Introduction: why cocoa and why the focus on CVD?
It is an appealing idea that a food commonly consumed for pure pleasure could also bring tangible benefits for health. Olive oil, green tea and red wine have been commonly researched in the pastReference Vissers, Zock and Katan1–Reference Cabrera, Artacho and Gimenez3 and now there is growing interest in cocoa. Cocoa is rich in polyphenols, similar to those found in green tea, and as polyphenols have been shown to have beneficial effects on CVD, it has resulted in heart health being the most common target for research on cocoa. There are several excellent recent reviews on cocoa and so this review is intended to be focused on lessons learned and on improving future researchReference Engler and Engler4, Reference Ding, Hutfless, Ding and Girotra5.
For cocoa, the terms that are used to describe the particular compounds of interest are flavanols (also known as flavan-3-ols or catechins). Flavanols are a subclass of flavonoids which are, in turn, a subclass of polyphenols. Flavanols can be monomeric and those found in cocoa beans are ( − )-epicatechin and (+)-catechin (their isomers may also be present in small quantities), dimeric (the most common in cocoa are B2 and B5, both made of two units of epicatechin with differing linkages) or they can be polymeric combinations of these monomers, and chains of up to and over 10 units have been found in cocoaReference Wollgast and Anklam6. These polymers are known as procyanidins. For ease of writing, the term of cocoa polyphenols here encompasses the monomers and the procyanidins. All polyphenols exert an antioxidant action in vitro Reference Cook and Samman7, Reference Zhu, Huang and Chen8, however, this does not mean that all polyphenols have an antioxidant effect in vivo. The use of the term antioxidant in the present report reflects this and is not intended to imply that all cocoa polyphenols have a proven antioxidant benefit in vivo.
Chocolate and cocoa are two different terms and are not interchangeable. Cocoa is the non-fat component of cocoa liquor (finely ground cocoa beans) which is used in chocolate making or as cocoa powder (commonly 12 % fat) for cooking and drinks. Cocoa liquor contains approximately 55 % cocoa butter and together this comprises cocoa solids, often referred to on chocolate packaging. Chocolate refers to the combination of cocoa, cocoa butter, sugar, etc. into a solid food product.
A recent survey found that in Europe, 58 % of people ate milk chocolate, closely followed by dark chocolate (43 %)Reference Callebaut9. For the UK, these figures were 61 and 35 %, respectively. In the USA, milk chocolate is also considered the most popular, but the majority of their confectionery consumption (~87 %) is not as pure chocolate but rather enrobed with nuts, wafer, fruit, etc.Reference Vinson, Proch, Bose, Muchler, Taffera, Shuta, Samman and Agbor10. Cocoa taken as a beverage is also popular in some countries like Spain and so should also be taken into account when surveying intake of chocolate and cocoa products.
What has been done in the last 10 years?
Waterhouse and colleagues wrote a letter, which was published in 1996, that described an in vitro experiment that was to open up a whole new area of nutrition and healthReference Waterhouse, Shirley and Donovan11. Polyphenols were extracted from commercial cocoa and chocolate, and the polyphenol content and antioxidant activity against LDL oxidation was measured. They found a potent inhibition by the cocoa polyphenols. At 5 μmol/l total polyphenols (expressed as gallic acid equivalents), LDL oxidation was inhibited by 75 %, compared to red wine at 37–65 %. This was the first publication to state that the action and content of polyphenols from cocoa meant that it could be considered as a dietary source of antioxidants.
After this, research papers linking chocolate and health began to appear, and patents on polyphenol content in cocoa and potential benefit areas were released. In 1999, another letter was published, this time explaining the contribution of chocolate compared to tea in the Dutch population as sources of catechins, finding that although tea was still the major source (55 %), chocolate contributed significantly (20 %)Reference Arts, Hollman and Kromhout12. The first human bioavailability trial of polyphenols from chocolate found that with 40 g of black chocolate, epicatechin was indeed absorbed into the bloodReference Richelle, Tavazzi, Enslen and Offord13. Epicatechin was present in plasma as metabolites conjugated with glucuronide and sulphate groups. These compounds exhibited a T max of 2 h in the plasma and C max of over 100 ng/ml. The compounds were still measurable after 8 h.
