Concern has been expressed in recent years that Se may increase the risk of type 2 diabetes (T2D), and indeed a number of studies have implicated the selenoproteins, cytosolic glutathione peroxidase 1 (GPx1) and selenoprotein P1 (SEPP1) in insulin resistance( Reference Rayman and Stranges 1 – Reference Hellwege, Palmer and Ziegler 4 ). However, epidemiological data are conflicting. Although higher Se status has been associated with higher prevalence of T2D or fasting plasma glucose in five cross-sectional studies, three such studies have found the opposite association and two have found no association( Reference Rayman and Stranges 1 , Reference Navarro-Alarcon, Lopez and Perez-Valero 5 – Reference Rajpathak, Rimm and Morris 7 ). Results of longitudinal or prospective studies have not supported a causal role for Se in T2D( Reference Rayman and Stranges 1 ). With regard to trial evidence, five randomised-controlled trials (RCT) with Se as a single agent reported on the risk of T2D, although none had T2D as a primary end point( Reference Rayman and Stranges 1 , Reference Mao, Zhang and Huang 8 , Reference Rayman, Blundell-Pound and Pastor-Barriuso 9 ). Of these, only one showed an increased incidence of T2D in the Se arm, although only in participants in the top tertile of plasma Se at baseline( Reference Stranges, Marshall and Natarajan 10 ).
Studies on pregnant women are also contradictory; in pregnant women without gestational diabetes (GDM), activity of the cytosolic selenoenzyme, GPx1, in erythrocytes increased during pregnancy and was positively associated with fasting plasma glucose, plasma insulin, C-peptide and the homoeostasis model of assessment for insulin resistance (HOMA-IR) index, suggesting an adverse effect of Se, or at least of a selenoprotein( Reference Chen, Scholl and Leskiw 11 ). By contrast, the literature also shows that pregnant women with impaired glucose tolerance or GDM have much lower serum Se concentrations than women with normal pregnancies( Reference Tan, Sheng and Qian 12 – Reference Kilinc, Guven and Ezer 14 ) and that there is an inverse relationship between serum Se and blood glucose concentration( Reference Kilinc, Guven and Ezer 14 ). Furthermore, in a small group of pregnant women, the increase in plasma glucose following an oral glucose tolerance test administered at 12 weeks of gestation was inversely correlated with plasma Se concentration( Reference Hawkes, Alkan and Lang 15 ). Recently, an RCT of Se (200 μg/d) in seventy women who had already developed GDM found a significant reduction in fasting plasma glucose, serum insulin and HOMA-IR in those on Se( Reference Asemi, Jamilian and Mesdaghinia 16 ). However, to date, there has been no RCT of the effect of Se supplementation on the risk of GDM or insulin resistance in normal pregnant women.
We took advantage of having stored plasma samples from the Se in Pregnancy Intervention (SPRINT) RCT of Se supplementation in pregnancy to test the effect of Se supplementation on a marker of insulin resistance in UK pregnant women( Reference Rayman, Searle and Kelly 17 ). SPRINT was set up to assess the effect of Se supplementation on the risk of pre-eclampsia in pregnant women of low-to-moderate Se status( Reference Rayman, Searle and Kelly 17 ). Although we could not validly measure plasma glucose or insulin as we only had non-fasted samples, we were able to measure plasma adiponectin, a recognised marker of insulin resistance( Reference Kishida, Funahashi and Shimomura 18 ) and a strong independent predictor of the risk of GDM and T2D( Reference Hotta, Funahashi and Arita 19 – Reference Fasshauer, Bluher and Stumvoll 24 ). Adiponectin measurements are valid even in non-fasted plasma samples, as diurnal variability is minor and there is no noticeable effect of food intake( Reference Hotta, Funahashi and Arita 19 , Reference Li, Shin and Ding 20 , Reference Gavrila, Peng and Chan 25 , Reference Shand, Elder and Scott 26 ). Higher plasma adiponectin concentration is associated with lower risk( Reference Li, Shin and Ding 20 ).
Plasma adiponectin concentration falls substantially during the course of normal pregnancy( Reference Catalano, Hoegh and Minium 27 ). We hypothesised that supplementation with Se at a nutritional level would not exacerbate that fall, indicating a lack of an adverse effect of Se on insulin resistance. On the basis of our previous findings( Reference Rayman, Searle and Kelly 17 ), we speculated that Se supplementation might even lessen the fall in plasma adiponectin in women in the bottom quartile of Se status at baseline.
