Reported community prevalence rates for dementia and its subtypes in people aged 65 years and over show wide variation. A meta-analysis reported prevalence rates varying between 0.5 and 16.3% for mild dementia and 1.1-7.4% for moderate to severe dementia (Reference Jorm, Korten and HendersonJormet al, 1987). Dementia in Alzheimer's disease and vascular dementia are the most frequently identified subtypes in most autopsy and clinical studies, although the different diagnostic criteria used make it difficult to draw firm conclusions from the data (Reference Erkinjuntti, Ostbye and SteenhuisErkinjuntti et al, 1997). Dementia with Lewy bodies (DLB) is a relatively recently identified entity, and changing neuropathological and clinical diagnostic criteria as well as sampling bias have resulted in great variation in the reported frequency of this subtype (Reference McKeith, Galasko and WilcockMcKeith et al, 1995), ranging from 4.6% (Reference Forno and LangstonForno & Langston, 1988) to 24.7% (Reference Galasko, Hansen and KatzmanGalasko et al, 1994). The frontal lobe dementias (FLD) also have been described relatively recently (Reference BrunBrun, 1987). Although Oliva (Reference Oliva2000) estimated the distribution of this subtype at 10-20%, there is a dearth of epidemiological data on these dementias. There has been only one published study on the community prevalence of DLB and FLD, by Yamada et al (Reference Yamada, Hattori and Miura2001), which found the prevalence of DLB to be 0.1% and identified no cases of FLD. The aim of our study was to determine the frequency of subtypes of dementia according to standardised clinical criteria in a representative community population aged 65 years and over, with particular emphasis on DLB and FLD.
METHOD
Ethical approval was obtained from Camden & Islington Community Health Services National Health Service (NHS) Trust Local Research Ethics Committee.
Sample selection
Stage 1
Participants were recruited from Islington, north London, which has a Jarman under-privileged area score of 49, making it the sixth most deprived area in England and Wales (Reference JarmanJarman, 1983). Enumeration districts (smallest unit of population into which the UK is divided for the census) in Islington were selected randomly to provide a sampling frame. Following an introductory letter, a researcher visited each residence to ask whether a person aged 65 years or over was present and available for an interview.
The shortened version of the Comprehensive Assessment and Referral Evaluation (Short-CARE; Reference Gurland, Golden and TeresiGurland et al, 1984) was used to elicit psychiatric symptoms and diagnoses. This is a valid and reliable questionnaire with what are described as diagnostic scales for depression and dementia and a scale for activity limitation (designed to identify those who need help with day-today living). The scale for dementia has been validated against the outcome of worsening dementia or death.
Demographic data also were collected about each participant.
Stage 2
An experienced psychiatrist (T. S.) performed a follow-up assessment. Those identified as screen-positive for dementia, defined as a score of ≥7 on the dementia sub-scale of the Short-CARE, were asked to provide written consent to undergo more detailed evaluation. If the participant was unable to provide written consent, then the principal carer was asked to provide written assent instead.
The evaluation consisted of:
-
(a) A clinical history obtained from the participants and any carers, general practitioners (GPs), other health care professionals involved in their care, and medical and psychiatric notes. The history focused on the onset and duration of the illness, the rapidity of deterioration, the nature of progression, including the presence or absence of fluctuation, as well as the extent of impairment of the participant's functioning. Information was sought on past medical or psychiatric history, medication, use of alcohol or nicotine, occupational history, brain imaging and family history of dementia or other psychiatric illness.
-
(b) Mental state examination with particular emphasis on affective and psychotic symptoms and cognitive performance. This was obtained using both a clinical interview and the Geriatric Mental State (GMS) schedule (Reference Copeland, Kelleher and KelletCopeland et al, 1976).
-
(c) Further assessment of cognitive performance using the Mini-Mental State Examination (Reference Folstein, Folstein and McHughFolstein et al, 1975) and tests of frontal lobe function — Trail Making Test (Reference ReitanReitan, 1958), cognitive estimates (Reference Shallice and EvansShallice & Evans, 1978) and verbal fluency (Reference Borkowski, Benton and SpreenBorkowski et al, 1967), both category and letter fluency (‘FAS’) tasks.
