Despite the recent identification of several putative environmental and genetic risk factors for schizophrenia (Reference Walker, Kestler and BolliniWalker et al, 2004), no single factor or combination of factors can predict with certainty who will develop the disorder. The strongest known predictor of schizophrenic illness remains the presence of an affected first-degree biological relative, which confers an 8- to 12-fold increase in risk (Reference Faraone, Tsuang and TsuangFaraone et al, 1999). The examination of unaffected relatives during the premorbid period within a genetic high-risk paradigm may therefore be particularly useful for identifying antecedents of schizophrenia (Reference Stone, Faraone and SeidmanStone et al, 2005). Unfortunately, however, such studies are difficult and costly to conduct because of their longitudinal nature and the need to administer a comprehensive battery of tests to ensure that the core predictors of illness can be isolated. Furthermore, the rate of conversion to psychosis – even in this genetically enriched population – is low (approximately 10%). Consequently, only a limited number of large-scale and long-term genetic high-risk studies of schizophrenia have been initiated.
Deficiencies in several areas of functioning, including academic, behavioural, cognitive and social domains, have consistently been observed in the existing studies of high-risk individuals (for extended reviews, see Reference AsarnowAsarnow, 1988; Reference Stone, Faraone and SeidmanStone et al, 2005). Some of the most commonly reported deficits include poorer social functioning, more restricted interests (Reference SmallSmall, 1990; Reference Dworkin, Cornblatt and FriedmannDworkin et al, 1993), lower social competence (especially in peer relationships and hobbies/interests) and greater affective flattening (Reference Auerbach, Hans and MarcusAuerbach et al, 1993). In this context, the Harvard Adolescent High Risk Study of Schizophrenia was established to replicate these findings in children and adolescents at high genetic risk of schizophrenia, as well as to evaluate other aspects of personality, psychopathology, social functioning, neuropsychology and neurobiology (Reference Seidman, Thermenos and PoldrackSeidman et al, 2006b ) in this population. In this paper we compare dimensions of psychopathology, personality traits and social development observed at baseline among the adolescent and young adult children and siblings of patients with schizophrenia and control participants enrolled in this longitudinal study. Once putative schizophrenia precursors and predictors have been identified, replicated and refined, they must be evaluated for their potential as vulnerability markers or ‘endophenotypes’ of the illness that may be useful for future genetic studies. Towards that end we also examined markers that most strongly discriminated between control and high-risk participants in relation to various established and novel indices of genetic loading for schizophrenia.
METHOD
Ascertainment and diagnosis of probands and participants
The participants in this study consisted of two groups: the biological children and siblings of schizophrenia patient probands (high-risk group), and the biological children and siblings of control probands (control group). The high-risk group comprised 10 children and 19 siblings of 22 adult probands who met DSM–IV criteria (American Psychiatric Association, 1994) for schizophrenia, and 6 children of 4 probands who met DSM–IV criteria for schizoaffective disorder, depressive type. The control group comprised 55 children of 35 control probands who did not meet DSM–IV criteria for any mental illness (n=25) or who met criteria for major depressive disorder (n=8), or mood disorder owing to a general medical condition (n=1) or cannabis abuse (n=1). Relatives of control probands with these diagnoses were allowed in the study to avoid the use of a ‘supernormal’ control group, which could have inflated the magnitude of group differences and limited the generalisability of the study. Best-estimate diagnoses were formulated based on data collected with the Diagnostic Interview for Genetic Studies (DIGS; Reference Nurnberger, Blehar and KaufmannNurnberger et al, 1994) and the Family Interview for Genetic Studies (FIGS; Reference MaxwellMaxwell, 1996). The adult patient probands were ascertained through hospitals and out-patient clinics in and around Boston, Massachusetts, USA, and the adult control probands were drawn from respondents to local newspaper advertisements and announcements posted in the vicinity of these sites. Children and siblings of both sets of probands were subsequently ascertained through their related adult probands to determine their willingness to participate in the study.
Exclusion criteria were any lifetime diagnosis of psychotic illness, substance dependence or neurological disease, a history of head injury or medical illness with documented cognitive sequelae, sensory impairments, current psychotropic medication use or a full-scale IQ less than 70. Candidates for the control group were also excluded if any of their first- or second-degree biological relatives had a history of a psychotic disorder. The full-scale IQ of participants 17 years of age or older was determined using the Wechsler Adult Intelligence Scale, version III (Reference WechslerWechsler, 1997), and the IQ of younger participants was determined with the Wechsler Intelligence Scale for Children, version III (Reference WechslerWechsler, 1991). No one was excluded from the sample based on the IQ criterion. Participants aged 18 years and older gave informed consent, whereas participants less than 18 years old gave assent in conjunction with informed consent provided by their parents. All participants received an honorarium. The study was approved by the human subject research committees of all academic and recruitment sites.