Methods
Participants
Blood and plasma samples for this study originate from the SPRINT study (trial registration ISRCTN37927591, http://controlledtrials.com/ISRCTN37927591), which randomised 230 primiparous women in Oxford, UK, to treatment with Se (60 μg/d Se, as Se yeast) or placebo (placebo yeast) from their first hospital antenatal visit (mean gestational age 12·3 weeks) until delivery of the baby( Reference Rayman, Searle and Kelly 17 ). Blood samples, from which plasma was prepared, were collected at baseline (12 weeks), 20 and 35 weeks( Reference Rayman, Searle and Kelly 17 ).
Five women were excluded from the analysis: one woman was recruited in error (treated with thyroxine), two had diabetes before conception and two were outliers, one with respect to whole-blood Se and one with respect to adiponectin concentration. One woman had no remaining plasma for adiponectin analysis at 12 weeks. There were 111 women in the placebo group and 113 in the Se group at baseline, and 107 in the placebo group and 104 in the Se group at 35 weeks (see Participant Flow chart in previous publication( Reference Rayman, Searle and Kelly 17 )).
The study was conducted in accordance with the guidelines of the Declaration of Helsinki. All procedures involving human subjects were approved by the Milton Keynes Research Ethics Committee (REC reference no. 08/H0603/46). A non-substantial amendment for additional laboratory measurements in stored samples was approved by NRES Committee South Central, Berkshire (27 July 2011). Written informed consent was obtained from all subjects.
Anthropometric measurements
Weight at 12 and 35 weeks was measured to the nearest 0·1 kg while subjects were dressed in light clothing. Subjects stood barefoot while height was measured to the nearest 0·01 m by using a wall-mounted stadiometer (Holtain Ltd). BMI was calculated using weight (kg) divided by height squared (m2).
Laboratory analyses
Whole-blood Se and plasma glutathione peroxidase 3 (GPx3) activity at 12 and 35 weeks were determined by inductively coupled plasma MS and a spectrophotometric assay, respectively, as previously described( Reference Rayman, Searle and Kelly 17 , Reference Rayman, Bath and Westaway 28 ). The CV for the whole-blood Se assay was 0·25 % at 1·4 mmol/l and 0·17 % at 3·0 mmol/l( Reference Rayman, Bath and Westaway 28 ). For GPx3, the inter-assay variation was 0·43 % and the intra-assay variation was 1·25 %( Reference Vanderlelie, Venardos and Clifton 29 ).
Toenail Se at 16 weeks was measured using instrumental neutron activation analysis at the Interfaculty Reactor Institute in Delft, as previously described( Reference Rayman, Searle and Kelly 17 , Reference Rayman, Bode and Redman 30 ). The laboratory has an embedded quality-control system for quality assurance and management, which complies with the requirements of the International Standard ISO/IEC 17025:2005 and has been accredited by the Dutch Council for Accreditation since 1993.
Plasma SEPP1 concentration at 35 weeks was measured by ELISA in the laboratory of Raymond Burk, as previously described( Reference Rayman, Searle and Kelly 17 , Reference Rayman, Bode and Redman 30 ). For quality control, each ELISA was run with two standards: purified SEPP1 and a standard plasma sample( Reference Xia, Hill and Li 31 ).
Total plasma adiponectin concentration at 12 and 35 weeks was measured using a commercial ELISA kit (Quantikine®, Human Total Adiponectin/Acrp30 Immunoassay; R&D Systems Europe Ltd) according to the manufacturer’s instructions (http://www.rndsystems.com/pdf/drp300.pdf). The intra- and inter-assay CV were 2·5 and 6·8 %, respectively.
Statistical methods
To use parametric analysis, adiponectin concentration was transformed using the natural logarithm (ln) to approximate a normal distribution. Then, the difference at baseline between placebo and Se groups was tested by an independent samples t test. A paired samples t test was used to compare adiponectin concentrations at 12 and 35 weeks within placebo and Se groups.