-
(d) Comprehensive physical examination, including an assessment of frontal lobe release signs and Parkinsonian signs, using the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS; Reference Stern, Stern and HurtigStern, 1988), in the use of which the rater (T. S.) had been trained.
-
(e) The Modified Hachinski Score (Reference Hachinski, Ilif and ZilchaHachinskiet al, 1975).
-
(f) Laboratory investigations, including full blood count, serum urea and electrolytes, liver function tests, serum folate and vitamin B12, thyroid function tests and syphilis serology.
Diagnostic criteria for classification of dementia
Two raters (G. L. and T. S.) jointly made diagnoses in accordance with DSM—IV (American Psychiatric Association, 1994) and ICD—10 (World Health Organization, 1992) criteria. Alzheimer-type dementia, vascular dementia, dementia in Parkinson's disease and other/unspecified dementias were diagnosed using these criteria.
In addition, diagnoses were made as follows:
-
(a) Alzheimer's disease: National Institute of Neurological and Communicative Diseases and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS—ADRDA) criteria (Reference McKhann, Drachman and FolsteinMcKhann et al, 1984), which have been shown to have a high degree of diagnostic accuracy (Reference Lim, Tsuang and KukullLim et al, 1999).
-
(b) Vascular dementia: National Institute of Neurological Diseases and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neuroscience (NINDS—AIREN) criteria (Reference Roman, Tabemichi and ErkinjuntiiRoman et al, 1993), shown to have high specificity although low sensitivity for vascular dementia (Reference Gold, Giannakopoulos and Montes-Paixo-JuniorGold et al, 1997).
-
(c) Dementia with Lewy bodies: the consensus criteria for the diagnosis of DLB (Reference McKeith, Galasko and WilcockMcKeith et al, 1995), shown to have high sensitivity and specificity (Reference McKeith, Ballard and PerryMcKeith et al, 2000).
-
(d) Frontal lobe dementias: the Gregory and Hodges criteria (Reference Gregory and HodgesGregory & Hodges, 1993) for dementia of frontal type and the consensus criteria for frontotemporal dementia proposed by Neary et al (Reference Neary, Snowden and Gustafson1998) that are based on the Lund and Manchester criteria (Reference Brun, England and GustafsonBrun et al, 1994).
The rater carrying out the clinical assessments (T. S.) made clinical diagnoses prior to the application of the above criteria. In particular, the diagnosis of DLB was made in the presence of progressive dementia with prominent well-formed visual hallucinations if one or both of the following features were present: spontaneous motor features of parkinsonism; pronounced fluctuation in cognitive performance. Evidence of cerebrovascular disease on history, physical examination or neuroimaging did not rule out the diagnosis unless there was a clear temporal relationship between a cerebrovascular accident and the onset of symptoms. Similarly, no restriction was imposed on the duration of Parkinsonian symptoms prior to the onset of dementia. These separate ‘clinical’ diagnostic criteria were influenced by a post-mortem study by Hohl et al (Reference Hohl, Tiraboschi and Hansen2000), who reported a clinical diagnostic accuracy of 50% for DLB and found fewer hallucinations in the false-positive clinical cases, suggesting that hallucinations are an important diagnostic marker. This clinical approach to the diagnosis of DLB therefore differed from that taken by the consensus criteria in requiring the presence of visual hallucinations.
RESULTS
Stage 1
A total of 1085 participants were interviewed (response rate 85.3%).
Non-participants
Of the 197 people not interviewed, 64.3% were female. The reasons for non-participation were: 153 (77.7%) refused an interview, 16 (8.1%) could not be contacted, 15 (7.6%) did not speak English, 2 (1.0%) had other communication problems and for 11 (5.6%) a relative refused on their behalf.
Participants
The rest of the paper refers to those who participated. Ages ranged from 65 to 102 years, with a mean of 75 years, and 644 participants (59.4%) were female. A total of 1031 (95%) lived at home in privately owned, rented or sheltered accommodation, with the remaining 54 (5%) occupying residential or nursing care facilities; 507 participants (46.7%) lived alone.
Of the 1085 people screened, 107 (9.86%) scored as screen positive on the dementia scale of the Short-CARE and 71 (66.4%) of these were female. The age range was 65-102 years (median 74 years).