Psychopathology, personality trait and social development assessments
Each participant was administered a battery of tests to assess psychopathology, personality traits and indices of social development. This battery consisted of the following seven tests:
-
(a) Schedule for Affective Disorders and Schizophrenia for School-Age Children, Epidemiologic version (K–SADS–E; Reference Orvaschel, Puig-Antich and ChambersOrvaschel et al, 1982);
-
(b) Magical Ideation Scale (MIS; Reference Chapman, Chapman and KwapilChapman et al, 1994);
-
(c) Perceptual Aberration Scale (PAS; Reference Chapman and ChapmanChapman & Chapman, 1980);
-
(d) Revised Physical Anhedonia Scale (RPAS; Reference Chapman and ChapmanChapman & Chapman, 1980);
-
(e) Social Adjustment Inventory for Children and Adolescents (SAICA; Reference John, Gammon and PrusoffJohn et al, 1987);
-
(f) Symptom Checklist–90–Revised (SCL–90–R; Reference DerogatisDerogatis, 1993);
-
(g) Temperament and Character Inventory/Junior Temperament and Character Inventory (TCI/JTCI; Reference Cloninger, Svrakic and PrzybeckCloninger et al, 1993; Reference Luby, Svrakic and McCallumLuby et al, 1999).
A total of 36 summary items (Table 1) were selected from this test battery to serve as dependent measures. These 36 items were selected because they served either as an entry point for questionnaires with an opt-out format (e.g. positive history of delusions, positive history of alcohol use) or as the summary score for a group of related responses (e.g. total score on PAS, total score on a TCI/JTCI dimension).
Table 1 Psychopathology, personality traits and social development measures selected for analysis

| Test and item | Description (distribution) |
|---|---|
| K—SADS—E | |
| Alcohol use | Positive history of beer, wine or liquor use (categorical: yes/no) |
| Auditory hallucinations | Positive history of auditory hallucinations (categorical: yes/no) |
| Delusions | Positive history of delusions (categorical: yes/no) |
| Drug use | Positive history of illicit drug use or prescription medication misuse (categorical: yes/no) |
| Olfactory/tactile hallucinations | Positive history of olfactory or tactile hallucinations (categorical: yes/no) |
| Tobacco use | Positive history of cigar, cigarette or pipe use (categorical: yes/no) |
| Visual hallucinations | Positive history of visual hallucinations (categorical: yes/no) |
| MIS | |
| Magical ideation | Total score derived from 30 items (continuous) |
| PAS | |
| Perceptual aberration | Total score derived from 35 items (continuous) |
| RPAS | |
| Physical anhedonia | Total score derived from 61 items (continuous) |
| SAICA | |
| Positive involvement in free-time activities | Global rating of level of involvement in free-time activities (ordinal: four-point scale) |
| Positive involvement with opposite gender | Global rating of level of activity with the opposite gender (ordinal: four-point scale) |
| Positive involvement with peers | Global rating of level of activity with peers (ordinal: four-point scale) |
| Positive involvement with siblings | Global rating of level of activity with siblings (ordinal: four-point scale) |
| Problems in free-time activities | Global rating of severity of problems during free time (ordinal: four-point scale) |
| Problems with opposite gender | Global rating of severity of problems with the opposite gender (ordinal: four-point scale) |
| Problems with parents | Global rating of severity of problems with parents (ordinal: four-point scale) |
| Problems with peers | Global rating of severity of problems with peers (ordinal: four-point scale) |
| Problems with siblings | Global rating of severity of problems with siblings (ordinal: four-point scale) |
| Spends time alone | Rating of time spent alone v. with others (ordinal: four-point scale) |
| SCL—90—R | |
| Anxiety | T-score derived from 10 items (continuous) |
| Depression | T-score derived from 13 items (continuous) |
| Hostility | T-score derived from 6 items (continuous) |
| Interpersonal sensitivity | T-score derived from 9 items (continuous) |
| Obsession—compulsion | T-score derived from 10 items (continuous) |
| Paranoid ideation | T-score derived from 6 items (continous) |
| Phobic anxiety | T-score derived from 7 items (continuous) |
| Psychoticism | T-score derived from 10 items (continuous) |
| Somatisation | T-score derived from 12 items (continuous) |
| TCI/JTCI | |
| Cooperativeness | Total score derived from 20 (TCI) or 20 (JTCI) items (continuous) |
| Harm avoidance | Total score derived from 20 (TCI) or 22 (JTCI) items (continuous) |
| Novelty seeking | Total score derived from 20 (TCI) or 18 (JTCI) items (continuous) |
| Persistence | Total score derived from 20 (TCI) or 6 (JTCI) items (continuous) |
| Reward dependence | Total score derived from 20 (TCI) or 9 (JTCI) items (continuous) |
| Self-directiveness | Total score derived from 20 (TCI) or 20 (JTCI) items (continuous) |
| Self-transcendence | Total score derived from 20 (TCI) or 10 (JTCI) items (continuous) |
Statistical analyses
Demography
Continuously distributed demographic variables including age, education and parental socio-economic status (Reference HollingsheadHollingshead, 1975) were compared between high-risk and control groups by t-tests for independent samples; categorical demographic variables including gender, ethnicity and age group were compared between the groups by χ2 tests.
Multivariate data reduction and analyses
Principal components analysis with varimax rotation was performed to reduce the number of psychopathology, personality trait and social development variables to be considered in subsequent analyses. The number of factors retained from the principal components analysis was based on interpretation of the scree plot and a minimum eigenvalue of 2.0. Scores on the rotated factors were modelled as the dependent measures in a multivariate analysis of covariance (MANCOVA) with age group (age <17 years), risk group (high-risk or control) and gender – as well as the interactions of age and gender with group – as fixed predictors, and socio-economic status as a continuous covariate. Age was dichotomised at 17 years since this was the threshold age for determining if a participant would be administered the JTCI (<17 years old) or TCI (<17 years old); this approach is also consistent with that adopted for our prior analyses of cognitive functioning in this sample, which revealed a distinctive pattern of worse performance only in the subset of high-risk participants aged 17 years or over (Reference Seidman, Giuliano and SmithSeidman et al, 2006a ).