Table 1 shows human trials with interventions using cocoa in different forms from 2000 to 2007. For each study, the intervention and its polyphenol content (if available), the controls, subject type and the main outcomes are described. Each of these trials investigated at least one health-related end-point. The end-points selected at the beginning of this period were suppression of platelet activationReference Rein, Paglieroni, Wun, Pearson, Schmitz, Gosselin and Keen14 and improvement of plasma antioxidant activity and lipid oxidationReference Rein, Lotito, Holt, Keen, Schmitz and Fraga15. These end-points were logical as they had been shown previously to be affected positively by other sources of polyphenols such as red wineReference Kondo, Matsumoto, Kurata, Tanahashi, Koda, Amachi and Itakura16–Reference Rein, Paglieroni, Pearson, Wun, Schmitz, Gosselin and Keen20. The rate of publication has generally been increasing since 2000. There were two human trials published in 2001, three in 2002, five in 2003, three in 2004, six in 2005 and five in 2006 (one so far in 2007). More often than not, the studies yield at least one positive and significant result although, as more than one end-point is measured in most of the trials, secondary outcomes are often unchanged. These trials do not include those investigating the metabolism and pharmacokinetics of chocolate components. End-points included blood pressure, insulin sensitivity and resistance, endothelial function and flow-mediated dilation (FMD), platelet function, plasma antioxidant status and oxidative stress, plasma lipids (levels and oxidation), nitric oxide (NO) and haemolysis. There is one epidemiological study that correlates long-term cocoa intake with lower overall and cardiovascular mortality in elderly menReference Buijsse, Feskens, Kok and Kromhout21 and a prospective study in post-menopausal women which found a borderline inverse association of chocolate intake and CVD mortalityReference Mink, Scrafford, Barraj, Harnack, Hong, Nettleton and Jacobs22. The research is predominantly focused on effects on the vascular system, however, there are other areas of research on man in vivo which are not so extensively investigated, such as those concerned with cognitionReference Francis, Head, Morris and Macdonald23, cancerReference Ramljak, Romanczyk, Metheny-Barlow, Thompson, Knezevic, Galperin, Ramesh and Dickson24 and diabetesReference Grassi, Lippi, Necozione, Desideri and Ferri25.
Table 1 Human intervention trials with cocoa
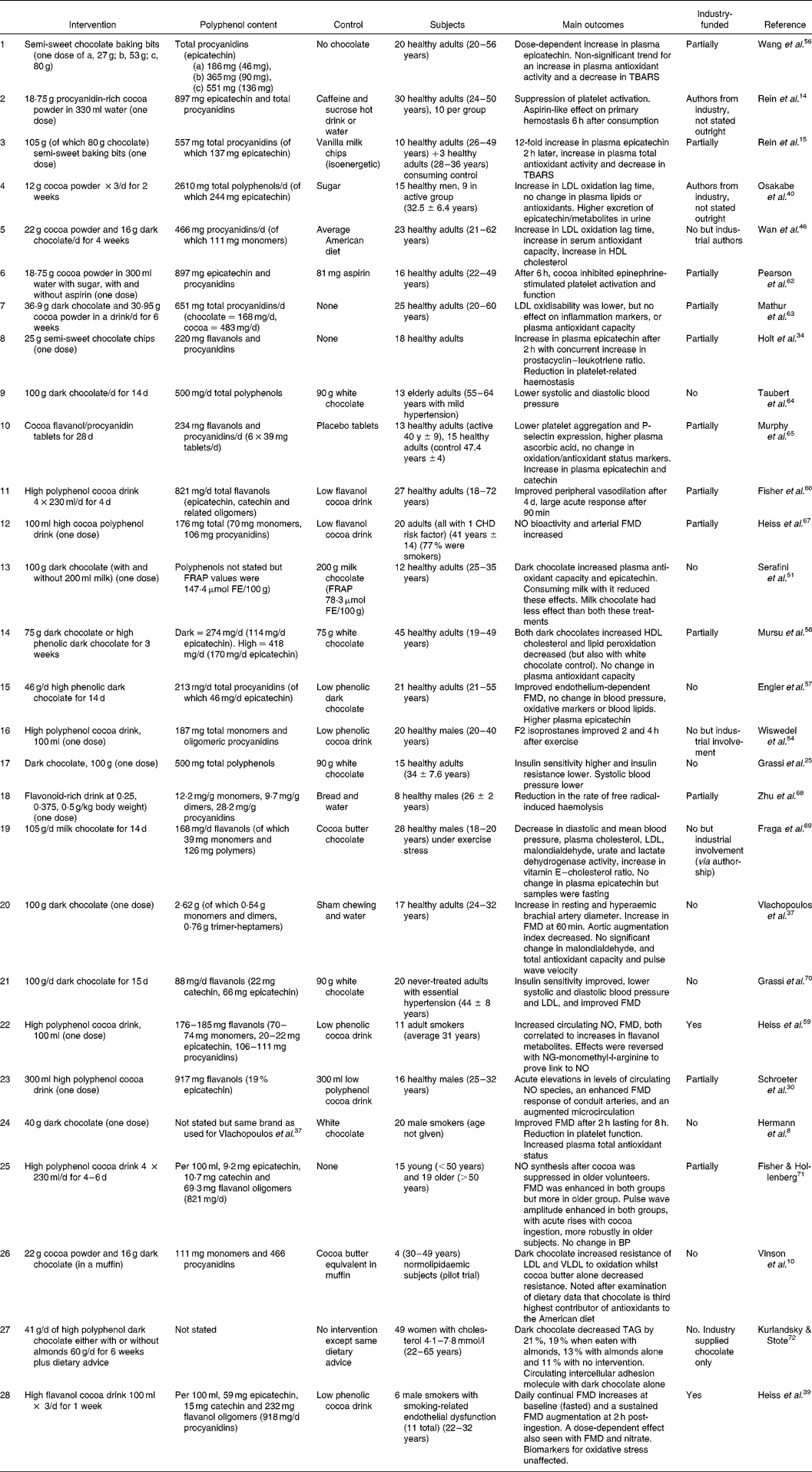
FE, Ferric equivalents; FMD, flow-mediated dilation; FRAP, ferric-reducing ability of plasma; TRAP, ferric reducing ability of plasma (or antioxidant potential ) TBARS, thiobarbituric acid reactive substances.