Sixty-nine people (64.5%; 95% CI 56-73%) lived in rented, sheltered or owner-occupied accommodation. Thus, the screened prevalence rate of dementia for those who lived in the community without 24-hour care was 6.7% (95% CI 6-9%). Thirty-eight people (35.5%; 95% CI 28-45%) lived in residential or nursing care. The screened prevalence rate for dementia in those with 24-hour care was therefore 70.4% (95% CI 57-81%). Sixty-seven people screened as having dementia (62.6%) were born in the UK, 17 (15.9%) in Africa or the Caribbean, 8 (7.5%) in Cyprus, Greece or Turkey, 5 (4.7%) in Ireland, 5 (4.7%) in other European countries and 5 (4.7%) in other countries outside Europe.
Stage 2
Hospital notes were obtained for 84 (78.5%) of the 107 people screened in stage 1 as having dementia and 64 (59.8%) subsequently were assessed in person; 10 people (9.3%) refused further evaluation, 16 (15%) had died and 17 (15.9%) were not traceable. Eight (80%) of the people who refused further evaluation were female. In three cases the people themselves refused, saying they did not want to answer any more questions. In all other cases a family member refused to allow their relative to be interviewed, the most common reason given being that the relative was unlikely to benefit from further assessment. The mean age was 82.3 years for people who had died, 81.6 years for those who were not contactable and 78.9 years for those who refused. Fourteen (87.5%) of the people who were dead at the time of attempted follow-up had lived at home when first seen, compared with two (12.5%) in residential care. Of those who refused, eight (80%) lived in rented or their own accommodation and one each in nursing and residential care.
Demography
The mean age of the 64 people evaluated further was 80.0 years and 40 (62.5%) of these were female: 39 (60.9%) lived in rented or owner-occupied accommodation, 2 (3.2%) in a nursing home and 23 (35.9%) in residential care. The mean time between interviews for stage 1 and stage 2 was 8.8 months.
Diagnoses
Sufficient information for clinical diagnoses to be made was obtained from the medical case notes, social services' reports, general practitioners and relatives of eight of the people who were not interviewed after the initial screening. Diagnoses thus were made for a total of 72 people (67.3% of the original 107 who scored above the screening cut-off for dementia).
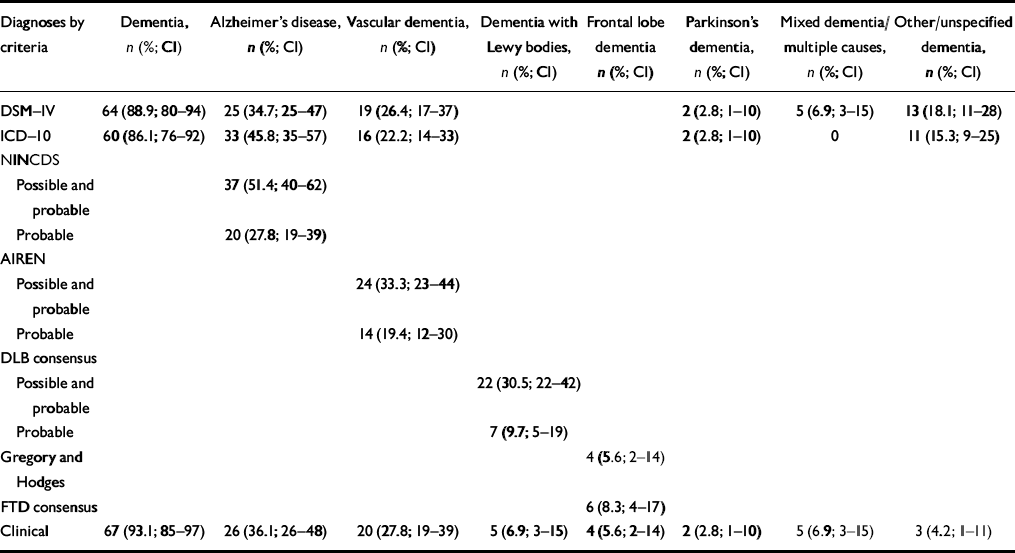
Table 1 indicates the numbers and proportions of people fulfilling the different diagnostic criteria. ‘Not applicable’ is used where a diagnostic category is not included in a particular classification system. ‘Alzheimer's disease’ includes all formulations for ‘dementia of the Alzheimer's type’ in DSM—IV and ‘dementia in Alzheimer's disease’ in ICD—10. Similarly, ‘vascular dementia’ includes all sub-categories under this heading in DSM and ICD. ‘Mixed dementia’ includes cases fulfilling DSM—IV criteria for ‘dementia due to multiple aetiologies’ and ‘other/unspecified dementia’, cases of ‘dementia in other medical conditions specified elsewhere’ and ‘dementia not otherwise specified’ in DSM—IV.