Univariate data analyses
Factors for which a significant risk-group difference was detected (high-risk v. control) were subsequently decomposed into their constituent items. Risk-group differences on these individual items were examined by analyses of covariance (ANCOVAs) with risk group as a fixed predictor, and age group, gender and socio-economic status (and their interactions with group) included as additional fixed predictors/continuous covariates if they significantly influenced the factor in the multivariate model. The significance of these post hoc analyses was determined by applying a family-wise correction for multiple testing using Simes’ method (Reference SimesSimes, 1986), which is a false discovery rate adjustment technique.
Genetic loading
Individual dependent measures that were found to be related to the genetic risk for schizophrenia (i.e. they were influenced by a main effect and/or interaction of risk group in univariate analyses) were examined in relation to various indices of genetic loading for the illness as a preliminary screen of their potential utility as phenotypes for genetic studies. There is no gold standard for quantifying genetic loading for a trait; therefore, we defined this parameter in a variety of ways (using three accepted methods and one novel method of our own design) and contrasted the results obtained with each method. In general, each method provides some index of how dense the individual's pedigree was with schizophrenia risk genes, using diagnosable schizophrenic illness as a proxy. All genetic loading indices were determined when considering individuals with either schizophrenia or schizoaffective disorder, depressive type, as affected. For each method, the numbers of affected and total members in the pedigree were provided by a family reporter, generally an adult relative of both the schizophrenia proband and the related high-risk participant.
The most basic quantification scheme implemented was the simplex/multiplex method of Faraone et al (Reference Faraone, Seidman and Kremen2000), in which each individual's family was identified as ‘simplex’ when the proband was the only affected member of the pedigree or as ‘multiplex’ when the proband and at least one other first-degree relative were affected. The remaining quantification schemes were more complex and yielded continuously distributed measures of genetic loading. For example, genetic loading was also quantified using published estimates (Reference Faraone, Tsuang and TsuangFaraone et al, 1999) to determine each individual's relative risk of schizophrenia given the number and degree of his or her biological relationships to affected members of the pedigree (the ‘relative risk’ method). A similar method (Reference Lawrie, Whalley and AbukmeilLawrie et al, 2001) accounting for the prevalence and heritability of the disorder was also employed (the ‘genetic liability’ method). These two methods assume that the traits under study map one-to-one on the risk genes for schizophrenia and thus show the same patterns of transmission and inheritance as the full disorder.
We also derived a novel index of genetic loading, calculated as:
where p is the expected identity-by-descent allele-sharing frequency between two individuals in a pedigree given their biological relationship, i represents the adolescent or young adult participant, j represents each affected member of the pedigree and k represents each unaffected member of the pedigree. In essence, this formulation (the ‘allele-sharing’ method) determines the relative proportion of alleles individual participants are expected to share with their affected biological relatives v. unaffected biological relatives while accounting for the overall pedigree size. Like the relative risk and genetic liability methods, this method assumes a tight correspondence between the traits under study and the risk genes for schizophrenia; but unlike those methods the power of the allele-sharing method is at its greatest when absolute penetrance of those genes is presumed. Values of genetic loading under this model ranged from 0 to 1, with higher values reflecting greater genetic loading. To illustrate, in the simple case where an individual comes from a pedigree with one affected brother, an affected father, an unaffected mother and an unaffected aunt, that person's genetic loading would be:
since the individual would be expected to share, on average, 50% (0.5) of his or her genes with both the affected brother and the affected father (numerator and denominator) and would also share 50% (or 0.5) of his or her genes with the unaffected mother and 25% (or 0.25) with the unaffected aunt (denominator only).
The effect of each genetic loading index was evaluated separately for each dependent measure that was influenced by a significant main effect and/or interaction of risk group in univariate analyses. The genetic loading index was included as a continuous covariate (or as a fixed factor in the case of the simplex/multiplex method) replacing risk group in the ANCOVA model that had previously revealed the significant main effect or interaction of risk group on the selected dependent measure. In all analyses of genetic loading we conservatively addressed the non-independence of observations within families by adjusting variance estimates with Huber's formula (Reference Schubert and McNeilSchubert & McNeil, 2003), a theoretical bootstrap that produces accurate statistical tests for clustered data (due to multiple individuals from the same family being entered into the study and analyses). The method enters cluster scores (the sum of scores within families) instead of individual scores into the formula for the estimate of the variance using the linearisation method (Reference Kish and FrankelKish & Frankel, 1974; Reference BinderBinder, 1983).
Technical information
Demographic data were available for all participants, whereas data on each dependent measure were available for 80–90 participants. The high-risk group was missing 4.8% of the data on these variables, whereas the control group was missing 2.0% of these data. Participants with missing data were removed from analyses by pairwise deletion. The type I error rate (α) for all analyses was set at 0.05. Corrections for multiple testing and variance adjustments for clustered data were conducted on a Windows-based personal computer with StataSE software, version 8.0, and all other statistical analyses were conducted on a Windows-based personal computer with the Statistical Package for the Social Sciences (SPSS version 13.0).
