Introduction
Accurate analysis of long-term monitoring data is essential for the effective management and conservation of wildlife populations (Fewster et al. Reference Fewster, Buckland, Siriwardena, Baillie and Wilson2000, BirdLife International 2004). Birds are used as a reference group for this topic, reflecting their innate interest to human cultures and the practical ease in surveying and monitoring them (Gregory and Van Strien Reference Gregory and Van Strien2010). Coastal waders are sensitive species in relation to the conservation of coastal zones (Ferns Reference Ferns1992), sites where anthropogenic activity causes many changes to the ecosystem. As a result, 50% of European waders have an unfavourable pan-European conservation status, and 47.7% of the breeding populations declined during the 1990–2000 period (BirdLife International 2004).
The Kentish Plover Charadrius alexandrinus inhabits coasts and inland wetlands in Eurasia and North Africa (Küpper et al. Reference Küpper, Augustin, Kosztolanyi, Burke, Figuerola and Székely2009, Sangster et al. Reference Sangster, Collinson, Crochet, Knox, Parkin, Svensson and Votier2011), with the breeding distribution in Europe being predominantly coastal (Delany et al. Reference Delany, Scott, Dodman and Stroud2009). This wader, included in Annex 1 of the European Union Directive on the conservation of wild birds (2009/147/CE), has demonstrated a marked decline in most breeding populations in Europe since the early twentieth century; this has mainly been attributed to human activities that cause disturbances at coastal breeding sites and destruction of breeding habitat (Delany et al. Reference Delany, Scott, Dodman and Stroud2009). The Spanish breeding population most likely declined in the last few decades, although the data are insufficient to define the national trend (Figuerola et al. Reference Figuerola, Amat, Díaz, Madroño, González and Atienza2004); in contrast, the Portuguese population has not shown any recent decline (Felgueiras Reference Felgueiras and Atlas2008). The Iberian Atlantic breeding population is located along the Portuguese coastline and south-west Spain (Figuerola and Amat Reference Figuerola, Amat, Martí and Moral2003, Felgueiras Reference Felgueiras and Atlas2008), whereas the northern and north-western Spanish population, with 101 pairs in 2010 (authors’ unpubl. data), is restricted to the Galician coast. This population, mostly consisting of resident birds (De Souza et al. Reference De Souza, Caeiro, Rosende, Monteagudo and Fafián1999, Domínguez and Vidal Reference Domínguez and Vidal2008), selects sparsely vegetated beaches for foraging, roosting, nesting and rearing young. The lifestyle of this population makes them especially vulnerable to oil spill pollution.
On November 13, 2002, the oil tanker Prestige, loaded with 77,000 tonnes of heavy fuel oil (M-100 type), had an accident near A Coruña (north-west Spain). From the morning of 14 November until 19 November, the tanker was towed by tugboat until it split in half and sank, spilling 63,000 tonnes of fuel oil into the ocean. Wind and currents dispersed the oil over a vast coastal area, producing one of the largest spills ever in Europe (Albaigés et al. Reference Albaigés, Morales-Nin and Vilas2006). Of the 723 Galician beaches, 503 showed clear signs of pollution (Junoy et al. Reference Junoy, Castellanos, Viéitez, De La Huz and Lastra2005) including all of the plover breeding beaches (Domínguez and Vidal Reference Domínguez and Vidal2007).
The clean-up activities on the coast (rocky and sandy intertidal areas) lasted from November 2002 to the summer of 2003 and continued throughout 2004 in the most affected areas. These tasks involved thousands of volunteers and heavy machinery on the polluted beaches. Although there are no accurate statistics on the amount of hydrocarbons on each beach, the presence of oil was obvious during the first months of 2003 (Junoy et al. Reference Junoy, Castellanos, Viéitez, De La Huz and Lastra2005, Domínguez and Vidal Reference Domínguez and Vidal2007, Bernabeu et al. Reference Bernabeu, Rey, Rubio, Vilas, Domínguez, Bayona and Albaigés2009), mainly on those beaches closest to the Prestige wreck. During the spring of 2003, there was a progressive decrease in the surface oil (Junoy et al. Reference Junoy, Castellanos, Viéitez, De La Huz and Lastra2005) due to both clean-up activities and its burial caused by the action of beach dynamics (Bernabeu et al. Reference Bernabeu, Rey, Rubio, Vilas, Domínguez, Bayona and Albaigés2009). However, the most affected beaches showed the persistence of buried oil five years after the Prestige oil spill (Bernabeu et al. Reference Bernabeu, Rey, Rubio, Vilas, Domínguez, Bayona and Albaigés2009).