Table 1 Diagnoses by clinical criteria (DSM non-demented cases included)1
| Diagnoses by criteria | Dementia, n(%; Cl) | Alzheimer's disease, n(%; Cl) | Vascular dementia, n(%; Cl) | Dementia with Lewy bodies, n(%; Cl) | Frontal lobe dementia n(%; Cl) | Parkinson's dementia, n(%; Cl) | Mixed dementia/multiple causes, n(%; Cl) | Other/unspecified dementia, n(%; Cl) |
|---|---|---|---|---|---|---|---|---|
| DSM-IV | 64 (88.9; 80-94) | 25 (34.7; 25-47) | 19 (26.4; 17-37) | 2 (2.8; 1-10) | 5 (6.9; 3-15) | 13 (18.1; 11-28) | ||
| ICD-10 | 60 (86.1; 76-92) | 33 (45.8; 35-57) | 16 (22.2; 14-33) | 2 (2.8; 1-10) | 0 | 11 (15.3; 9-25) | ||
| NINCDS | ||||||||
| Possible and probable | 37 (51.4; 40-62) | |||||||
| Probable | 20 (27.8; 19-39) | |||||||
| AIREN | ||||||||
| Possible and probable | 24 (33.3; 23-44) | |||||||
| Probable | 14 (19.4; 12-30) | |||||||
| DLB consensus | ||||||||
| Possible and probable | 22 (30.5; 22-42) | |||||||
| Probable | 7 (9.7; 5-19) | |||||||
| Gregory and Hodges | 4 (5.6; 2-14) | |||||||
| FTD consensus | 6 (8.3; 4-17) | |||||||
| Clinical | 67 (93.1; 85-97) | 26 (36.1; 26-48) | 20 (27.8; 19-39) | 5 (6.9; 3-15) | 4 (5.6; 2-14) | 2 (2.8; 1-10) | 5 (6.9; 3-15) | 3 (4.2; 1-11) |
The results reveal Alzheimer's disease to be the most common cause of dementia by both DSM—IV and ICD—10 criteria. In addition, the majority of the sample fulfilled NINCDS—ADRDA criteria for either possible or probable Alzheimer's disease. The second most common diagnosis, again by both DSM and ICD criteria, was vascular dementia, with one-third meeting the NINDS—AIREN criteria for possible or probable vascular dementia. The majority of people diagnosed with unspecified dementia were those with severe dementia on whom insufficient collateral information was available with regard to onset and course of the illness for a probable aetiology to be identified.
Almost 10% of the sample fulfilled the consensus criteria for probable DLB, 30.5% fulfilling criteria for either probable or possible DLB. Of the cases of probable DLB, four (57.1%) met the DSM—IV criteria for Alzheimer's disease and one (14.3%) each met the DSM criteria for vascular dementia, dementia due to Parkinson's disease and unspecified dementia. Identical results were seen when probable DLB cases were compared with their ICD—10 diagnoses. In contrast, a clinical diagnosis of DLB based on the criteria described above, namely with emphasis on visual hallucinations and more flexible assessment of the time of onset of symptoms and contribution of vascular factors, was made in five cases (6.9%). All five cases fulfilled the consensus criteria for probable DLB.
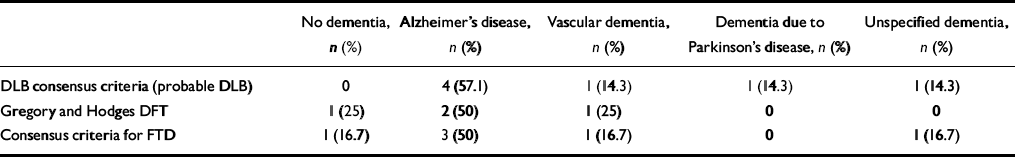
Four people (5.6%) met the Gregory and Hodges criteria for FLD, compared with six (8.3%) who fulfilled the consensus criteria for frontotemporal dementia. All four Gregory and Hodges cases also met the consensus criteria. A comparison of diagnoses made using these two sets of criteria with the DSM—IV diagnoses is shown in Table 2.