Intention-to-treat analysis
The difference in the change in adiponectin between placebo and Se groups from 12 to 35 weeks was tested by the Mann–Whitney U test. On the basis of effects seen previously in this study in women in the bottom Se status quartile (whole-blood Se)( Reference Rayman, Searle and Kelly 17 ), we repeated the test in the group of women in the bottom quartile of whole-blood Se at baseline. Because a previous study saw adverse effects only in those in the top tertile of baseline plasma Se(10), we also carried out the same analysis in those of high status – that is, women in the top quartile in the current study. We also explored the difference in adiponectin concentration between placebo and Se groups at 35 weeks by ANCOVA, by adjusting for baseline ln-adiponectin concentration, as adiponectin concentration at baseline and 35 weeks were highly correlated (r 0·755; P<0·001).
For the sample size per treatment group and an uncorrected two-sided significance level of 0·05, the trial had 80 % power to detect a 25·1 % difference in adiponectin change during pregnancy between Se and placebo groups.
Cross-sectional associations
To evaluate the cross-sectional associations between Se status and adiponectin concentration at baseline (12 weeks), we divided the population into quartiles by the various Se status parameters. The median value of each quartile was used as an ordinal variable to test the significance of the linear trend across quartiles by multiple linear regression analysis. Adjustment was made for baseline BMI, as BMI is known to be associated with adiponectin concentration( Reference Haghiac, Basu and Presley 32) as was also the case in our study. No adjustment was made for gestational age despite the fact that it affects adiponectin concentration(27), as the range of gestational ages was very narrow (median 12 (interquartile range (IQR) 12, 13) weeks).
As the magnitude and range of both Se status and adiponectin concentration changed substantially over the course of the trial, we carried out a cross-sectional analysis at 35 weeks, stratified by treatment group. ln-transformed adiponectin concentration at 35 weeks was adjusted for baseline ln-transformed adiponectin and BMI at 35 weeks.
Statistical analyses were conducted using IBM SPSS Statistics version 20. P values<0·05 (two-tailed) were considered significant.
Results
Baseline characteristics
Table 1 shows the baseline adiponectin concentrations in the group overall and in Se and placebo groups separately, and the P value for comparison between the groups. There was no significant difference in adiponectin concentration between treatment groups at baseline. We previously reported the lack of any significant difference in parameters of Se status (whole-blood Se, GPx3 activity, toenail Se) and other participant characteristics (age, ethnicity, gestational age, smoking, drinking, BMI) between treatment groups at baseline( Reference Rayman, Searle and Kelly 17 , Reference Rayman, Bath and Westaway 28 ).
Table 1 Baseline adiponectin concentrations in the group overall and in selenium and placebo groups separately, and P value for comparison between groups (Medians and interquartile ranges (IQR); geometric means and 95 % confidence intervals)
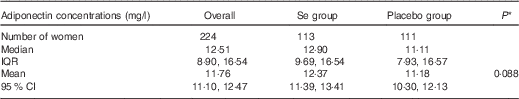
* P value for difference between Se and placebo groups by independent samples t test.
Effect of treatment
At 35 weeks, median whole-blood Se in the Se group was significantly higher than in the placebo group (1·87 (IQR 1·67, 2·15) v. 1·16 (IQR 1·05, 1·30) μmol/l; P<0·001), although there was no significant difference in GPx3 activity between the two groups (P=0·140). Compared with baseline, in the women supplemented with Se, whole-blood Se and GPx3 activity at 35 weeks significantly increased by 42·1 % (P<0·001) and 6·7 % (P=0·026), respectively. In the placebo group, between baseline and 35 weeks, whole-blood Se significantly decreased by 12·8 % (P<0·001) but GPx3 activity did not change (P=0·874).
Intention-to-treat analysis
Adiponectin concentration significantly decreased during pregnancy in both placebo and Se groups, by 23·7 and 25·5 %, respectively (P<0·001 for both). However, there was no significant difference in the change in adiponectin during pregnancy between the two groups (P=0·938), nor when the analysis was restricted to women in the bottom or top quartile of whole-blood Se at baseline (Table 2). At 35 weeks, adiponectin concentration was highly and significantly correlated with that at baseline in the overall population (r 0·755; P<0·001). When 35-week adiponectin concentration was adjusted for baseline concentration, ANCOVA found no significant difference in adiponectin concentration between placebo and Se groups (P=0·332).