Although the acute effects of the spill in the form of Kentish Plover mortality did not appear to be substantial (no carcasses recovered; SEO/Birdlife 2003), many breeding birds were partially oiled during the winter and spring of 2003 (Domínguez and Vidal Reference Domínguez and Vidal2009). Furthermore, high levels of polycyclic aromatic hydrocarbons (PAHs) appeared in their eggs during 2004-2007, though the sources were both petrogenic and pyrogenic (Vidal et al. Reference Vidal, Domínguez and Luis2011). The sub-lethal effects of oil have been described for different species as resulting in habitat degradation, exposure to residual oil, decrease in prey availability and disturbance from clean-up activities that cause changes in habitat use and foraging and breeding performance (Maccarone and Bizorad Reference Maccarone, Bizorad and Burger1994, Sharp et al. Reference Sharp, Cody, Turner, Rice, Spies, Wolfe and Wright1996, Andres Reference Andres1997, Reference Andres1998, Golet et al. Reference Golet, Seiser, Mcguire, Roby, Fischer, Kuletz, Irons, Dean, Jewett and Newman2002, Maccarone and Bizorad Reference Maccarone and Bizorad2005, Domínguez and Vidal Reference Domínguez and Vidal2007).
Many management actions were implemented after the 2003 breeding period to promote nest survival and maximise recruitment. These efforts were focused on the most affected areas, with an emphasis on the two main breeding beaches in Galicia: Carnota and Corrubedo. Conservation actions included selective control during 2003 and 2004 of the main nest predator, the Carrion Crow Corvus corone (Domínguez and Vidal Reference Domínguez and Vidal2003), nest wardening (from 2003 to 2006), nest protection (symbolic fencing and predator exclosures) (from 2003 to 2010) and artificial incubation (from 2005 to 2006). These management strategies resulted in decreased nest loss due to predation and clean-up activities, achieving a slight increase in hatching success over previous years (Domínguez and Vidal Reference Domínguez and Vidal2007). In this study, we used a 23-year dataset (1988–2010) that tracked the abundance and distribution of breeding Kentish Plovers to evaluate population trends and identify the underlying effects of the oil spill.
Study area and methods
Annual censuses of Kentish Plover breeding pairs were undertaken along the Galician coast (Figure 1). Between 1988 and 2010, a total of 36 beaches were surveyed, although not all of the beaches were surveyed every year. The years with complete coverage were 1988 (De Souza and Domínguez 1989), 1991–92 (De Souza Reference De Souza1993a,b), 1999 and 2002–2010 (this study). The years between 1989 and 1998 and 2000–2001 included partial coverage (De Souza Reference De Souza1993a, De Souza et al. Reference De Souza, Caeiro, Monteagudo, Rosende and De Souza1996) with surveys of a variable number of beaches. No beaches were surveyed in 1994 and 1997.

Figure 1. Study area showing the course of the Prestige since it initially split in half until its final sinking. Stretch A (< 50 km from the Prestige wreck), including the beaches: A Barra, Traba, Nemiña, Rostro, Caldebarcos-Carnota, Lariño, Louro, Testal and Aguieira; Stretch B (50–100 km from the Prestige wreck), including the beaches: Doniños, Baldaio, Queiruga, Río Sieira, Xuño-Basoñas, Espiñeirido, Balieiros, Corrubedo, Couso, Aguiño, Castro, Sálvora, Coroso, Corna, Carragueiros, Xidoiro, Mexilloeira, A Lanzada and Rodas; and Stretch C (> 100 km from the Prestige wreck), including the beaches: Altar, Pampillosa, Morouzos, Vilarrube, Frouxeira, San Xurxo, América and Camposancos.