Bioavailability
Richelle et al. Reference Richelle, Tavazzi, Enslen and Offord13 first demonstrated the appearance of epicatechin in blood after consumption of black (dark) chocolate; and 3 years later a study demonstrated the presence of a dimer in the plasma within 30 min post-consumption of flavanol-rich cocoaReference Holt, Lazarus, Sullards, Zhu, Schramm, Hammerstone, Fraga, Schmitz and Keen26. Cocoa polyphenols are therefore absorbed but factors such as low C max in the plasma, a short half-life and rapid excretion all add to a relatively low bioavailabilityReference Manach, Williamson, Morand, Scalbert and Remesy27. In general, the smaller the polyphenol, the higher the concentration in the blood, and the higher chance that it will reach its target organ in the body. Intestinal perfusion studies have shown that B2 and B5 can cross the enterocytes but to a very limited extentReference Spencer, Schroeter, Shenoy, Srai, Debnam and Rice-Evans28. Larger units than the dimer are unlikely to be able to cross the gut barrier, although they could have an action within the gut lumen or be cleaved by colonic bacteria before absorption of the resulting metabolites. Monomers such as epicatechin are metabolised to O-methylated forms or conjugated as glucuronides and sulphates, with 3′-O-methylepicatechin being investigated for its potential protective effectsReference Spencer, Schroeter, Rechner and Rice-Evans29. This area of research is still relatively new and the breakdown products of procyanidins have not been fully identified nor characterised for possible effects. For the moment, taking only current knowledge into account, it would be logical that for chocolate or cocoa to confer health benefits, it should have a high percentage of the smaller (monomeric) polyphenols. In accordance with this, a recent study concluded that the epicatechin content was likely to be the main explanation for cocoa's association with healthReference Schroeter, Heiss and Balzer30.
Bioavailability can also be affected by the matrix in which the cocoa polyphenols are delivered. In the previous human trials such matrices have been semi-sweet chocolate baking bits, cocoa powder, dark chocolate, tablets, drinks, milk chocolate and even in a muffin. It is possible that these different matrices affect the release of the polyphenols from the food, making them more or less available for absorption.
Of the twenty-eight trials listed in Table 1, fifteen trials used a one-off dose of polyphenols and thirteen trials used a chronic intervention style, with periods of supplementation lasting from 4 d to 6 weeks. There are benefits and shortcomings of both types of trials, depending on what exactly is being investigated. However as the effect of polyphenols is often short lived, once an effect has been seen in the short term, it would be logical to see if this effect can be maintained over a longer period. There may even be adaptation to a regular supply of a certain polyphenol, resulting in a more efficient uptake and therefore a greater possibility of an effect.
One aspect of intervention studies is that inter-subject variability of bioavailability may obscure the true meaning of results. Most (non-bioavailability) studies assume a postprandial T max of 120 min. However, studies can show such variation in C max at this timeReference Rein, Lotito, Holt, Keen, Schmitz and Fraga15, that either the C max occurs earlier or later and so is missed, or there truly is large interpersonal variation in absorption of a compound such as epicatechin. It may also happen that a person with a high plasma value at 2 h may not have a correspondingly high response in whichever health-related biomarker is measured, obscuring any potential correlation between apparent bioavailability and bioefficacy. The consequence of this is that a single measurement of plasma levels at 2 h cannot be considered a measurement of bioavailability, but rather only a check for compliance, limiting the usefulness of this measure.