Table 2 Diagnoses of cases of dementia with Lewy bodies (DLB) and frontal lobe dementia (FLD) by DSM—IV (DSM non-demented cases included)
| No dementia, n(%) | Alzheimer's disease, n(%) | Vascular dementia, n(%) | Dementia due to Parkinson's disease, n(%) | Unspecified dementia, n(%) | |
|---|---|---|---|---|---|
| DLB consensus criteria (probable DLB) | 0 | 4 (57.1) | 1 (14.3) | 1 (14.3) | 1 (14.3) |
| Gregory and Hodges DFT | 1 (25) | 2 (50) | 1 (25) | 0 | 0 |
| Consensus criteria for FTD | 1 (16.7) | 3 (50) | 1 (16.7) | 0 | 1 (16.7) |
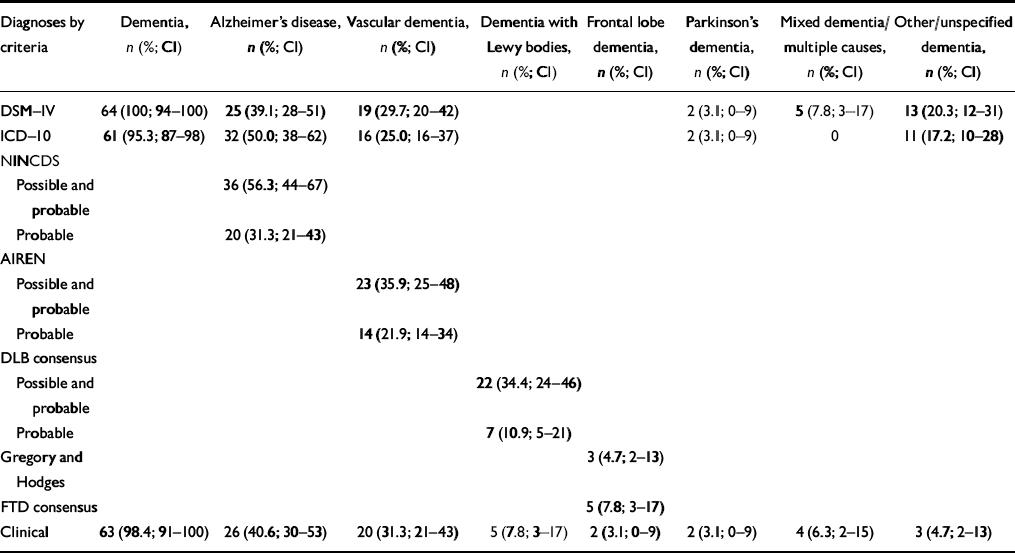
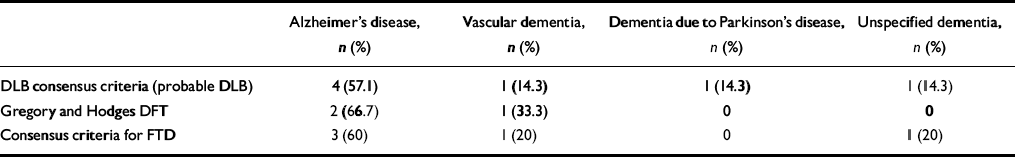
Eight people (11.1%) did not meet the DSM—IV criteria for dementia. Two did not have memory impairment, one of whom had an FLD and one of whom had English as her second language. Three were depressed and three had cognitive dysfunction but no significant impairment in social or occupational function due to cognitive deficit. Table 3 shows the numbers and proportions of people fulfilling the different diagnostic criteria once those not meeting DSM dementia criteria have been excluded. No DLB consensus cases of probable or possible DLB have been excluded (i.e. all of these cases met the DSM criteria for dementia). The proportions of people with probable DLB and probable plus possible DLB are thus higher, namely 10.9% and 34.4%, respectively. As noted above, one person fulfilling both sets of criteria for FLD was excluded. Table 4 indicates the DSM diagnoses of the remaining Gregory and Hodges and FTD consensus criteria cases of DFT/FTD.
