The nutritional management of patients with severe acute pancreatitis represents a significant challenge due to the underlying pathophysiological processes and altered nutritional status of these patients. For example, cardiovascular, respiratory, renal and endocrine dysfunction, together with disturbances in gastrointestinal motility, can make the delivery of nutritional support problematic(Reference Curtis and Kudsk1–Reference Smout3). In addition, patients can present with altered nutritional status including overweight (for example, in gallstone pancreatitis) and various micronutrient deficiencies (for example, in alcohol-induced pancreatitis)(Reference Petrov4, Reference Liamis, Gianoutsos and Elisaf5).
Parenteral nutrition (PN) was for many years regarded as the ideal method of nutritional management in patients with severe acute pancreatitis as it provides essential nutrients whilst minimising pancreatic stimulation. However, more recently, a number of randomised controlled trials (RCT) and subsequent meta-analyses(Reference Petrov, van Santvoort and Besselink6–Reference Al-Omran, Albalawi and Tashkandi9) have consistently demonstrated that enteral nutrition (EN) significantly reduces infectious complications, surgical interventions and mortality in predicted severe acute pancreatitis. Therefore, EN is now established as a key component in the early management of patients with severe acute pancreatitis(Reference Forsmark and Baillie10–12).
At the same time, EN and PN may lead to various gastrointestinal and metabolic complications. The pooled-effect incidence has been investigated in surgical and critically ill patients, with two meta-analyses demonstrating a significantly higher risk of complications during the delivery of EN compared with PN(Reference Braunschweig, Levy and Sheean13, Reference Peter, Moran and Phillips-Hughes14) and one meta-analysis showing no difference in incidence between the two(Reference Gramlich, Kichian and Pinilla15). Obviously, these inconsistent findings cannot be extrapolated to patients with severe acute pancreatitis, in whom the risk of adverse effects with the use of EN and PN remains to be established.
Therefore, we systematically reviewed and statistically aggregated the data from RCT of the complications attributable to EN v. PN in patients with predicted severe acute pancreatitis.
Methods
Search strategy
Eligible studies were identified via MEDLINE, Scopus and the Cochrane Controlled Clinical Trials Register Database. The final closeout date for the search process was 1 December 2009. All searches included the following keywords: ‘acute pancreatitis’, ‘enteral nutrition’, ‘parenteral nutrition’ and ‘randomised controlled trial’. Bibliographies of previous review articles were searched for other relevant publications. Additionally, the abstracts of major gastroenterology meetings from 2005 to 2009 were screened manually.
Selection criteria
We included RCT meeting all of the following criteria:
(1) reported in English;
(2) studied adults with predicted severe acute pancreatitis defined on the basis of generally accepted criteria;
(3) evaluated the efficacy of exclusive PN via central venous catheter v. exclusive EN via nasojejunal tube;
(4) assessed the incidence of at least one complication of nutrition, including diarrhoea, abdominal bloating or hyperglycaemia. In each case, the definition of the complication was taken as that given in the primary trial.
Data extraction and quality assessment
The bibliographic data, information regarding the study quality, patients' baseline characteristics, and complications of EN and PN were extracted using a standardised data extraction sheet. Each patient population was used only once such that if the same population appeared in more than one report, only that providing the most complete data was chosen. Where insufficient detail was contained within a report, the authors were contacted for further information.
Methodological quality of included studies was assessed using a previously published quality score(Reference Petrov, Correia and Windsor16), consisting of eight criteria (patient selection, comparability of groups at baseline, allocation sequence, concealment of allocation, blinding, description of interventions, description of co-interventions, and description of withdrawals) and resulting in a quality score ranging from 0 to 16 points.
Statistical analysis
Statistical heterogeneity was assessed graphically by Galbraith's radial plot (see below) and numerically by the Q statistic, which is a χ2 with the corresponding degrees of freedom, as well as corresponding P value (values below 0·05 indicated statistical heterogeneity). Meta-analysis was conducted with a fixed-effects (Peto) model, since the estimates of treatment effect obtained from all trials belonged to the same distribution. Both intention-to-treat and per-protocol analyses were conducted. In the intention-to-treat analysis, the dropouts were considered as adverse events and added to the number of observed events. In the per-protocol analysis, the dropouts were not evaluated and were subtracted from the total number of subjects randomised.