Another fairly new area for cocoa and bioavailability is that of the chiral nature of polyphenols and the effect of chirality on bioavailability. For instance, the (+) form of catechin tends to dominate in cocoa beans, and the ( − ) form in chocolateReference Gotti, Furlanetto, Pinzauti and Cavrini31. One paper found that chocolate tended to contain predominantly ( − )-epicatechin and ( − )-catechin, with only small amounts of (+)-catechin and negligible (+)-epicatechinReference Donovan, Crespy, Oliveira, Cooper, Gibson and Williamson32. The same paper indicated that the (+) form of catechin was almost 10 times more absorbed than the ( − ) form using a rat perfusion model, which may explain why catechin from cocoa is not as well absorbed as from other foodsReference Donovan, Bell, Kasim-Karakas, German, Walzem, Hansen and Waterhouse33, Reference Holt, Schramm, Keen, Lazarus and Schmitz34.
Mechanism of action
Multiple approaches have been used to investigate the mechanism of action of cocoa polyphenols including clinical, pre-clinical and in vitro studies. Cocoa polyphenols have been investigated predominantly for their effect on the vascular system, with NO concentrations being a central target (Fig. 1). One of these effects is on endothelial function, which is an extremely promising biomarker to calculate heart attack riskReference Schindler, Hornig, Buser, Olschewski, Magosaki, Pfisterer, Nitzsche, Solzbach and Just35, Reference Vita36. Several clinical studies have shown improved endothelial function after cocoa consumptionReference Vlachopoulos, Aznaouridis, Alexopoulos, Economou, Andreadou and Stefanadis37–Reference Heiss, Finis, Kleinbongard, Hoffmann, Rassaf, Kelm and Sies39, but it is not known if these improvements are due to a subtle combination of mild effects rather than a single targeted effect. Other effects related to reduced CVD risk include decreased susceptibility of LDL to oxidationReference Osakabe, Baba, Yasuda, Iwamoto, Kamiyama, Takizawa, Itakura and Kondo40, and inhibition of platelet activation and aggregationReference Rein, Paglieroni, Wun, Pearson, Schmitz, Gosselin and Keen14. As shown in Table 1, although many different biomarkers have been measured, the results consistently show changes in biomarkers related to oxidative status and/or vascular function (thiobarbituric acid reactive substances (TBARS), LDL oxidation, F2-isoprostanes, platelet aggregation and FMD).
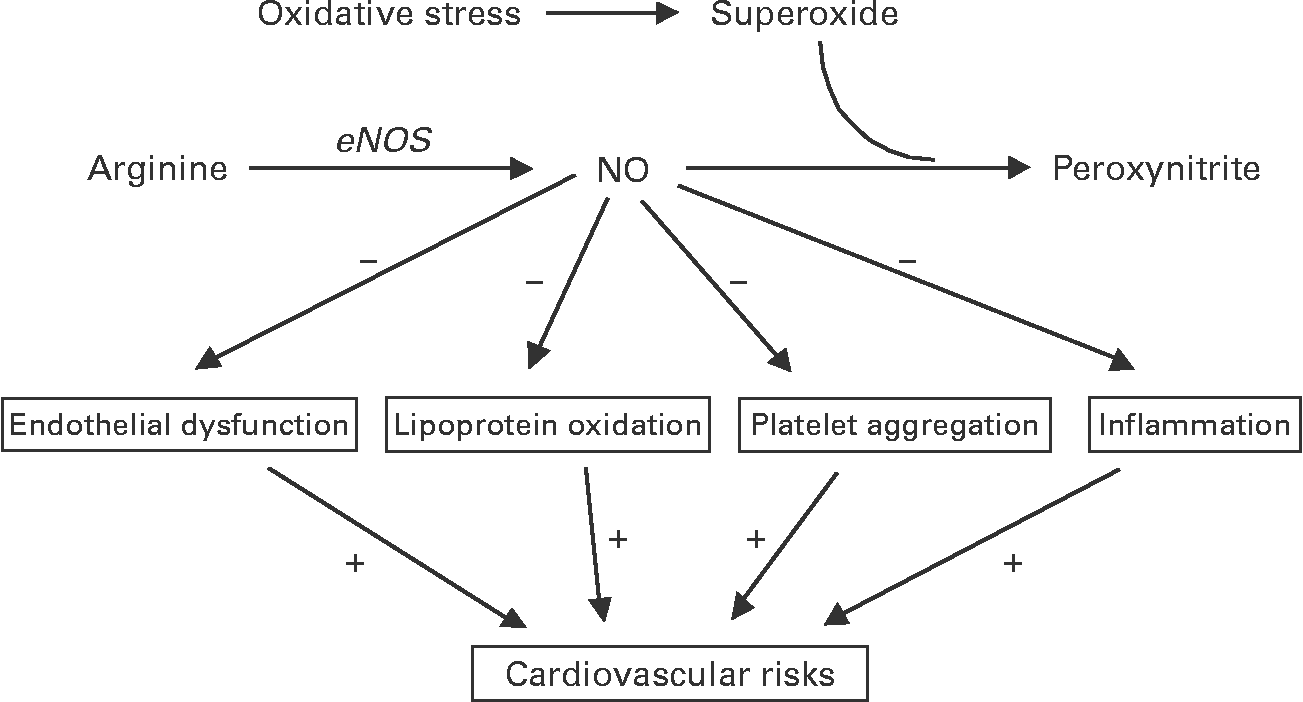
Fig. 1 Diagram to show the how cocoa polyphenols might affect the vascular system, with nitric oxide (NO) as the target. eNOS, endothelial nitric oxide synthase.