Table 3 Diagnoses by clinical criteria (DSM non-demented cases excluded)1
| Diagnoses by criteria | Dementia, n(%; CI) | Alzheimer's disease, n(%; CI) | Vascular dementia, n(%; CI) | Dementia with Lewy bodies, n(%; CI) | Frontal lobe dementia, n(%; CI) | Parkinson's dementia, n(%; CI) | Mixed dementia/multiple causes, n(%; CI) | Other/unspecified dementia, n(%; CI) |
|---|---|---|---|---|---|---|---|---|
| DSM—IV | 64 (100; 94-100) | 25 (39.1; 28-51) | 19 (29.7; 20-42) | 2 (3.1; 0-9) | 5 (7.8; 3-17) | 13 (20.3; 12-31) | ||
| ICD—10 | 61 (95.3; 87-98) | 32 (50.0; 38-62) | 16 (25.0; 16-37) | 2 (3.1; 0-9) | 0 | 11 (17.2; 10-28) | ||
| NINCDS | ||||||||
| Possible and probable | 36 (56.3; 44-67) | |||||||
| Probable | 20 (31.3; 21-43) | |||||||
| AIREN | ||||||||
| Possible and probable | 23 (35.9; 25-48) | |||||||
| Probable | 14 (21.9; 14-34) | |||||||
| DLB consensus | ||||||||
| Possible and probable | 22 (34.4; 24-46) | |||||||
| Probable | 7 (10.9; 5-21) | |||||||
| Gregory and Hodges | 3 (4.7; 2-13) | |||||||
| FTD consensus | 5 (7.8; 3-17) | |||||||
| Clinical | 63 (98.4; 91-100) | 26 (40.6; 30-53) | 20 (31.3; 21-43) | 5 (7.8; 3-17) | 2 (3.1; 0-9) | 2 (3.1; 0-9) | 4 (6.3; 2-15) | 3 (4.7; 2-13) |
Table 4 Diagnoses of cases of dementia with Lewy bodies (DLB) and frontal lobe dementia (FLD) by DSM—IV (DSM non-demented cases excluded)
| Alzheimer's disease, n(%) | Vascular dementia, n(%) | Dementia due to Parkinson's disease, n(%) | Unspecified dementia, n(%) | |
|---|---|---|---|---|
| DLB consensus criteria (probable DLB) | 4 (57.1) | 1 (14.3) | 1 (14.3) | 1 (14.3) |
| Gregory and Hodges DFT | 2 (66.7) | 1 (33.3) | 0 | 0 |
| Consensus criteria for FTD | 3 (60) | 1 (20) | 0 | 1 (20) |
Three people (4.7%) who met the DSM criteria for dementia did not fulfil the ICD—10 criteria because they had not been suffering clearly from the disorder for 6 months or more.
Figure 1 indicates the degree of diagnostic overlap for cases meeting the DSM criteria for dementia. For example, a person fulfilling the consensus criteria for probable DLB but also the NINCDS—ADRDA, DSM and/or ICD criteria for Alzheimer's disease would fall in the area of overlap between DLB and Alzheimer's disease. The category ‘unspecified dementia’ has been excluded because this does not represent a diagnostic entity. The single cases of ‘pure’ DLB and FLD (i.e. those not lying in an area of overlap) had been diagnosed as unspecified dementia by DSM and/or ICD. Cases of mixed dementia have been included in the area of overlap between Alzheimer's disease and vascular dementia, because these were judged to be the combined aetiologies in each instance.