The results were expressed as OR and risk difference with the corresponding 95 % CI. The summary estimates were graphically displayed by means of the forest plot and Galbraith's radial plot: a plot of z-statistic (vertical axis) against 1/standard error (horizontal axis)(Reference Leandro17). In the Galbraith plot, every RCT is represented by a number such that points close to the origin (0,0) indicate imprecise trials and points far from the origin indicate the more precise trials, and consequently have more weight in the meta-analysis. The plot also contains three continuous parallel lines. The central one depicts the pooled estimate on the scale; the CI is indicated by the segment of arc parallel to the scale. The other two lines, originating from 0 ± 2, indicate a ‘homogeneity area’ within their limits. If one or more points (RCT) are outside of this area, they are considered ‘heterogeneous’. The possibility of publication bias was investigated by means of the funnel plot and the test of funnel plot asymmetry. All calculations and graphs were done using specialist meta-analysis software (MetAnalysis 1.2; Tecnopharma S.r.l., Genoa, Italy).
Results
The process for identifying eligible studies is shown in Fig. 1. A total of thirteen RCT of EN v. PN were found(Reference McClave, Greene and Snider18–Reference Mizock32). Of these, eight RCT were excluded: two because they reported a mixed population of patients with mild and severe acute pancreatitis and did not present separate data on those with severe disease(Reference McClave, Greene and Snider18, Reference Windsor, Kanwar and Li19), five because they did not include complications of EN or PN as outcome measures(Reference Abou-Assi, Craig and O'Keefe20–Reference Wu, Ji and Wang24), and one because PN was delivered via peripheral venous catheter(Reference Eckerwall, Axelsson and Andersson25). Eventually, five RCT in which a total of 181 patients were randomised to receive either PN or EN were included in the analysis(Reference Kalfarentzos, Kehagias and Mead26–Reference Casas, Mora and Fort30). Seven patients were withdrawn after randomisation, leaving ninety-two (53 %) patients receiving PN and eighty-two (47 %) patients receiving EN. The funnel plot and the test of funnel plot asymmetry did not yield any evidence of a publication bias. The study characteristics for the included trials are shown in Table 1 and the composition of the feeding formulas used is presented in Table 2.

Fig. 1 Flow chart illustrating the selection process. RCT, randomised controlled trial; EN, enteral nutrition; PN, parenteral nutrition.
Table 1 Study characteristics for the included trials

PN, parenteral nutrition; EN, enteral nutrition.
* Median and range.
Table 2 Composition of the feeding formulas used in the included trials

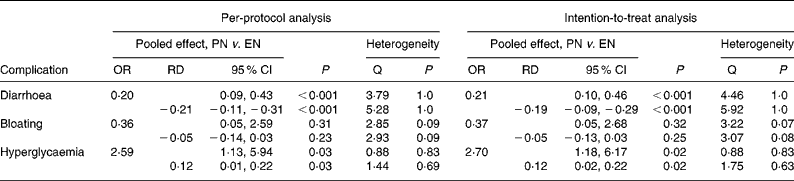
PN, in comparison with EN, reduced the odds of having diarrhoea by 80 % by per protocol, and by 79 % (Fig. 2) by intention to treat (both P < 0·001). The test for heterogeneity yielded statistically non-significant results for both analyses (Table 3). The mean difference in the risk of diarrhoea between PN and EN was 21 % by per protocol, and 19 % by intention to treat (both P < 0·001; Fig. 3). The Galbraith plot identifies the most precise study(Reference Petrov, Kukosh and Emelyanov29) and demonstrates that all the studies are within the homogeneity area (Fig. 3). The test for heterogeneity yielded statistically non-significant results in both analyses (Table 3).

Fig. 2 Forest plot of OR of diarrhoea associated with parenteral v. enteral nutrition (per-protocol analysis).
Table 3 Results of the meta-analysis comparing complications of parenteral nutrition (PN) and enteral nutrition (EN) in patients with severe acute pancreatitis
RD, risk difference.

Fig. 3 Galbraith plot of risk difference of diarrhoea associated with parenteral v. enteral nutrition (intention-to-treat analysis).
PN, when compared with EN, reduced the odds of abdominal bloating by 64 % (P = 0·31) by per protocol, and by 63 % (P = 0·32) by intention to treat, although these were not statistically significant (Table 3).
PN, when compared with EN, was associated with a 2·6-fold greater odds of hyperglycaemia requiring administration of insulin (P = 0·03) when per-protocol analysis was applied (Fig. 4) and a 2·7-fold greater odds when intention-to-treat analysis was applied (P = 0·02). The test for heterogeneity yielded statistically non-significant results in both analyses (Table 3). A mean difference in the risk of hyperglycaemia between the PN and EN groups was 12 % in both per-protocol (P = 0·03) and intention-to-treat (P = 0·02) analysis (Fig. 5). The Galbraith plot demonstrates that all studies were within the homogeneity area (Fig. 5), yielding statistically non-significant results for heterogeneity (Table 3).

Fig. 4 Forest plot of OR of hyperglycaemia associated with parenteral v. enteral nutrition (per-protocol analysis).