RESULTS
Demographic variables
High-risk and control participants were well-matched on several key demographic variables (Table 2) such as ethnicity (χ2 (4)=3.70, P=0.449), gender (χ2 (1)=0.04, P=0.847) and level of education (t (88)=0.12, P=0.906). However, high-risk participants were of significantly lower socio-economic status (t (88)=3.38, P=0.001) and were significantly older than the control group (t (88)=2.23, P=0.028); consequently, a greater percentage of high-risk participants compared with control participants fell into the older age group when age was dichotomised at 17 years (χ2 (1)=7.49, P=0.006). These results warranted the control of these factors and covariates in subsequent statistical models.
Table 2 Sample demographics
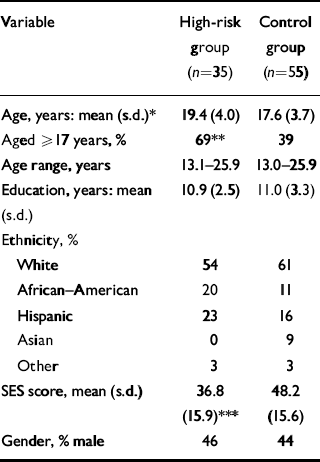
| Variable | High-risk group (n=35) | Control group (n=55) |
|---|---|---|
| Age, years: mean (s.d.)* | 19.4 (4.0) | 17.6 (3.7) |
| Aged ≥ 17 years, % | 69** | 39 |
| Age range, years | 13.1-25.9 | 13.0-25.9 |
| Education, years: mean (s.d.) | 10.9 (2.5) | 11.0 (3.3) |
| Ethnicity, % | ||
| White | 54 | 61 |
| African—American | 20 | 11 |
| Hispanic | 23 | 16 |
| Asian | 0 | 9 |
| Other | 3 | 3 |
| SES score, mean (s.d.) | 36.8 | 48.2 |
| (15.9)*** | (15.6) | |
| Gender, % male | 46 | 44 |
Multivariate data reduction and analyses
Principal components analysis of the 36 test items yielded a scree plot that indicated the presence of three predominant factors, each of which had an eigenvalue over 2.0. The three-factor solution explained 44.35% of the variance among the 36 individual variables (Table 3). The conceptualisation of factor 1 as representing ‘psychopathology’ is straightforward, given its almost exclusive composition of symptom summary items from the SCL–90–R. Factor 2 is a more heterogeneous factor representing personality traits from the TCI/JTCI, alcohol and drug use measures from the K–SADS–E, a social performance score from the SAICA and summary scores from the MIS, PAS and RPAS. Many of these items – especially those with the highest loadings – measure levels of achievement, influence over or by others, and mastery of the self, a characteristic referred to by Bakan (Reference Bakan1966) as ‘agency’ and so the term is adopted here. Factor 3 is also heterogeneous, comprising items from the SAICA, K–SADS–E and TCI/JTCI; however, 8 of the 13 items loading primarily on this factor are indices of social performance and dysfunction from the SAICA, with strong positive loadings from negative performance items and strong negative loadings from positive performance items, and this factor was therefore designated as ‘social difficulties’. The multivariate profile of scores on these three factors was significantly influenced by age group (F (3,62)=27.71, P <0.001) and risk group (F (3,62)=5.20, P=0.003) but not by socio-economic status (F (3,61)=2.51, P=0.067) or gender (F (3,60)=2.05, P=0.116). The interaction of risk group and age group was also significant (F (3,62)=3.60, P=0.018), but no other significant interaction was observed in the multivariate model.
