, Armonk, New York, version 22.0). Quantitative data were expressed as means ± standard deviation (s.d.) and compared between different groups using students' t test. Qualitative data were expressed as n (%) and compared between different groups using Pearson's chi-square test or Fisher's exact probability test. Significance was defined using P < 0.05.
Uni-and multi-variable logistic regression analysis was performed to identify risk factors for concurrent EPTB and PTB, or exclusively EPTB. Uni- and multi-variable cox regression analysis was used to identify significant prognostic factors. Factors with P < 0.1 were uni-variately significant and were then entered into multi-variable cox regression analysis.
Results
This study collected a total of 3488 TB patients, including 2086 patients with concurrent PTB and EPTB (EPTB and PTB group, 59.8%), and 1402 patients with exclusively EPTB (EPTB group, 40.2%). Differences between the EPTB and PTB group and the EPTB group were statistically significant in age (42.82 ± 19.14 years vs. 48.82 ± 19.48 years, P < 0.001), gender (P < 0.001), incidences of co-morbidities (P = 0.002), severe symptoms (P = 0.015), whole-course management (P = 0.007) and mortality (P = 0.001). However, nationality was not significantly different between the two groups (P = 0.053, Table 1).
Table 1. Demographic and clinical characteristics of two groups

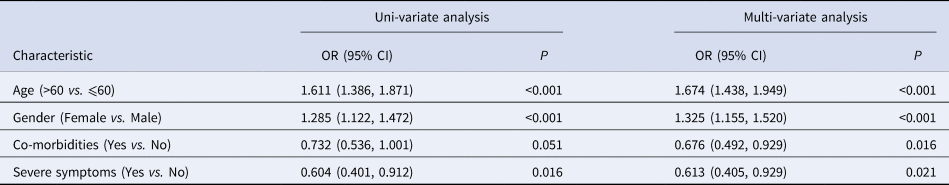
The factors associated with exclusively EPTB were analysed by using uni-variable logistic regression analysis and multi-variable logistic regression analysis. Uni-variable analysis suggested that patients with age >60 years (OR = 1.611; 95% CI = 1.386–1.871, P < 0.001) and female gender (OR = 1.285, 95% CI = 1.122–1.472, P < 0.001) were positively correlated with exclusively EPTB, whereas severe symptoms were negatively correlated with exclusively EPTB (OR = 0.604, 95% CI = 0.401–0.912, P = 0.016, Table 2). No significant correlation was observed for co-morbidities (P = 0.051, Table 2). According to multi-variable logistic regression analysis, age >60 years (OR = 1.674, 95% CI = 1.438–1.949, P < 0.001) and female gender (OR = 1.325, 95% CI = 1.155–1.520, P < 0.001) possessed positive correlations with exclusively EPTB, whereas co-morbidities (OR = 0.676, 95% CI = 0.492–0.929, P = 0.016) and severe symptoms (OR = 0.613, 95% CI = 0.405–0.929, P = 0.021) possessed negative correlations with exclusively EPTB (Table 2).
Table 2. Identification of factors associated with exclusively EPTB by uni- and multi-variable logistic regression analysis
During the therapeutic process, 48 (2.3%) and 12 (0.9%) deaths among patients were reported in the EPTB and PTB group and the EPTB group, respectively. Uni-variable Cox regression analysis showed that in the EPTB patients with concurrent PTB, age >60 years (OR = 15.545, 95% CI = 7.276–33.211, P < 0.001), co-morbidities (OR = 4.230, 95% CI = 2.107–8.491, P < 0.001) and extra- pulmonary involvement (P = 0.022) increased the risk of death, while female gender (OR = 0.106, 95% CI = 0.038–0.296, P < 0.001) decreased the risk of death (Table 3). Furthermore, according to results of multi-variable Cox regression analysis, age >60 years increased the risk of mortality with a HR value of 11.059 (95%CI = 5.097–23.999, P < 0.001, Table 3), while being female decreased the risk of mortality with a HR value of 0.178 (95%CI = 0.063–0.505, P = 0.001, Table 3). In contrast, only age was identified to be an independent prognostic factor (P = 0.002) for patients with exclusively EPTB by uni-and multi-variable Cox regression analysis, with age >60 years increasing the risk of mortality by 23.994 folds (95%CI = 3.093–186.151, Table 4).