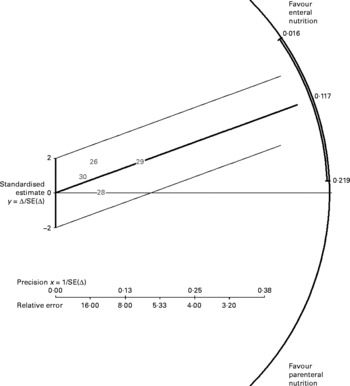
Fig. 5 Galbraith plot of risk difference of hyperglycaemia associated with parenteral v. enteral nutrition (intention-to-treat analysis).
Discussion
This is the first systematic review of complications attributable to EN and PN in patients with predicted severe acute pancreatitis. It demonstrates that EN was associated with a significant increase in the odds of diarrhoea, whereas PN was associated with a significant increase in the odds of hyperglycaemia.
Hyperglycaemia is a common complication in critically ill patients, including those with severe acute pancreatitis. The finding from one RCT(Reference Van den Berghe, Wouters and Weekers31) indicates that hyperglycaemia increases the risk of infectious complications and mortality in surgical patients on the intensive care unit (ICU), providing a rationale for tight glucose control in such patients. Although this trial has not specifically addressed the impact of nutrition on blood glucose control, it has been demonstrated that patients receiving PN require significantly higher insulin doses in order to achieve euglycaemia in comparison with patients receiving EN.
In line with these findings, the results of our meta-analysis on patients with predicted severe acute pancreatitis confirmed a higher hyperglycaemic potential of PN over EN. The exact mechanism remains to be established, but elevated plasma glucose concentrations might be due to either accelerated disturbances of carbohydrate utilisation during PN or increased concentration of endogenous insulin during EN(Reference Mizock32–Reference Petrov and Zagainov34). It is also worth noting that there was no significant difference in nutrient delivery between those patients receiving EN or PN in any of the trials. Thus, the higher incidence of hyperglycaemia in those receiving PN is unlikely to be markedly attributed to hyperalimentation in this group. In any case, the use of EN instead of PN in patients with severe acute pancreatitis may minimise the episodes of hyperglycaemia. The impact of tight blood glucose control on infectious complications in patients with acute pancreatitis receiving total EN needs to be assessed in a RCT.
Diarrhoea is a common complication of EN in the ICU(Reference Jolliet, Pichard and Biolo35, Reference Wiesen, Van Gossum and Preiser36). Although it is rarely considered a life-threatening complication, diarrhoea may increase the risk of dehydration and incontinence, and thus increase the risk of wound infection as well as being burdensome to patients and nursing staff(Reference Sabol and Carlson37–Reference Majid, Emery and Whelan39). However, the actual incidence of diarrhoea during EN (29 %) in the included RCT was still relatively low, particularly given that most patients were on the ICU and were receiving antibiotics, both of which are associated with greater incidence(Reference Whelan40).
The increase in gastrointestinal luminal contents during EN, which of course does not occur during PN, will inevitably contribute to increased stool output. However, many other factors may be related to the increased risk of diarrhoea in patients receiving EN, above merely increasing luminal contents. For example, formulas with high osmolality have been associated with increased risk of diarrhoea(Reference Wiesen, Van Gossum and Preiser36, Reference Sabol and Carlson37) and some of those used in the included RCT were hyperosmolar (Table 2). Furthermore, the concurrent administration of antibiotics might have a confounding effect, with almost all patients in our systematic review receiving antibiotics, which are known to increase the risk of diarrhoea during EN(Reference Wiesen, Van Gossum and Preiser36, Reference Sabol and Carlson37). In addition, a number of RCT have demonstrated that EN in predicted severe acute pancreatitis is associated with the reduced blood glucose concentrations(Reference Eckerwall, Axelsson and Andersson25, Reference Kalfarentzos, Kehagias and Mead26), which in themselves may accelerate intestinal motility(Reference de Boer, Masclee and Lam41, Reference Rayner, Samsom and Jones42) and thus exacerbate diarrhoea in those receiving EN.
A number of approaches to minimising the risk of diarrhoea in patients receiving EN have been investigated. A recent meta-analysis of thirteen RCT comparing fibre and fibre-free EN formulas, incorporating a total of 683 patients, showed a significant reduction of diarrhoea in those receiving fibre formulas(Reference Elia, Engfer and Green43). However, this benefit was mainly observed in non-ICU and surgical patients. A systematic review(Reference Petrov, Loveday and Pylypchuk44) of the feeding formulas used in patients with acute pancreatitis found that the effect of fibre formulas has been evaluated in only one RCT(Reference Karakan, Ergun and Dogan45), demonstrating no diarrhoea in fifteen (0 %) patients receiving a fibre formula, compared with two of fifteen (13 %) patients receiving a fibre-free formula. However, this difference was not statistically significant, perhaps reflecting the limited sample size.