Table 2 Effect of selenium supplementation on change in plasma adiponectin concentration between 12 and 35 weeks of gestation in women with samples at both 12 and 35 weeks, in all participants and in the bottom and top quartiles of whole-blood selenium (whole group) at baseline (Geometric means and 95 % confidence intervals; medians and interquartile ranges (IQR))

* P value for comparison between Se and placebo treatment groups for median change in adiponectin by Mann–Whitney U test.
† P value from paired t test, 12 v. 35 weeks.
Two women (1·8 %) in the placebo group were diagnosed with GDM in late pregnancy but none in the Se group; however, clearly no significance can be attached to this finding.
Cross-sectional association between parameters of selenium status and adiponectin concentration at baseline
To investigate cross-sectional association between parameters of Se status and adiponectin concentration, we divided participants by quartile of Se status at baseline. Adiponectin concentration was not associated with any parameter of Se status (Table 3). Although the geometric mean of plasma adiponectin was higher in the top than in the bottom quartile of Se status for whole-blood Se, GPx3 activity and toenail Se, the difference did not reach significance (adjusted P for linear trend=0·252, 0·776 and 0·235, respectively).
Table 3 Cross-sectional association between selenium status parameters and adiponectin concentration at baseline (12 weeks) (Geometric means and 95 % confidence intervals; medians and interquartile ranges (IQR))

GPx3, glutathione peroxidase 3.
* P for trend from linear regression models of ln-adiponectin concentration adjusted for baseline BMI.
Cross-sectional association between adiponectin concentrations and selenium status parameters in the placebo group at 35 weeks
As adiponectin concentration fell substantially from 12 to 35 weeks, we explored whether there might be a cross-sectional association with Se status at that lower level of adiponectin that represents a higher level of insulin resistance or risk of GDM. We therefore carried out a cross-sectional analysis of the effect of 35-week Se status parameters on 35-week baseline-adjusted ln-adiponectin concentration in each group separately. Although there was a near-significant positive association of GPx3 activity with adiponectin concentration in both placebo and Se groups, it did not reach significance (P=0·061 and 0·077, respectively, Table 4). None of the other Se status parameters was associated with adiponectin concentration.
Table 4 Cross-sectional association between parameters of selenium status and adiponectin concentration at 35 weeks by treatment group (Geometric means and 95 % confidence intervals; medians and interquartile ranges (IQR))

GPx3, glutathione peroxidise 3; SEPP1, selenoprotein P1.
* P for trend from linear regression models of ln-adiponectin concentration adjusted for baseline ln-adiponectin concentration and BMI at 35 weeks.
Discussion
The lack of a difference between the effect of Se and placebo supplementation on the change in plasma adiponectin concentration from 12 to 35 weeks (P=0·938) confirmed our hypothesis that supplementation with Se at a nutritional level would not adversely affect plasma adiponectin. Although we had speculated that Se supplementation to women in the bottom quartile of Se status at baseline might benefit them by reducing the fall in plasma adiponectin, we did not see any such benefit (P=0·515).
The significant fall that we observed in plasma adiponectin from 12 to 35 weeks gestation is in line with previous findings( Reference Catalano, Hoegh and Minium 27 ) and corresponds to an impairment of peripheral insulin sensitivity in late pregnancy that allows increased glucose availability to the feto-placental unit( Reference Catalano, Hoegh and Minium 27 , Reference Catalano, Tyzbir and Roman 33 ).
Taking plasma adiponectin as a proxy for insulin resistance( Reference Kishida, Funahashi and Shimomura 18 ), our results are reassuring insofar as they demonstrate the lack of an adverse effect of low-dose Se on insulin resistance in pregnancy, at least in a pregnant population of relatively low Se status such as that of the UK( Reference Rayman 34 ). The only other RCT that used plasma adiponectin as biomarker of insulin resistance/T2D similarly found no diabetogenic effect, despite the Se dose levels being considerably higher, i.e., 100, 200 and 300 μg/d( Reference Rayman, Blundell-Pound and Pastor-Barriuso 9 ); that trial was also carried out in a UK population. In fact, the only trial that has found an increased risk of T2D was in a US population of considerably higher Se status than that of the UK, and it gave quite a substantial dose of Se, i.e., 200 μg/d( Reference Stranges, Marshall and Natarajan 10 ).
We divided the various parameters of Se status into quartiles to see whether we could identify any cross-sectional associations at baseline, as had been seen in the previous trial of Se with plasma adiponectin as an outcome( Reference Rayman, Blundell-Pound and Pastor-Barriuso 9 ). Although the geometric mean of plasma adiponectin was higher in the top than in the bottom quartile for all Se status parameters, the difference did not reach significance.