How much chocolate is enough?
Chocolate is predominantly a food for pleasure, and many people incorporate it into part of a healthy, varied and balanced diet. However, there is controversy over whether it should be recommended for its health benefits. Furthermore, it is difficult establish how much chocolate and what type to recommend for health benefits. High cocoa content dark chocolate tends to be richest in polyphenols, although each chocolate is different in polyphenol contentReference Cooper, Campos Giménez, Jiménez Alvarez, Nagy, Donovan and Williamson41. Polyphenols are known to be destroyed by harsh processing of the cocoa bean and so percentage cocoa content should be considered a guideline only to polyphenol content. There are no long-term intervention studies addressing the health benefits of chocolate consumption. Most previous short-term studies have given a single ‘dose’ of chocolate, which is probably more than one person would normally consume, and demonstrated an effect: decreased plasma leukotriene–prostacyclin ratios in human plasma and aortic endothelial cellsReference Schramm, Wang, Holt, Ensunsa, Gonsalves, Lazarus, Schmitz, German and Keen42, activation of endothelial nitric oxide synthase and enhanced endothelium relaxation in vitro Reference Karim, McCormick and Kappagoda43, inhibition of human cytokine transcription and secretionReference Mao, Van de Water, Keen, Schmitz and Gershwin44, and inhibition of mammalian 15-lipoxygenase activityReference Schewe, Sadik, Klotz, Yoshimoto, Kuhn and Sies45. These multiple effects lend credence to the opinion that a ‘simple’ antioxidant mechanism in vivo is not likely, but more probably via inhibition of inflammatory pathways, leading to reduced risk of a chronic disease state. By how much the change in biomarkers listed earlier influence the actual risk of CVD is difficult to quantify at present. Endothelial function is an excellent indicator of CVD riskReference Vita36, but effects of cocoa polyphenols are generally short lived, i.e. for the duration of the presence of catechins in plasma. Some studies have shown an effect on general antioxidant markers in vivo, and antioxidant assays reflect specific biochemical parameters in the plasma. For example, the Trolox equivalent antioxidant capacity assay is dominated by the presence of albumin and uric acid, and the polyphenol itself at low micromolar concentrations would not have a significant effect by itself on the Trolox equivalent antioxidant capacity value. However, on a regular daily basis, the overall effects may potentially accumulate. Very recently it has been shown that the effects of cocoa polyphenols on FMD (but not markers of oxidative stress) can be cumulative if taken in high doses on a daily basis for 1 week and with a return to baseline after a week washoutReference Heiss, Finis, Kleinbongard, Hoffmann, Rassaf, Kelm and Sies39. Seven of the studies used approximately 100 g of chocolate and two used 920 ml of a cocoa drink in 1 d, which would be difficult to justify every day long term. There have been very few dose–response studies and so it is difficult to judge exactly how much chocolate is needed for an ‘antioxidant’ effect. However, for other effects, very little might be needed. In smokers, 40 g of dark chocolate improved FMD and platelet function (no polyphenol content was stated)Reference Hermann, Spieker and Ruschitzka38. The most recent study on smokers with endothelial dysfunction found that the dose needed for a half-maximal FMD at 2 h post-consumption was 616 mg total flavanolsReference Heiss, Finis, Kleinbongard, Hoffmann, Rassaf, Kelm and Sies39. Another study found an increase in the prostacyclin–leukotriene ratio and a reduction in platelet-related haemostasis in healthy people with just 25 g of semi-sweet chocolate bits containing 220 mg flavanols and procyanidinsReference Holt, Schramm, Keen, Lazarus and Schmitz34. The polyphenol content is of more importance and it is essential that, in future, all published trials give a full characterisation of the chocolate or cocoa used and the calculated dose. This characterisation should include a breakdown of the types of polyphenols, especially monomer content. For example, one study indicated that their dose was 500 mg total polyphenols in 100 g dark chocolate/dReference Taubert, Berkels, Roesen and Klaus64 whereas another gave 22 g cocoa powder and 16 g dark chocolate/d containing 466 mg procyanidinsReference Wan, Vinson, Etherton, Proch, Lazarus and Kris-Etherton46. Although both these sets of information are useful to some extent, they could be improved by the use of a more comparable parameter such as epicatechin. It is also important to specify the methodology behind the measurement, i.e. HPLC or colorimetry.