Probiotics may also be used to prevent diarrhoea in patients receiving EN through their potential suppression of enteropathogenic colonisation, immune stimulation and modulation of colonic metabolism(Reference Whelan40). However, the efficacy of different probiotic strains may vary and a recent large RCT demonstrated that a specific probiotic product (Lactobacillus acidophilus, L. casei, L. salivarius, L. lactis, Bifidobacterium bifidum, B. lactis) may even be harmful in patients with predicted severe acute pancreatitis(Reference Besselink, van Santvoort and Buskens46). At the same time, a systematic review found three other trials, using different probiotics products, in patients with pancreatitis and, although none of these measured the effect on diarrhoea, there were no statistically significant increases in adverse events in the probiotic groups(Reference Whelan and Myers47). Further research regarding the efficacy and safety of fibre formulas and probiotics in acute pancreatitis is warranted(Reference Petrov, Loveday and Pylypchuk44).
A number of other complications are related to the delivery of nutrition, albeit not actually attributable to the PN or EN itself, including catheter infections and tube extubation. In the studies reviewed, twelve of ninety-two (13 %) patients receiving PN developed central venous catheter infections, whereas none were reported in EN. Meanwhile, eight of eighty-two (10 %) patients receiving EN experienced extubation of their feeding tube, which inherently results in inadequate nutrient delivery(Reference Whelan, Hill and Preedy38) and requires reinsertion which may increase nasal trauma.
There are some limitations of the present systematic review and meta-analysis. First, the observed results might be influenced by the quality of nutritional practice and adherence to nutrition protocols rather than whether EN or PN were used. However, all the included trials were conducted in tertiary academic centres and nutrition protocols aiming to standardise delivery and management were developed before the commencement of each RCT. Second, the meta-analysis focuses only on the nutrition-related complications as reported by the authors of the primary trials. However, many clinical trials lack comprehensive monitoring and reporting of adverse events, leading to their under-reporting in the literature(Reference Ioannidis48). Therefore, in the present meta-analysis, there may be other complications that were not monitored or reported (for example, fatty liver disease). Third, the definition of diarrhoea used in clinical trials is notoriously inconsistent(Reference Lebak, Bliss and Savik49) and many included primary trials did indeed not provide a definition. Not using a predetermined definition of diarrhoea, and thus relying on clinical judgment for its diagnosis, is not ideal, as different health professionals may define and report diarrhoea differently(Reference Whelan, Judd and Taylor50). In addition, as none of the trials was blinded, an episode of loose stool may be more likely to be considered diarrhoea when a patient was receiving EN compared with one receiving PN. Fourth, for the purpose of the present study we constrained ourselves to studies comparing EN with PN delivered via a central venous catheter. This resulted in the exclusion of one RCT comparing nasogastric EN with peripheral PN(Reference Eckerwall, Axelsson and Andersson25). This was excluded, as the incidence of procedure-related complications differs between peripheral and central PN(Reference Couse, Pickford and Mitchell51). In addition, peripheral PN is infrequently used in a routine ICU practice. Moreover, the excluded trial(Reference Eckerwall, Axelsson and Andersson25) compared peripheral PN with nasogastric EN, as opposed to nasojejunal EN (the only such trial that we have identified). So, inclusion of this trial would add a significant heterogeneity to this meta-analysis and thus compromise the validity of our findings. Having excluded this trial, we have constrained ourselves to the trials of nasojejunal EN v. central PN and reached a statistical homogeneity (as evidenced by Galbraith's radial plot and Q statistic). Finally, limitations of the scoring systems used to predict severity of acute pancreatitis are well known(Reference Rau, Schilling and Beger52, Reference Frossard, Hadengue and Pastor53) and therefore some of the patients in the present systematic review may indeed have had a mild or moderate course of disease. Future clinical studies in acute pancreatitis have to be based on actual rather than predicted severity(Reference Petrov and Windsor54).
In conclusion, the present systematic review and meta-analysis revealed a significantly higher incidence of diarrhoea in patients receiving EN and hyperglycaemia in patients receiving PN. Taking into account that previous meta-analyses consistently demonstrated that EN, when compared with PN, is associated with a significantly lower incidence of pancreatic infectious complications and mortality, the former should be regarded as the primary method of nutrient delivery in patients with severe acute pancreatitis.
Acknowledgements
Ethics approval was not required.
The authors are grateful to Dr Casas and Dr Farré (Hospital de la Santa Creu i Sant Pau, Barcelona, Spain) and to Dr Karakan (Gazi University, Ankara, Turkey) for providing additional information on their trials.
Both authors were involved in conducting the study and drafting the manuscript; M. S. P. was additionally involved in planning the study and is the guarantor of the article.
The authors have no conflicts of interest and no extra funding support to declare.