In our own surveys, the beaches were surveyed by 1–2 people, between mid-May and mid-June. Adult pairs with territorial behaviour, a nest or with unfledged chicks were considered breeding pairs. Surveys conducted by other observers used a similar methodology (De Souza and Domínguez 1989, De Souza Reference De Souza1993a,b).
Trend analysis
We employed TRIM v3.54, a program developed for the analysis of count data derived from wildlife monitoring schemes (Pannekoek and Van Strien Reference Pannekoek and Van Strien2005). The TRIM statistical procedure assumes that changes in particular sites are representative of the sites that are not counted, and this assumption seems reasonable (Gregory et al. Reference Gregory, Van Strien, Vorisek, Gmelig, Noble, Foppen and Gibbons2005), so missing counts from particular beaches were estimated from changes in all other beaches, or beaches with the same characteristics by using covariates (Gregory et al. Reference Gregory, Van Strien, Vorisek, Gmelig, Noble, Foppen and Gibbons2005, Gregory and Van Strien Reference Gregory and Van Strien2010). TRIM uses a Generalised Estimating Equation approach to correct possible violations to the assumption of Poisson distributions due to over-dispersion or serial correlation (Gregory and Van Strien Reference Gregory and Van Strien2010).
The TRIM program frequently recommends not using data with more than 50% missing counts. In our case, the missing counts for 1988–2010 were 49.1% but only 9.8% for 2002–2010. Only those sites with count data for 50% or more of the number of years in which the survey occurred were used for the assessment of trends (Underhill and Prys-Jones Reference Underhill and Prys-Jones1994).We calculated the trend from 1988 to 2010 using a TRIM linear trend model, which permits an analysis of trends before and after a certain point in time. We began the analysis using a model with change points at a set of 11 years as the time points and used the stepwise selection procedure to identify change points with significant changes in slope based on Wald tests. The 11 years selected were those with good survey coverage and that allowed the models to run. The base year was 2003 because it was the first breeding year after the Prestige oil spill.
To model the trend, we used five categorical variables related to beach characteristics and the Prestige oil spill: 1) beach position - inner, located in the interior part of the estuaries, or outer, exposed to the open sea; 2) beach length ≤1 km, 1–3 km or 3–6 km; 3) the location of the stretch of coast according to its distance from the Prestige wreck (UTM wreck coordinates, 29T 469460/4766800) and the degree of influence after the catastrophe (Junoy et al. Reference Junoy, Castellanos, Viéitez, De La Huz and Lastra2005, Franco et al. Reference Franco, Viñas, Soriano, De Armas, González, Beiras, Salas, Bayona and Albaigés2006, Serrano et al. Reference Serrano, Sánchez, Preciado, Parra and Frutos2006, Soriano et al. Reference Soriano, Viñas, Franco, González, Ortiz, Bayona and Albaigés2006) (Figure 1); stretch A (< 50 km, great impact), stretch B (50–100 km, medium impact) or stretch C (> 100 km, low impact); 4) the presence/absence of an intertidal area (linked to an inner lagoon or estuary) next to the beach; and 5) human impact (high, medium, low) (Marm Reference Marm2010). The values for the first three covariates were obtained from digital cartography with the aid of GIS software (ArcGis 9.3). It was not possible to measure quantitatively the human use of each beach, thus we used a simple measure of the touristic use designed by the Spanish government (Marm Reference Marm2010).