No cross-sectional association was found between 35-week SEPP1 and plasma adiponectin, although an association had been found previously between serum adiponectin and SEPP1; however, that association was in patients with T2D( Reference Misu, Ishikura and Kurita 35 ). It is, nonetheless, of interest that we found a near-significant positive correlation of GPx3 activity with adiponectin concentration in both placebo and Se groups at 35 weeks, a time when adiponectin concentration had fallen substantially. GPx3 concentration has been found to be significantly lower in women with T2D than in those with normal glucose tolerance, and GPx3 expression has been shown to be down-regulated in T2D muscle( Reference Chung, Kim and Youn 36 ). Furthermore, the ability of the thiazolidinediones to prevent insulin resistance induced by oxidative stress in human skeletal muscle cells is exclusively mediated by GPx3( Reference Chung, Kim and Youn 36 ). We may have been limited by our study size in not seeing a significant correlation with GPx3. It may be relevant that the near-significant effect of GPx3 that we saw appears to be independent of the effect of Se, per se. This may suggest that an SNP in the GPx3 gene affects adiponectin concentration in pregnancy. We are currently investigating such a potential GPx3 genotype effect.
Our study was limited by being a secondary analysis of a trial that was set up for another purpose, and hence our sample size would not have been adequate to detect a small treatment effect. A further limitation was that we only had one marker of insulin resistance owing to a lack of fasted samples; however, adiponectin is a well-recognised marker of insulin resistance( Reference Kishida, Funahashi and Shimomura 18 ) and it has been shown to be an independent predictor of the risk of GDM( Reference Lacroix, Battista and Doyon 23 , Reference Fasshauer, Bluher and Stumvoll 24 ).
In conclusion, a nutritional dose of Se given to pregnant women of modest Se status in an RCT had no apparent adverse effect on the concentration of adiponectin, a biomarker of insulin resistance, nor did there appear to be a beneficial effect, although some previous studies might have predicted benefit( Reference Tan, Sheng and Qian 12 – Reference Asemi, Jamilian and Mesdaghinia 16 ); however, the dose was small. Given the known positive associations between higher Se status or Se supplementation and healthy pregnancy, this is good news for women in countries such as the UK and Europe who are unlikely to increase the risk of insulin resistance by taking a modest Se supplement( Reference Hawkes, Alkan and Lang 15 , Reference Rayman, Searle and Kelly 17 , Reference Rayman, Bath and Westaway 28 , Reference Rayman, Bode and Redman 30 , Reference Rayman, Wijnen and Vader 37 – Reference Negro, Greco and Mangieri 39 ).
Acknowledgements
The authors are extremely grateful to all the women who took part in this study, and the team at the John Radcliffe Hospital, especially research midwife, Libby Searle, who recruited the women. We thank Christine Sieniawska of the Trace Element Unit, Southampton University Hospital NHS Trust for analysis of Se in whole blood and Mehmet Sarilar and Menno Blaauw of TU Delft Reactor Instituut who analysed the toenails.
The SPRINT study was supported by a Wellcome Trust Project grant no. 083918/Z/07/Z (M. P. R., C. W. G. R.); adiponectin measurement was supported by Nestle Nutrition Institute Fellowship; J. M. was supported by Shenyang Science and Technology Project (F12-193-9-28). S. C. B. was supported by an MRC Population Health Scientist Fellowship. Toenail analysis at TU Delft Reactor Instituut was supported by the European Commission under the 7th Framework Programme through the ‘Research Infrastructures’ action of the ‘Capacities’ Programme (NMI3-II grant no. 283883). The funders had no role in the design, analysis or writing of this article.
M. P. R. was the SPRINT study principal investigator and C. W. G. R. was responsible for the recruitment of the women at the John Radcliffe Hospital. M. P. R. and J. M. designed the current research project. J. J. V. and A. V. P. were responsible for measuring GPx3 activity. J. M. performed adiponectin measurement and performed the statistical analysis. S. C. B. advised on the data analysis. M. P. R. wrote the paper with help from J. M., M. P. R. had primary responsibility for final content. All authors read and approved the final manuscript.
There are no conflicts of interest.