Table 3. Identification of factors associated with risk of death using uni- and multi-variable Cox regression analysis for patients with concurrent EPTB and PTB
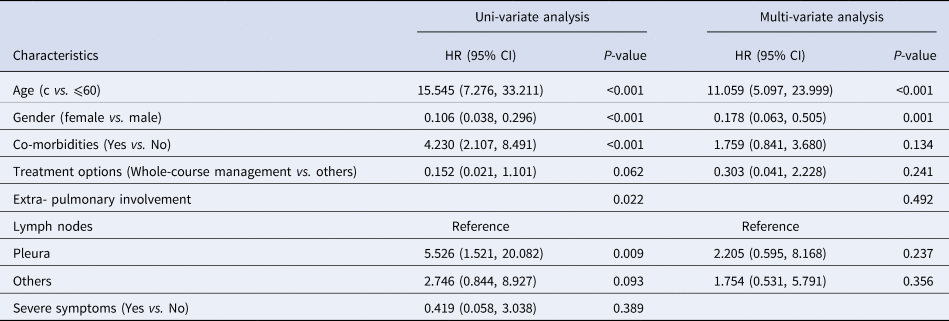
Table 4. Identification of factors associated with risk of death using uni-and multi-variable Cox regression analysis for patients with exclusively EPTB

Discussion
Approximately 15–25% of TB infections involve extrapulmonary sites and cause EPTB through haematogenous and lymphatic dissemination of M. tuberculosis dissemination [Reference Moule and Cirillo17]. Pleural, lymphatic and musculoskeletal TBs are among the most common sites of EPTB [Reference Leonard and Blumberg18]. Clinical signs and symptoms of EPTB vary largely with the affected organ, the disease aggressiveness and the host immune response, and its imaging representations may be vague [Reference Norbis19]. According to different EPTB locations and drug resistance, the treatment regimen may need to be adapted, such as elongating treatment time to 9–12 months for CNS and skeletal TB, and addition of steroids for meningeal and pericardial TB [Reference Natali20]. Surgical treatment plays a role in establishing the diagnosis of EPTB and the management of relevant complicated complications [Reference Fry21].
China has the third highest number of TB cases following India and Indonesia, accounting for 8.4% of global TB cases [Reference Chakaya22]. In the present study, we reviewed 3488 EPTB patients in Shanghai Pulmonary Hospital during the period from January 2015 to December 2020. Among them, 2086 patients (59.8%) were diagnosed with both EPTB and PTB, while 1402 patients (40.2%) were diagnosed with EPTB alone. Compared to the patients with exclusively EPTB, the patients with both EPTB and PTB appeared to be significantly younger, had a higher proportion of being male, more likely to suffer from complications or severe symptoms and had a poorer prognosis.
A transversal study conducted in northern Portugal reports that age older than 40 years and female gender are independent risk factors for EPTB [Reference Sanches, Carvalho and Duarte23]. A Turkey study consistently shows that women are more frequently affected by EPTB, while men are more commonly affected by PTB [Reference Sunnetcioglu24]. Yet, younger female patients from rural areas are at a higher risk of EPTB in Chinese TB patients [Reference Pang10]. It indicates that associations between age and EPTB may vary with geographical and ethnic parameters. Our study found that EPTB alone was prone to occur in female patients older than 60 years, while co-existence of EPTB and PTB was prone to be observed in male patients younger than 60 years, which were in congruence with previous results of a multi-centre observational study in China by Kang et al. [Reference Kang11].