Fat and sugar are major components of chocolate, and provide significant energy that needs to be taken into account when assessing possible risks and benefits of recommending chocolate consumption for health purposes. Chocolate contains fatty acids such as stearic, oleic and palmitic acids. These particular fats appear to have a neutral effect on blood lipid levelsReference Kris-Etherton, Derr, Mitchell, Mustad, Russell, McDonnell, Salabsky and Pearson47, Reference Kris-Etherton, Derr, Mustad, Seligson and Pearson48, i.e. they do not raise blood cholesterol levels. Chocolate, especially of the milk variety, contains high amounts of sugar which obviously increases the energy value and has possible implications for dental health and diabetes if eaten in large amounts, although carbohydrates might play a role in improving uptake of polyphenolsReference Schramm, Karim, Schrader, Holt, Kirkpatrick, Polagruto, Ensunsa, Schmitz and Keen49. Cocoa itself is much easier to recommend on a health basis as it is not high in sugar and fat. Populations that take cocoa compared to genetically similar groups with less consumption, i.e. island- v. mainland-dwelling Kuna Indians of Panama, have been shown to excrete more NO metabolites, which is an indicator of higher NO production, which is in turn associated with lower incidence of CVDReference Schroeter, Heiss and Balzer30. A more recent evaluation of the causes of mortality between these two populations found a substantially lower number of deaths between 2000 and 2004 from NO-dependent diseases such as CVD, cancer and diabetes mellitus in the island v. mainland Kuna IndiansReference Bayard, Chamorro, Motta and Hollenberg50.
Dark or milk?
This is a question that is often asked when considering health effects. Many countries around the world predominantly consume cocoa as part of milk chocolate rather than dark. Also a cocoa drink may be made with either water or milk. So we can question whether these people are getting similar benefits as those countries where dark chocolate or water-based cocoa drinks are mainly consumed.
The polyphenols in chocolate come from the cocoa liquor. Hence, as milk chocolate generally contains less cocoa liquor than dark chocolate, it will contain less polyphenols. White chocolate contains no cocoa liquor and hence no polyphenols at all. However, this is complicated by the fact that polyphenols can be destroyed during the processing of the raw cocoa depending on the manufacturing methods used. So a chocolate may contain 70 % cocoa solids but due to processing only contain the same content of polyphenols as a normal milk chocolate. How would a consumer know that the dark chocolate they are buying is a good source of polyphenols?
Of the twenty-eight clinical trials, only two used milk chocolate. One study published in Nature showed that 100 g plain dark chocolate resulted in an increase in total antioxidant capacity but was markedly reduced when consumed with 200 ml whole milk, or taken as milk chocolate (200 g)Reference Serafini, Bugianesi, Maiani, Valtuena, De Santis and Crozier51. It was also shown that absorption of epicatechin from chocolate was significantly less when consumed with milk or as milk chocolate. The hypothesis is that milk proteins bind to cocoa polyphenols, which in turn prevents their absorption in the gastrointestinal tract. However, this study generated much controversy in the literature. Studies after this have not found this reduction in epicatechin bioavailability when cocoa was consumed with milkReference Schroeter, Holt, Orozco, Schmitz and Keen52, but also have not been able to definitively explain why the original paper found these results. Experimental differences, such as giving cocoa powder in a drink comparing either water or milk as a matrix, rather than as a solid chocolate may be one possible reason. The fat differences between milk chocolate and a cocoa and milk drink are considerable and may play a roleReference Serafini and Crozier53. Matrix effects are becoming increasingly important for food as new EU legislation Directive 2000/13/EC that came into effect in January 2007 may make it essential that any food labelling a high content of a beneficial compound must be able to show evidence that it is bioavailable from that food product, and is also effective in its implied benefit.
As many of the human studies used liquid-based cocoa for their interventions and found positive effects, it indicates that cocoa polyphenols taken as a liquid can be bioavailable, though no direct comparison with solid cocoa or chocolate has been made to date. As most studies have investigated dark chocolate to avoid the possibility that milk might interfere, it is hard to infer that milk chocolate will be just as effective as dark, even when strictly controlling for overall polyphenol intake. One study did use milk chocolate and found a positive effect on blood pressure, plasma cholesterol and markers of oxidative stress on young exercising malesReference Wiswedel, Hirsch, Kropf, Gruening, Pfister, Schewe and Sies54. One other study has shown bioavailability from a milk cocoa beverage common for children in SpainReference Roura, Andés-Lacueva, Jauregui, Badia, Estruch, Izquierdo-Pulido and Lamuela-Raventos55. However, we feel that this issue has not been resolved, as there has been no definitive study confirming the bioavailability with solid milk chocolate. It is important to resolve this issue as milk chocolate is much more popular in many countries.