Lastly, a set of 19 models was generated, including a model without covariates and other models with 1 to 5 categorical variables. The most parsimonious model was selected using the Akaike criterion (AIC). The values were rescaled using the model with the minimum AIC:∆i = AIC – AICmin. In general, models with a ∆I ≤ 2 have strong support, models having a ∆i of approximately 4 to 7 have considerably less support, and those with values > 10 have essentially no support (Burnham and Anderson Reference Burnham and Anderson1998). The Akaike weights were obtained as follows:
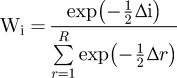 $${\rm{W}}_{\rm{i}} = {{\exp \left({ - {1 \over 2}\Delta {\rm{i}}} \right)} \over {\mathop\sum\limits_{r = 1}^R {\exp \left({ - {1 \over 2}\Delta r} \right)} }}$$
$${\rm{W}}_{\rm{i}} = {{\exp \left({ - {1 \over 2}\Delta {\rm{i}}} \right)} \over {\mathop\sum\limits_{r = 1}^R {\exp \left({ - {1 \over 2}\Delta r} \right)} }}$$The sum of all of the weights equal to the unit and the value of each wi indicate that the model i is the best overall Kulback-Leibler model (Anderson et al. Reference Anderson, Burnham and Thompson2000). Significant covariates in the final model were established using the Wald test, and significant values (P < 0.05) indicate that the trends differ significantly between the covariate categories. The values of covariates can vary both across sites and across time points. Population trends were established using the classification proposed in TRIM 3.5 (Pannekoek and Van Strien Reference Pannekoek and Van Strien2005).
Results
Between 1988 and 2010, the Galician Kentish Plover breeding population increased from 48 pairs restricted to 21 beaches to 101 pairs dispersed throughout 35 beaches. This population showed a change in the distribution of birds along the coast after the Prestige oil spill. This change was evident in the increased importance of stretches B and C with respect to stretch A (Figure 2). Until 2003, the birds mainly occupied wide beaches (average 1988–2003, 53.48% ± 2.32%) and displayed a preference for stretch A (average 1988–2003, 51.6% ± 2.16%). From 2003 until the present, higher percentages of occupation were detected on the medium-length beaches (average 2004–2010, 53.45% ± 1.86%), mainly in stretch B (average 2004–2010, 47.64% ± 1.05%).
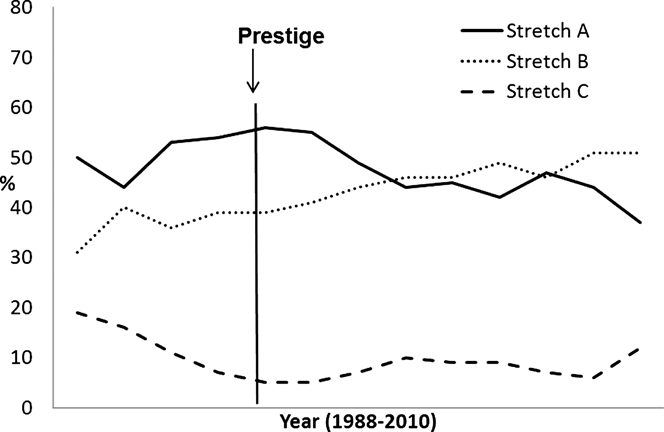
Figure 2. Percent distribution of Kentish Plover breeding population along the Galician Coast from 1988 to 2010.