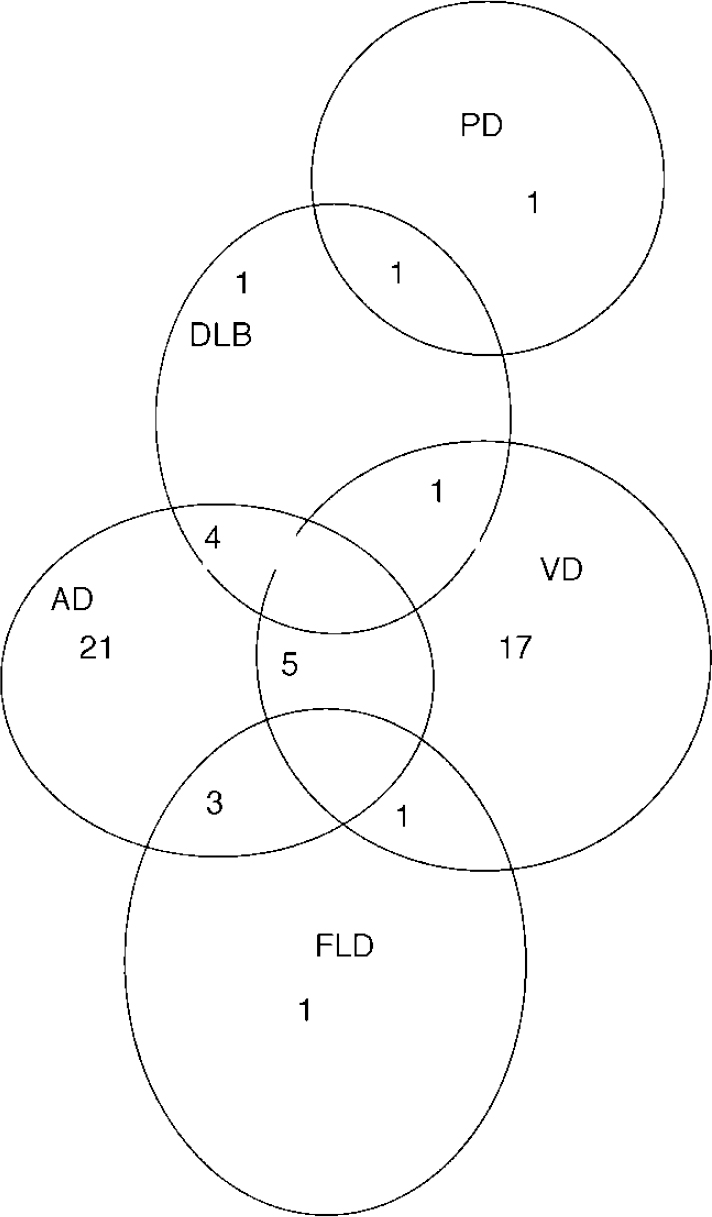
Fig. 1 Overlap of diagnoses: AD, Alzheimer's disease; DLB, dementia with Lewy bodies; FLD, frontal lobe dementia; PD, dementia in Parkinson's disease; VD, vascular dementia.
DISCUSSION
Main findings
Ours is the first study in the Western world to report the distribution of DLB and FLD as subtypes of dementia in the community. The community prevalence of screen-positive dementia was 9.86%, and this figure fell to 6.7% when people living in residential or nursing homes were excluded. This prevalence rate is comparable to that of 6.4% reported by Lobo et al (Reference Lobo, Launer and Fratiglioni2000) in a recent comparison of European prevalence studies. If we exclude those cases not meeting the DSM—IV criteria for dementia and we use the best-validated diagnostic criteria for each subtype of dementia (NINCDS—ADRDA for Alzheimer's disease, NINDS—AIREN for vascular dementia, consensus criteria for DLB and consensus criteria for FTD), then the distribution of the subtypes is as follows: probable Alzheimer's disease, 31.3% (95% CI 21-43); probable vascular disease, 21.9% (95% CI 14-34); probable DLB, 10.9% (95% CI 5-21); and FLD, 7.8% (95% CI 3-17). The results indicate that although Alzheimer's disease remains most common form of dementia, there are several other types that occur sufficiently frequently to be seen in routine clinical practice. The distribution of DLB in our study contrasts markedly with the findings of the only other published study of this type, by Yamada et al (Reference Yamada, Hattori and Miura2001), in which the prevalence of DLB in a Japanese population was 0.1%. Given that the prevalence of dementia in Yamada et al's study was 3.8%, the distribution of DLB as a subtype may be calculated as 2.63%. Yamada et al furthermore identified no cases of FLD in their study population. The reasons for the differences in results are unclear.
Limitations
These figures are not immutable because the distribution of the different subtypes differs considerably, depending on the criteria used. Our results are restricted by the numbers of people identified at screening as having dementia on whom we could not make a diagnosis. The Short-CARE has not been validated as an instrument on people who have dementia without significant memory impairment and we do not know to what extent this may have influenced our findings, although we did detect some people with this syndrome. In addition, it is a limitation of the study that post-mortem diagnoses were not available.