Designing smart clinical intervention trials
Of the twenty-eight human intervention trials, twenty-one were with apparently healthy adults (one including exercise as a stress parameter and one comparing older v. younger adults). The other seven involved elderly hypertensives, healthy smokers, smokers with endothelial dysfunction, subjects with one CHD risk factor, women with high cholesterol and essential hypertensives. Although most of the twenty-one trials had at least one positive measurable change in a health biomarker, one could question the usefulness of healthy and well-nourished subjects for testing the efficacy of antioxidant supplementation. The age ranges used within the healthy adult trials were also wide, often over 30 years. As polyphenolic antioxidants may possibly be more useful in the ageing population, since ageing can be considered to partly involve an ‘oxidative stress’, it is probable that effects in the older segment of subjects may be missed by grouping all the data together.
When antioxidants such as polyphenols are given in an optimal dose to a person with a sufficient dietary status, supplementation is unlikely to make much of a measurable difference to their health status (Fig. 2), for example, on plasma antioxidant status or blood pressureReference Wang, Schramm, Holt, Ensunsa, Fraga, Schmitz and Keen56–Reference Mursu, Voutilainen, Nurmi, Rissanen, Virtanen, Kaikkonen, Nyyssonen and Salonen58. However, if the person is subject to a stress event, such as inflammation, smoke inhalation or sunburn, or suffers from chronic antioxidant deficiencies, then antioxidants may be able to counteract the effect of the stress to return this individual closer towards a healthy status. Hence using people who are at risk of a disease (e.g. through elevated blood pressure, ageing or poor diet) to look for an effect of polyphenolic antioxidants is more likely to provide a measurable result and therefore reveal the true potential of the compound being studied.
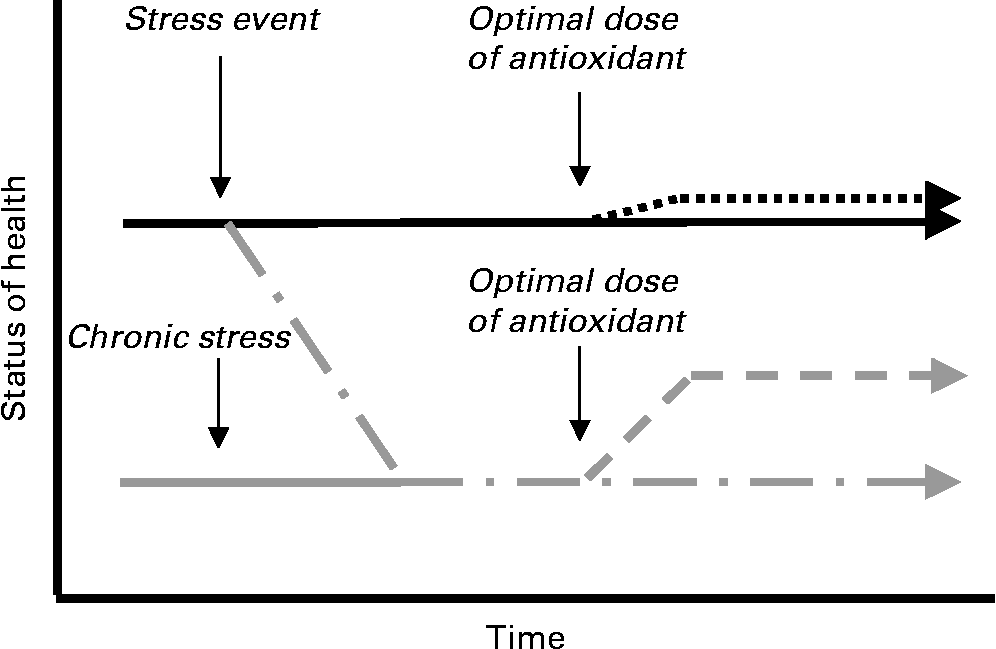
Fig. 2 A simplified representation of the hypothetical action of antioxidants on the health status of an apparently healthy person or a person under chronic stress. ![]() , The status of health, e.g. in relation to inflammation;
, The status of health, e.g. in relation to inflammation; ![]() , the status of health when the person is under chronic stress;
, the status of health when the person is under chronic stress; ![]() , only a small improvement in health status with an optimal dose of an antioxidant under conditions of minimal stress;
, only a small improvement in health status with an optimal dose of an antioxidant under conditions of minimal stress; ![]() , the worsening health status after a stress event such as UV exposure, smoke inhalation, inflammation or oxidative stress;
, the worsening health status after a stress event such as UV exposure, smoke inhalation, inflammation or oxidative stress; ![]() , the improvement in health status when an optimal dose of an antioxidant is given whilst in a state of stress.
, the improvement in health status when an optimal dose of an antioxidant is given whilst in a state of stress.