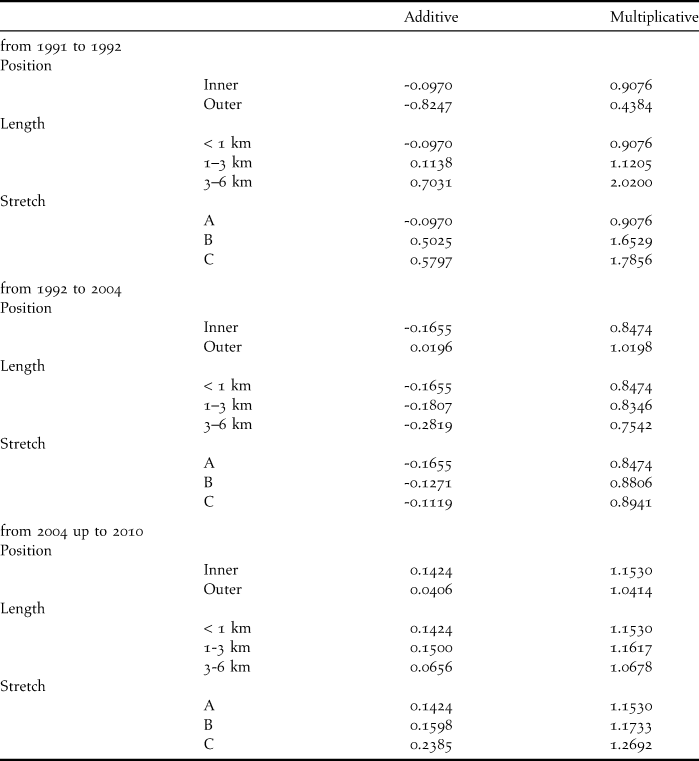
The TRIM analysis classified the trend as a moderate increase (Table 1 and Figure 3), with an annual change of + 1.58% per year. The best-fit model included four covariates, and another model with three covariates also had strong support (∆i < 2). In addition, two other models had minor support (Table 1). In the most parsimonious model, three covariates were significant: beach position (Wald = 13.07, df = 3, P = 0.0045), beach length (Wald = 12.76, df = 6, P = 0.0471) and the location of the stretch of coast in relation to the Prestige wreck (Wald = 13.46, df = 6, P = 0.0363). The presence of an intertidal area next to the beach was also included in the best-fit model but was not significant (Wald = 3.06, df = 3, P = 0.383). There were three years demonstrating significant points of change: 1991 (Wald = 17.18, df = 7, P = 0.0163), 1992 (Wald = 18.10, df = 7, P = 0.0115) and 2004 (Wald = 15.12, df = 7, P = 0.0345). Between 1991 and 1992, the population trend showed an annual decrease for the shortest beaches of stretch A, both inner and outer positions (Table 2). From 1992 to 2004, a widespread decrease was observed, except for those populations located on outer beaches. During the last period, 2004–2010, the population trend showed an overall increase for all the covariate categories, which was more marked for the medium-length beaches of stretches B and C (Table 2).

Figure 3. Model-based and imputed index of the north-west Spain Kentish Plover breeding population during 1988–2010.
Table 1. Summary of TRIM model selection results for the 1988–2010 period. Models with ∆i < 10 are shown and ranked by descending Akaike weights (wi). Covariates: 1, beach position; 2, beach length; 3, stretch of the coast in relation to the Prestige wreck; 4, intertidal area next to the beach; 5, beach human pressure. LR, Likelihood Ratio. OMSI, Overall Multiplicative Slope imputed. *according to the classification proposed in TRIM 3.5.
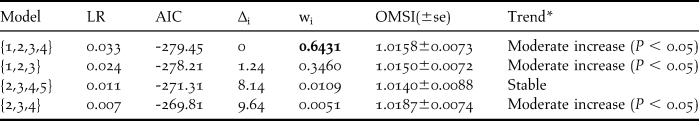
Table 2. Effects of significant covariates on the slope of the time intervals in the most parsimonious model. The multiplicative slope stands for the annual change. The additive slope is the natural logarithm of the multiplicative slope.
Discussion
Using trend data to assess the impact of, and the recovery from, such a perturbation as an oil spill could be confounded by the effects of natural temporal and geographic variation that are inherent in wildlife populations (Wiens and Parker Reference Wiens and Parker1995). Accordingly, pre- and post-spill data for abundance and distribution can be used to distinguish the effects of an environmental perturbation from natural spatial and temporal variation (Stewart-Oaten et al. Reference Stewart-Oaten, Bence and Osenberg1992). In our case, the confounding effects of natural variability could be reduced because of the existence of long-term, pre-spill data for population trends of Kentish Plovers on the Galician coast. Grouping beaches according to the degree of impact after the catastrophe allowed us to complete the classic before-after-control-impact (BACI) approach (Osenberg et al. Reference Osenberg, Schmitt, Holbrook, Abu-Saba and Flegal1994). We assumed the following: 1) in the absence of an oil spill, the Kentish Plover populations in the three coastal stretches would exhibit similar trends, and thus should have been affected to a similar extent by natural perturbations; and 2) no natural, density-dependent mechanisms affected the bird population trend on the Galician coast (e.g. changes in the carrying capacity of the environment between 1988 and 2010).