Table 3 Factor structure and loadings after principal components analysis and varimax rotation (only loadings greater than 0.300 on a secondary factor are shown)

| Test and item | Factor 1 Psychopathology | Factor 2 Agency | Factor 3 Social difficulties |
|---|---|---|---|
| SCL—90—R | |||
| Obsession—compulsion | 0.901 | ||
| Anxiety | 0.863 | ||
| Depression | 0.863 | ||
| Interpersonal sensitivity | 0.861 | ||
| Psychoticism | 0.855 | ||
| Paranoid ideation | 0.845 | 0.323 | |
| Hostility | 0.809 | ||
| Phobic anxiety | 0.795 | ||
| Somatisation | 0.795 | ||
| MIS Magical ideation | 0.579 | 0.302 | |
| K—SADS—E Auditory hallucinations | 0.381 | ||
| SAICA Spends time alone | 0.244 | ||
| TCI/JTCI | |||
| Cooperativeness | -0.891 | ||
| Self-directiveness | -0.881 | ||
| K—SADS—E Alcohol use | -0.689 | ||
| TCI/JTCI | |||
| Self-transcendence | 0.567 | ||
| Harm avoidance | 0.565 | 0.352 | |
| K—SADS—E Drug use | -0.551 | ||
| RPAS Physical anhedonia | 0.472 | ||
| PAS Perceptual aberration | 0.442 | ||
| SAICA Positive involvement with opposite gender | 0.419 | -0.358 | |
| TCI/JTCI Novelty seeking | 0.399 | ||
| K—SADS—E Olfactory/tactile hallucinations | 0.321 | ||
| SAICA | |||
| Problems with peers | 0.729 | ||
| Problems in free-time activities | 0.622 | ||
| Problems with opposite gender | 0.567 | ||
| Problems with siblings | 0.562 | ||
| K—SADS—E | |||
| Delusions | 0.507 | ||
| Visual hallucinations | 0.490 | ||
| SAICA Positive involvement with peers | -0.474 | ||
| K—SADS—E Tobacco use | -0.395 | 0.425 | |
| SAICA Positive involvement with siblings | -0.400 | ||
| TCI/JTCI | |||
| Persistence | -0.389 | ||
| Reward dependence | -0.372 | -0.383 | |
| SAICA | |||
| Positive involvement in free-time activities | -0.231 | ||
| Problems with parents | 0.177 |
The significant main effect of age group observed in the multivariate model reflected two opposing main effects of age at the level of individual factor scores, wherein older participants scored significantly higher than younger ones on ‘psychopathology’ (factor 1; F (1,64)=8.49, P=0.005), but significantly lower on ‘agency’ (factor 2; F (1,64)=48.53, P <0.001). In contrast, the significant main effect of risk group observed in the multivariate model was driven by similar main effects of the variable on ‘agency’ (F (1,64)=4.04, P=0.049) and ‘social difficulties’ (F (1,64)=12.10, P=0.001), with high-risk participants scoring significantly higher than control participants on both factors. In addition to the main effect of risk group, ‘social difficulties’ were also significantly influenced by the interaction of age group with risk group (F (1,64)=5.47, P=0.022). Decomposition of this interaction indicated that ‘social difficulties’ remained relatively stable across age groups among the controls, whereas they increased dramatically with age group among high-risk participants (Fig. 1). As a consequence, a significant risk-group difference on factor 3 was observed between the older subsample of high-risk participants and control subjects (F (1,31)=12.72, P=0.001), but no risk-group difference was observed in the younger subsample (F (1,33)=1.18, P=0.285).
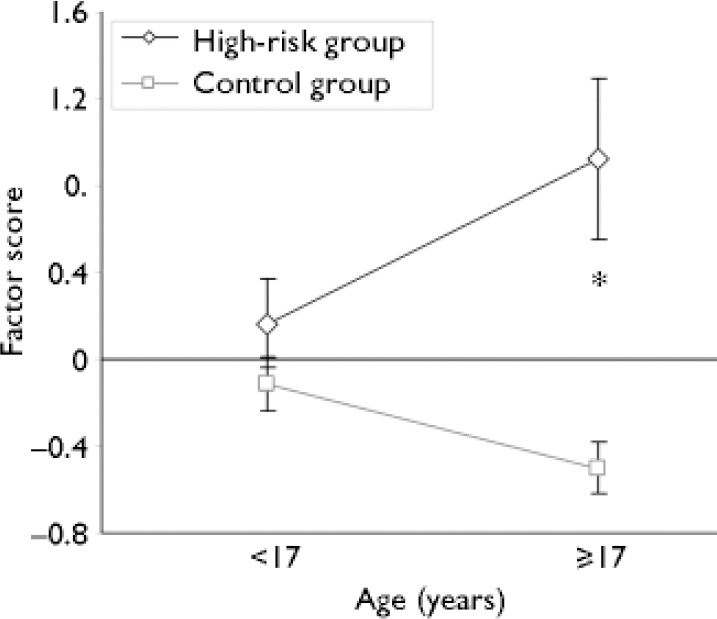
Fig. 1 Social difficulties as a function of age and group. Values represent mean (s.e.m.) scores on factor 3 (social difficulties). The interaction of age and group was significant (F (1,64)=5.47, P=0.022). *P=0.001 for comparison with control group of the same age.
Univariate analyses
Because significant risk-group differences were observed on ‘agency’ and ‘social difficulties’ (factors 2 and 3), we next identified the individual test items that significantly differentiated high-risk and control participants. Among ‘agency’ variables, high-risk participants exhibited significantly less cooperativeness (F (1,78)=6.29, P=0.014) and self-directiveness (F (1,78)=4.72, P=0.033) than control participants, and significantly more physical anhedonia (F (1,86)=8.94, P=0.004). However, only the risk-group difference on physical anhedonia remained significant after correcting for the multiple comparisons of high-risk and control groups on each of the ‘agency’ variables (corrected α threshold significance value for 11 comparisons=0.005).
Among ‘social difficulties’ variables, high-risk participants exhibited significantly less positive involvement with peers (F (1,87)=10.18, P=0.002) and a correspondingly greater frequency of problems with peers (F (1,85)=5.89, P=0.017), siblings (F (1,79)=10.39, P=0.002) and members of the opposite gender (F (1,86)=4.48, P=0.037). After correcting for multiple comparisons, the risk-group differences in level of positive involvement with peers and frequency of problems with siblings remained significant (corrected α threshold significance value for 13 comparisons=0.004).
Because a significant interaction of risk group and age group was observed for ‘social difficulties’, we also performed a separate set of univariate analyses on variables loading on this factor in the older and younger subsamples of high-risk and control participants. The younger subgroup of high-risk participants did not appear impaired on any measure relative to controls; in fact, the younger high-risk participants exhibited significantly more positive involvement with peers (F (1,42)=4.29, P=0.044) and less problems with siblings (F (1,37)=6.06, P=0.019) than similarly aged control participants. However, neither of these differences remained significant after correction for multiple testing (corrected α threshold significance value for 13 comparisons=0.004).