A relatively high proportion (11.1%) of people identified by initial Short-CARE screening did not have dementia according to DSM—IV. For three of these eight people there was insufficient evidence of impairment in social or occupational functioning for dementia to be diagnosed, despite clear evidence of cognitive impairment in multiple domains. Because this was an epidemiological study, we employed a screening instrument and, subsequently, diagnostic criteria that produced categorical outcomes. Caution, however, clearly needs to be exercised in the use of these criteria in clinical practice in order to avoid excessively strict adherence to the inclusion and exclusion requirements at the expense of common sense.
Clinical implications
Previous studies of the frequency of DLB have concentrated on hospital in-patient or out-patient populations and, in particular, on the epidemiologically unrepresentative cohorts that come to post-mortem. It is highly likely that many of the figures from published studies on the distribution of other dementia subtypes are contaminated by DLB cases, particularly as neither of the two major classification systems currently in use, DSM—IV and ICD—10, include the diagnosis of DLB. Most cases of ‘probable’ DLB in this study were diagnosed as having Alzheimer's disease using DSM—IV and ICD—10. Almost one-third of the sample fulfilled DLB consensus criteria for either ‘probable’ or ‘possible’ DLB. This suggests that the consensus criteria are broad, at least with regard to the diagnosis of ‘possible’ DLB. The ‘clinical’ criteria used to diagnose DLB, which simultaneously were more restrictive in requiring the presence of visual hallucinations and more flexible in leaving unspecified the time relationship between the onset of parkinsonism and that of dementia, yielded a lower rate for this disorder.
As with DLB, neither DSM—IV nor ICD—10 permits the diagnosis of FLD, although both include Pick's disease as a possible aetiology. Because other aspects of cognition and activities of daily living are relatively preserved in early FLD, our rates may be conservative in that some cases may have been missed using a screening test that relies on orientation, memory and independence in activities of daily living (Reference Gregory and HodgesGregory & Hodges, 1996). There are suggested ways of overcoming this problem using additional bedside tests (Reference Gregory, Orrell and SahakianGregory et al, 1997). In our study, FLD was diagnosed more often using consensus criteria than the Gregory and Hodges criteria. This may be due to disagreement about the terminology used to describe these dementias: some groups prefer ‘frontotemporal dementia’ (Reference Brun, England and GustafsonBrunet al, 1994) whereas others regard this term as including disparate conditions and so prefer ‘dementia of frontal type’ (Reference Gregory, McKenna and HodgesGregory et al, 1998). Both sets of criteria used in our study are weighted towards a purely degenerative aetiology of FLD and exclude the possibility of a vascular contribution to symptoms.
Our results indicate that it is possible to determine a probable aetiology in most cases of dementia. The relatively small degree of overlap of the Alzheimer's disease and vascular dementia categories, as shown in Fig. 1, indicates that the diagnostic systems in current use lead to the same diagnostic conclusions for most individuals. This, however, is only true for Alzheimer's disease and vascular dementia.
The ability to distinguish particular subtypes of dementia is important for several reasons. It enables clinicians to identify associated risk factors, to implement specific treatment strategies, to inform patients and relatives more accurately of the prognosis of each one and to provide relevant services. This is particularly pertinent in the light of the development of treatments such as the cholinesterase-inhibiting drugs for Alzheimer's disease. The demand for these drugs, together with their high cost, requires accuracy of diagnosis and standardisation of diagnostic criteria. The inability of DSM and ICD to identify DLB and FLD, which are relatively common forms of dementia, means that they do not suffice to categorise these conditions. Both DLB and FLD should be incorporated in future editions of standard diagnostic criteria.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Prevalence rates of dementia subtypes vary depending on the diagnostic criteria used.
-
• Dementia with Lewy bodies (DLB) and frontal lobe dementias (FLD) are relatively common and will be encountered frequently in clinical practice.
-
• Future editions of standard diagnostic systems such as DSM—IV and ICD—10 should include DLB and FLD as categories.
LIMITATIONS
-
• Not all people scoring above the screening cut-off point for dementia were demented on subsequent assessment.
-
• No diagnosis was made for almost one-third of people identified as demented at screening.
-
• Post-mortem confirmation of diagnoses was not available.








eLetters
No eLetters have been published for this article.