The main trend in the Galician population of Kentish Plover was a decline up to 2004, followed by a moderate increase to the present. The model selected 2004 as the year when the main trend changed, suggesting that declines in abundance were more pronounced immediately after the spill and that recovery has been occurring from 2004 to the present. These results reflect a fast recovery after the catastrophe, similar to that observed for other bird populations after the Exxon-Valdez oil spill (Bowman et al. Reference Bowman, Schempf and Hodges1997, Day et al. Reference Day, Murphy, Wiens, Hayward, Harner and Lawhead1997a,b, Murphy et al. Reference Day, Murphy, Wiens, Hayward, Harner and Smith1997).
During the last period (2005–2010), population trends showed an overall increase, especially in the medium-length beaches of stretches B and C. Apparently, the birds reacted to pollution by changing their location. The changes occurring in the spatial distribution of the population likely represented a response to a change in the availability of trophic resources and intense human occupation related to the clean-up activities at the most affected beaches. The Prestige oil spill clearly had initial impacts on breeding beaches (Junoy et al. Reference Junoy, Castellanos, Viéitez, De La Huz and Lastra2005, Franco et al. Reference Franco, Viñas, Soriano, De Armas, González, Beiras, Salas, Bayona and Albaigés2006, Serrano et al. Reference Serrano, Sánchez, Preciado, Parra and Frutos2006, Domínguez and Vidal Reference Domínguez and Vidal2007). Prior to the oil spill, the beaches most frequented by plovers corresponded to those situated in stretch A, sites where the oil and the clean-up activities lasted for a longer period of time. Overall, the Kentish Plover population declined for many of the beaches of stretch A (more polluted) and increased for those beaches belonging to stretch B (less polluted). These changes were simultaneous, suggesting a redistribution of these plovers along the Galician coast according to the level of the exposure of the beaches to the Prestige pollution. These movements were similar to those observed for bird populations studied after other oil spills, with increasing numbers appearing in oil-free areas due to displacement from oiled areas (Day et al. Reference Day, Murphy, Wiens, Hayward, Harner and Lawhead1997a, Castège et al. Reference Castège, Hémery, Roux, D’elbée, Lalanne, D’amico and Mouchès2004, Banks et al. Reference Banks, Sanderson, Hughes, Cranswick, Smith, Whitehead, Musgrove, Haveock and Fairney2008, Iverson and Esler Reference Iverson and Esler2010) or to less time spent foraging on contaminated substrates than on unoiled substrates (Andres Reference Andres1999). Breeding habitat loss or degradation can decrease the extent of philopatry (Haig and Oring Reference Haig and Oring1988, Wiens and Cuthbert Reference Wiens and Cuthbert1988, Paton and Edwards Reference Paton and Edwards1996, Cohen et al. Reference Cohen, Fraser and Catlin2006) and may have caused the birds to seek better breeding habitats, such as the unusually good habitat that has occurred in the last several years. The mobility of the birds enables them to respond quickly to changes in local habitat conditions; therefore, the birds themselves determine the condition and suitability of their habitat. The availability of suitable habitat is a prerequisite for recovery from any spill-related impact on population abundance (Morrison Reference Morrison and Johnston1986).
Human use, which can vary depending on the year, was not a significant covariate, as it did not apparently influence the population trend and changes in habitat use by Kentish Plovers along the Galician coast. However, the human use categories used were overly coarse, only referring to general tourist use, and did not reflect the use associated with the oil clean-up in the two years following the catastrophe. For this reason, caution should be exercised in interpreting this covariate in the temporal dynamics of the population. Detailed information on trophic resources before and after the oil spill was not available; indeed, this might have been useful to understand the observed changes.