As expected based on the significant risk group by age group interaction for ‘social difficulties’, risk-group differences on variables loading on this factor were even more pronounced in the older subsample than in the full sample. Thus, despite the decreased power afforded by the smaller sample size of older high-risk and control participants relative to the full sample, more items were found to differ significantly between the two older groups. For example, the older group of high-risk participants exhibited significantly less positive involvement with peers (F (1,43)=5.00, P=0.031) and significantly more problems with peers (F (1,43)=12.66, P=0.001), siblings (F (1,40)=4.69, P=0.036) and members of the opposite gender (F (1,43)=7.47, P=0.009) compared with similarly aged control participants. In addition, these high-risk individuals exhibited significantly less reward dependence (F (1,38)=4.67, P=0.037) than similarly aged control participants. Of these comparisons, only the risk-group difference in frequency of problems with peers remained significant after correction for multiple testing (corrected α threshold significance value for 13 comparisons=0.004).
All significant differences observed between risk groups in this study are summarised along with corresponding effect size estimates in Table 4.
Table 4 Significant risk group differences
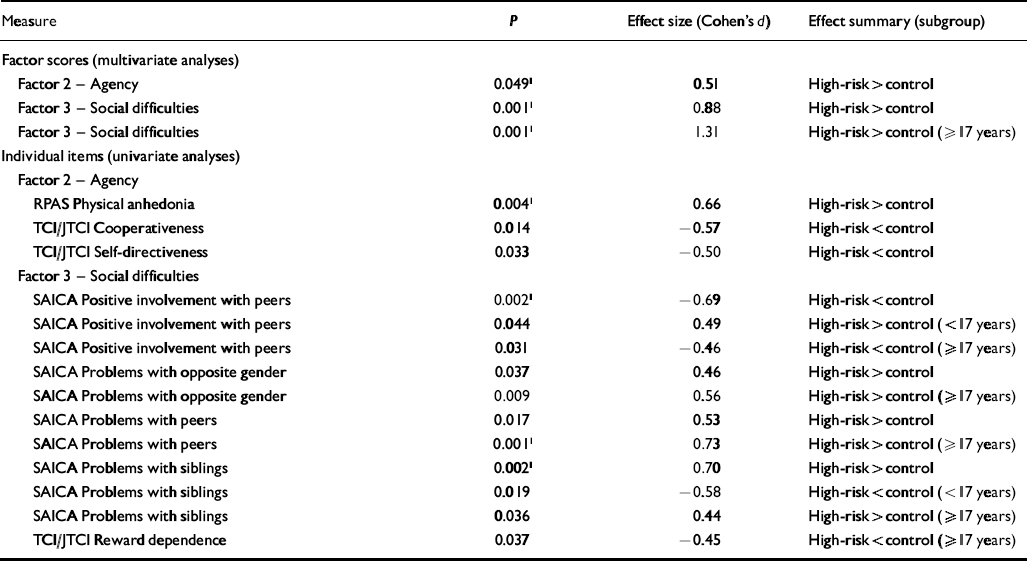
| Measure | P | Effect size (Cohen's d) | Effect summary (subgroup) |
|---|---|---|---|
| Factor scores (multivariate analyses) | |||
| Factor 2 — Agency | 0.0491 | 0.51 | High-risk>control |
| Factor 3 — Social difficulties | 0.0011 | 0.88 | High-risk>control |
| Factor 3 — Social difficulties | 0.0011 | 1.31 | High-risk>control (≥17 years) |
| Individual items (univariate analyses) | |||
| Factor 2 — Agency | |||
| RPAS Physical anhedonia | 0.0041 | 0.66 | High-risk>control |
| TCI/JTCI Cooperativeness | 0.014 | -0.57 | High-risk<control |
| TCI/JTCI Self-directiveness | 0.033 | -0.50 | High-risk<control |
| Factor 3 — Social difficulties | |||
| SAICA Positive involvement with peers | 0.0021 | -0.69 | High-risk<control |
| SAICA Positive involvement with peers | 0.044 | 0.49 | High-risk>control (<17 years) |
| SAICA Positive involvement with peers | 0.031 | -0.46 | High-risk<control (≥17 years) |
| SAICA Problems with opposite gender | 0.037 | 0.46 | High-risk>control |
| SAICA Problems with opposite gender | 0.009 | 0.56 | High-risk>control (≥17 years) |
| SAICA Problems with peers | 0.017 | 0.53 | High-risk>control |
| SAICA Problems with peers | 0.0011 | 0.73 | High-risk>control (≥17 years) |
| SAICA Problems with siblings | 0.0021 | 0.70 | High-risk>control |
| SAICA Problems with siblings | 0.019 | -0.58 | High-risk<control (<17 years) |
| SAICA Problems with siblings | 0.036 | 0.44 | High-risk>control (≥17 years) |
| TCI/JTCI Reward dependence | 0.037 | -0.45 | High-risk<control (≥17 years) |
Genetic loading
As described above, several individual variables within the ‘agency’ and ‘social difficulties’ factors were found to relate to the genetic predisposition toward schizophrenia as evidenced by significant main effects of risk group and/or interactions of risk group with other variables such as age group. Therefore, the extent to which these putative vulnerability markers linearly related to genetic liability within high-risk individuals was examined in a more quantitative manner. Of those variables constituting the ‘agency’ factor and showing a significant relationship to risk group (physical anhedonia, cooperativeness and self-directiveness), none was related to genetic loading using any of the four quantification methods (all P >0.198). Of those variables constituting the ‘social difficulties’ factor and showing some evidence of a significant relationship to risk group (positive involvement with peers, problems with opposite gender, problems with peers, problems with siblings and reward dependence), only reward dependence was influenced by genetic loading quantified using the relative risk method (F (1,20)=5.87, P=0.025). However, problems with peers and problems with the opposite gender increased significantly with genetic loading when using either the simplex/multiplex method (problems with peers: F (1,20)=4.37, P=0.049; problems with opposite gender: F (1,20)=12.32, P=0.002) or the genetic liability method (problems with peers: F (1,20)=4.40, P=0.049; problems with opposite gender: F (1,20)=11.25, P=0.003). None of the five variables on factor 3 was significantly related to genetic loading using the allele-sharing method.