EPTB are common in immuno-compromised patients, such as DM patients [Reference Gaifer25]. DM is a risk factor for TB that strongly impacts the diagnosis and treatment of TB, with the proportion of DM in all TB patients varying from 15% to 25% [Reference Ferdous2, Reference Jacob26]. Meanwhile TB is a common co-morbidity of depression and may precipitate depression through immune-inflammatory response [Reference Jacob27]. EPTB involving central nervous system or causing pericarditis or military tuberculosis can be life-threatening, while osteoarticular and urogenital tuberculosis can be disabling or severely impair functions [Reference Figueiredo, Lucon and Srougi28, Reference Vohra and Dhaliwal29]. In the present study, we found that EPTB patients with concurrent PTB were more likely to suffer from co-morbidities (psychological disorder and DM) and severe symptoms relative to patients with exclusively EPTB. It implies that immune function is weaker in the patients with concurrent EPTB and PTB compared to the patients with exclusively EPTB.
In the present study, 48 patients (2.3%) with concurrent EPTB and PTB and 12 patients (0.9%) with exclusively EPTB died during the therapeutic process. In concordance with a previous study [Reference Uchida30], in EPTB patients with or without PTB, age was an independent prognostic factor and increased the risk of mortality by 11.059 fold and 23.994 fold, respectively. Nonetheless, only in the EPTB patients with concurrent PTB, gender was identified to be an independent prognostic factor, and being female decreased the risk of mortality by 82.2%. It implies that male patients with concurrent EPTB and PTB older than 60 years are prone to have a poor prognosis.
With improved sensitivity and specificity than conventional methods, Xpert MTB/RIF (Xpert) is a fully automated real-time hemi-nested PCR system on the GeneXpert platform that allows for detection of complex DNA of M. tuberculosis, and drug susceptibility testing for rifampicin resistance [Reference Di Tanna12]. There is evidence that Gene Xpert is superior to acid-fast bacilli (AFB) smear and culture for the detection of urinary TB using urine specimens [Reference Pang31]. Xpert MTB/RIF is recommended to be an initial diagnostic tool for EPTB [Reference Tadesse32]. Gene Xpert, Bectec960 culture, and AFB were used for the diagnosis of TB and EPTB in our study.
Some limitations should be noted. Firstly, our study collected data of EPTB cases from one hospital only. Secondly, EPTB cases should be further classified according to extra-pulmonary sites prior to further analysis their clinical characteristics. Thirdly, HIV infection, a common co-morbidity of TB [Reference Letang33], was not focused in the current study. Future studies covering a larger scope of China are warranted to confirm and further our findings concerning Chinese EPTB patients.
Conclusion
Our study summarised epidemiological characteristics of EPTB cases in a hospital in China from January 2015 to December 2020. It showed that 2086 EPTB patients (59.8%) were diagnosed with concomitant PTB. Patients with concomitant TB and EPTB were more likely to be younger males and have high probabilities of comorbidities (psychological disorder and DM) and severe symptoms with a poor outcome. Age >60 years and female gender are risk factors for exclusively EPTB, while co-morbidities and severe symptoms are risk factors for concomitant PTB and EPTB. Female gender is a positive prognostic factor for concomitant TB and EPTB. This study has important implications for surveillance, diagnosis and management of EPTB in China. Concomitant PTB should be screened in EPTB patients to help develop effective treatment regimens.
Data
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
None.
Financial support
This work was funded by Shanghai Clinical Research Center for infectious disease (tuberculosis) (Grant ID: 19MC1910800), Shanghai key clinical specialty construction project – Tuberculosis department (Grant ID: Shslczdzc 03001) and Shanghai Leading medical talents Training Program (Grant ID: 2019LJ13).
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
The study was approved by the Ethnical committee of Shanghai Pulmonary Hospital, School of Medicine, Tongji University. Obtaining written informed consent from each participant preceded this study.
Consent for publication
Not applicable.
Author contributions
Conception and design of the research: Y. F.; acquisition of data: Q. Z. and L. L.; analysis and interpretation of data: Y. F. and Y. Z.; statistical analysis: Y. F. and W. S.; drafting the manuscript: Y. F.; revision of the manuscript for important intellectual content: W. S. All authors read and approved the final manuscript.