In addition to the observed redistribution, an increase in the number of breeding pairs was found for the last period. This population growth can be attributed to the combination of three main factors, the first two directly related to the oil spill event, as follows:
1) Successful management strategies were undertaken for many of the Galician Kentish Plover breeding beaches after the Prestige oil spill. Although chicks usually do not return to specific natal sites, they often return to a local region (De Souza et al. Reference De Souza, Caeiro, Monteagudo, Rosende and De Souza1996, Domínguez and Vidal Reference Domínguez and Vidal2008). Potential breeding beaches within the vicinity of the spill area could be colonised by young adults, which exhibit much greater propensity than experienced breeders to colonise new breeding sites (Amat Reference Amat, Carrascal and Salvador2003, Colwell et al. Reference Colwell, Mcallister, Millett, Transou, Mullin, Nelson, Wilson and Levalley2007, Domínguez and Vidal Reference Domínguez and Vidal2008).
2) Successful results were due to dispersion. Higher dispersion of breeding pairs results in a good chance of avoiding the negative effects of density dependence (e.g. higher predation and lower breeding success) (Page et al. Reference Page, Stenzel, Winkler and Swarth1983, Mayer and Ryan Reference Mayer and Ryan1991, Paton and Edwards Reference Paton and Edwards1996, Plissner and Haig Reference Plissner and Haig2000, Yasué and Dearden Reference Yasué and Dearden2006) and reduces vulnerability to demographic and environmental stochasticity (Baillie et al. Reference Baillie, Sutherland, Freeman, Gregory and Paradis2000).
3) Migration of birds from other sites occurred. These movements were corroborated by recovery of individuals from elsewhere, mainly from the Portuguese coast, on Galician breeding beaches (Domínguez and Vidal Reference Domínguez and Vidal2008). Along the Iberian Atlantic coast, a metapopulation in highly fragmented landscapes, with small local populations and a high risk of extinction, could form in different patches at variable distances due to the process of local extinction and migrant colonisation (Domínguez and Vidal Reference Domínguez and Vidal2008).
Apparently, the impact of the Prestige oil spill on habitat conditions (as measured by habitat occupancy) was not congruent with the impact on the population trend, most likely because the birds could respond to spill-related changes in their habitat by moving elsewhere, perhaps to return later. The initial negative impact on the habitat use was evident for stretch A, suggesting initial effects on the suitability of the habitat. At present, this impact is persisting in that the percent distribution of birds did not recover to the pre-spill conditions.
Population trends and changes in breeding distribution are particularly useful biological indicators for assessing the state of the ecosystem (Boyd and Murray Reference Boyd and Murray2001, Piatt et al. Reference Piatt, Harding, Shultz, Speckman, Van Pelt, Drew and Kettle2007). Therefore, the lack of return to the pre-Prestige distribution seems to indicate the lack of complete recovery in environmental quality in the most affected regions.
Implications for conservation and management
Population trends in individual species are used to assess their conservation status, which is then translated into the goals of future management plans (Van Roomen et al. Reference Van Roomen, Koffijberg, Noordhuis, Soldaat, Boere, Galbraith and Stroud2006). Therefore, knowledge of population dynamics and habitat changes of the Kentish Plover can contribute to the development of management strategies for this vulnerable species. Preserving breeding sites is a challenging task due to the species’s spatio-temporal dynamics: new sites can be colonised, and previously occupied sites can be abandoned. Dispersal has been considered an important process in the Galician Kentish Plover population, so protecting only isolated areas would be insufficient to maintain the populations within them.
Acknowledgements
The field work in 1999 was funded by the project XUGA20003A97; from 2002 to 2010, the work was partly funded by the Consellería de Medio Ambiente (Xunta de Galicia) and the Fundación Arao. We are grateful to Luis Enrique Rego, Isabel Quintero, and Manuel Romeu for their participation in some of the censuses conducted and to Arco van Strien for his assistance concerning the use of TRIM. We thank two anonymous reviewers for their useful suggestions.