DISCUSSION
In the realm of social development, children and siblings of patients with schizophrenia reported less frequent positive interactions with peers and a greater frequency of problems with peers, siblings and members of the opposite gender during adolescence and young adulthood. These high-risk participants also exhibited less cooperativeness, self-directiveness and reward dependence than the control group and experienced greater levels of physical anhedonia. Deficits in all of these domains were either more pronounced in or exclusive to high-risk participants over 17 years old. Increased genetic loading for schizophrenia was associated with greater deficits in reward dependence and more systematically with higher frequencies of problems with peers and members of the opposite gender.
Integration with prior high-risk studies
Our results replicate several observations reported in other cohorts of individuals at high genetic risk of schizophrenia. As reviewed by Asarnow (Reference Asarnow1988), some form of personality trait or (more typically) social dysfunction has been observed in all high-risk studies of schizophrenia in which such measures have been evaluated. High-risk groups in the Edinburgh (Reference Lawrie, Whalley and AbukmeilLawrie et al, 2001; Reference Johnstone, Ebmeier and MillerJohnstone et al, 2005) and Helsinki (Reference Niemi, Suvisaari and HaukkaNiemi et al, 2004) high-risk studies exhibited profound social withdrawal or inhibition which also strongly predicted the subsequent emergence of psychosis. The composite indices of social withdrawal and inhibition used in those studies differ in level of detail from the discrete items assessed with the SAICA in our study; however, higher scores on social withdrawal and inhibition factors might closely relate to the reduction in positive interactions with peers observed in our high-risk sample. High-risk individuals in the New York High Risk Project also displayed social impairments including elevated levels of problem behaviour at school and at home (Reference Moldin, Gottesman and Erlenmeyer-KimlingMoldin et al, 1990), which closely resembles the increased frequency of problems with peers, siblings and members of the opposite gender identified in our study. Importantly, these social impairments only emerged at mid-adolescence in both the New York project (age 15–16 years) and in our study (age 17 years or greater), suggesting a possible critical period for the emergence of this particular deficit in relation to risk of subsequently developing schizophrenia.
Personality differences between individuals at high genetic risk of schizophrenia and control group members have also been reported in other samples (Reference Moldin, Gottesman and Erlenmeyer-KimlingMoldin et al, 1990; Reference Bolinskey, Gottesman and NicholsBolinskey et al, 2001; Reference Miller, Byrne and HodgesMiller et al, 2002; Reference Stone, Faraone and SeidmanStone et al, 2005). Our results confirm that high-risk participants have different personality traits from those of control participants, and also identify the specific traits of cooperativeness, reward dependence and self-directiveness as particularly informative risk indicators. Our findings of greater physical anhedonia among high-risk individuals also have precedent among the existing high-risk studies. For example, people at high genetic risk of schizophrenia in the New York project showed increased levels of physical anhedonia, a feature not shared by those at genetic risk of affective disorders (Reference Freedman, Rock and RobertsFreedman et al, 1998). Interestingly, a path analysis of those data indicated that physical anhedonia mediated the relationship between genetic risk of schizophrenia and later social dysfunction. In light of this result, it will become critical to monitor the emergence of physical anhedonia and social dysfunction among our younger subsample of high-risk participants (<17 years) who as yet show no difference from control participants in these domains.
Extensions to prior high-risk studies
This study extends our understanding of people at high genetic risk in several ways. First, whereas most prior work identified broad, psychometrically defined constructs that differentiated high-risk and control groups, we took this approach a step further by examining factors more closely to identify the individual test items that drove group differences. Second, the identification of risk-group differences restricted to a more narrowly defined, older subgroup (≥17 years) represents progress toward the goal of identifying a critical period for the emergence of personality traits and social dysfunction in individuals harbouring a strong genetic risk of schizophrenia. The observation of these effects only in the older subgroup of high-risk participants could merely reflect stochastic differences between the older and younger cohorts. Alternatively, if these effects emerge subsequently in our younger cohort, these might indicate faulty developmental processes or the emergence of some developmentally triggered degenerative process.
Third, it is noteworthy that increased psychopathology observed among high-risk participants in other cohorts (e.g. Reference Ott, Roberts and RockOtt et al, 2002) was not apparent in our study. Although high-risk individuals in our study did have higher scores on factor 1 and on several individual SCL–90–R items within that factor, these differences dissipated when covariates such as age, gender and socio-economic status were included in the multivariate and univariate statistical models, thus underscoring the importance of recognising and controlling for potential confounds in case–control study designs such as this. Fourth and finally, we identified three traits (reduced reward dependence, and increased frequency of problems with peers and members of the opposite gender) that not only differentiated high-risk participants from controls but also showed a gradient of increasing impairment with genetic loading for schizophrenia within the high-risk group. Under the prevailing multifactorial polygenic model of the aetiology of schizophrenia, such traits might have a higher likelihood of reflecting genetic defects in families with one or more member with schizophrenia, and thus may prove to be among the most suitable for inclusion in composite alternate phenotypes of the disorder for use in future genetic studies.
Clinical implications
This study identified several behaviours and psychological traits that differ between individuals at high genetic risk of schizophrenia and the control group. The easily observable nature of some deficits (e.g. problems with peers, siblings and members of the opposite gender) may furnish them with considerable utility in the clinical setting as early warning signs of emergent psychosis among individuals with a positive family history of schizophrenia. If these social difficulties in particular are found to predict the eventual emergence of full schizophrenia-spectrum illness among the high-risk individuals in our longitudinal study, they may serve as useful targets for early intervention efforts as well, since these particular behaviours may be easier than personality traits or physical anhedonia to identify and alter. For the purposes of early identification and intervention based on the presence of social difficulties, our findings also make clear that the collection of accurate information regarding family history of schizophrenia is critical for quantifying risk. Last, if the biological foundations of these more elemental phenotypes can be understood, they may provide insight into the pathological mechanisms underlying the manifestation of full schizophrenia, its subtypes or other conditions in the schizophrenia spectrum.
Limitations of the study
The results of this study must be considered in light of some limitations. First, we implemented a conservative analytic strategy that began with data reduction through principal components analysis, but the tests and variables selected for inclusion were chosen a priori from a much larger panel. Thus, inclusion of different tests or different variables from those tests in the present analyses might have had major effects on the factor structure and thus the pattern of significant group differences observed.
Second, the power of these analyses was not optimal for detecting small effect sizes. Thus, although the given sample sizes afforded more than 80% power to detect risk-group differences in excess of 0.4 standard deviations, smaller but nonetheless important effects would have had a low likelihood of detection in this sample. Continued ascertainment of both participant groups (but especially high-risk participants) should augment the power of this sample. Longitudinal follow-up of the existing samples will ultimately allow for the use of more powerful within-subject statistical modelling techniques, which should also facilitate the detection of smaller significant differences between risk groups.
Third, in addition to the limitations on inferential power imposed by the sample size, the analyses of genetic loading were also subject to an additional limitation: recall bias. Thus, all genetic loading quantification schemes used in this study relied upon how much of the pedigree was recalled and reported by the family's reporter, and how well the reporter recognised and recalled the pedigree members who were affected with a schizophrenic illness. Thus, if reporters underestimated or overestimated the number of affected individuals in their families, the genetic loading index of their related high-risk participant would be biased downwards or upwards respectively. If this recall variation differed systematically between reporters whose related high-risk participants performed well and those whose related high-risk participants performed poorly, an effect of genetic load might appear where none existed, or the converse. However, as not all individual variables within a factor showed an effect of genetic loading, these potential sources of bias may be either offset or have minimal practical importance.
Fourth, this was a family study wherein probands and participants had both genetic and environmental factors in common. Thus, the observed group differences and the effects of genetic loading may not reflect the effects of risk genes shared between high-risk individuals and patients with schizophrenia, but rather their exposure to common environmental factors that influenced the dependent measures.
Future directions
Children and siblings of people with schizophrenia are approximately ten times more likely to develop schizophrenia or a related disorder than are individuals in the general population. Consequently, these individuals require careful monitoring. Even if they do not develop psychosis, our results suggest that they are at high risk of social dysfunction and the expression of abnormal personality traits, and thus of a lowered quality of life. Longitudinal tracking of these individuals will allow us to specify more definitively critical periods for the emergence of schizophrenia precursors and to possibly shed light on developmental triggers for the illness, as well as determine which measures are the best predictors of the transition to schizophrenia, and which predict more stable deficits. In addition, these risk markers can be combined with neuropsychological and neuroimaging abnormalities observed in these same individuals (reported elsewhere) to develop more powerful and flexible composite risk phenotypes. Future follow-up studies of this sample will help us clarify psychopathological processes in schizophrenia, develop accurate predictors of psychosis and identify treatment targets for early intervention and prevention programmes.
Acknowledgements
We thank Dr Heidi Thermenos for helpful comments on the manuscript. This work was supported in part by grants R01MH043518 (L.J.S., M.T.T.), R25MH060485 (M.T.T.), R01MH065562 (L.J.S., M.T.T.) and R01MH063951 (L.J.S.) from the National Institute of Mental Health, grants from the National Association for Research in Schizophrenia and Depression (M.T.T., L.J.S.), and a grant from the Mental Illness and Neuroscience Discovery Institute (L.J.S.).








eLetters
No eLetters have been published for this article.