Obviously, the use of a control group always strengthens human intervention trials. However, if biological effects are to be attributed to cocoa polyphenols rather than another component of the cocoa, then the perfect control would be a dark chocolate that contains everything other than polyphenols. Most trials have been unable to do this, as it is not that simple to make or find. Controls have ranged from white chocolate to bread and water. This may show the effect is due to cocoa but not necessarily to cocoa polyphenols. Some trials have been able to source a control which purports to be low in polyphenols such as trials with cocoa drinksReference Schroeter, Heiss and Balzer30, Reference Heiss, Finis, Kleinbongard, Hoffmann, Rassaf, Kelm and Sies39, Reference Wiswedel, Hirsch, Kropf, Gruening, Pfister, Schewe and Sies54, Reference Heiss, Kleinbongard, Dejam, Perre, Schroeter, Sies and Kelm59 and one which uses a low polyphenol dark chocolateReference Engler, Engler and Chen57. Perhaps the best trial for this to date used epicatechin as a positive control and found the effects from both cocoa and epicatechin to be of a similar magnitude, hence allowing the effects to be more strongly attributed to epicatechinReference Schroeter, Heiss and Balzer30. However, the majority have not been able to control fully for this aspect, and for these trials, the question does remain as to whether the effects seen are from cocoa polyphenols, from some other component such as caffeine or magnesium, or indeed from a synergistic effect of several components from cocoa.
Potential for research bias?
As with any research there is the potential for bias. The field of cocoa polyphenols has been dominated by industrially funded research for the last 10 years. Of all the twenty-eight listed publications, fifteen had partial or full industrial funding and a further four had industrial involvement of some type (supply of chocolate, etc.) not including those that were helped by the American Cocoa Research Institute which is a non-profit organisation dedicated to supporting cocoa research and consisting of many industrial members. There are several reasons why this picture might appear skewed and these are discussed in a recent commentary on this subjectReference Katan60. In short, an industry-funded study is likely to be conducted with a foodstuff already considered to be a likely possibility for success as prior in vitro research and product development would have narrowed down the potential candidates. If there has been a null or negative result in a study, then it is likely the industry would possess the resources to try again with a modified study. In addition, journals tend to be less interested in publishing null results and so this can distort the overall scientific area. However, investigators already avoid such studies and rarely apply for (and more rarely receive) grants that are designed to observe little to no effect, even from public agencies. On the other hand, industry support is certainly less likely to be requested for studies into potentially negative effects unless there is a health and safety issue. However, the other important issue is that if industry had not been involved, would the area of interest exist and would valuable faculty research capacity be so directed? This may be especially true of cocoa polyphenol research which has been obviously dominated by industry-funded studies since its inception. Overall, the main point to consider is that all the papers described here were published in peer-reviewed journals and therefore must be considered trustworthy and reliable; otherwise there is a need to investigate the integrity of the review process. One way for industry (and academia) to improve transparency of ongoing human trials would be to formally register with one of the public domain agencies, such as with the National Institutes of Health (www.clinicaltrials.gov), at the beginning of any study. The advantage is that a null or negative study would still be public knowledge, and this could help bring more balance to this area of research.
Future research directions
For the future, we recommend that since cocoa is accepted as a dietary source of polyphenols, future studies should focus on specific mechanisms of action, i.e. inflammatory pathways, and not direct antioxidant effects, with more diversification on non-vascular end-points. Human intervention trials should be conducted that use a relevant amount (e.g. about 40 g/d, 10 % of a 8369 kJ/d (2000 kcal/d) diet) of chocolate, an amount most people could readily incorporate into their diet. In addition, the composition of the cocoa or chocolate must be carefully defined with regard to the proportions of polyphenols in the monomeric, oligomeric and polymeric forms, as well as the concentrations of the fats, sugars and other components such as proteins from milk solids. Further studies on milk chocolate to settle the bioavailability debate are most definitely required.
Separating the effects of specific compounds could also prove fruitful as there is less information on the larger procyanidins and their health effects. There is also the question of attributing beneficial effects to cocoa polyphenols v. cocoa as a whole. Other compounds in cocoa are known to be bioactive such as caffeine and theobromineReference Usmani, Belsivi, Oatel, Crispino, Birrell, Korbonits, Korbonits and Barnes61.
The bona fide health effect of cocoa polyphenols will not be answered short of a large-scale epidemiological study or long-term interventions. The only epidemiological papers to date gave intriguing resultsReference Buijsse, Feskens, Kok and Kromhout21, Reference Mink, Scrafford, Barraj, Harnack, Hong, Nettleton and Jacobs22 but without more corroboration the question will remain unanswered. Whilst long-term interventions will be difficult or impossible to blind, it should proceed with the best controls possible, because without them, conclusion of the benefits of chocolate on changing disease risk will remain tenuous. To obtain results in a reasonable time frame and with the most likelihood of a significant result, we suggest targeting future trials to populations that are under antioxidant stress or deficiency due to a poor diet, chronic disease, ageing or have an elevated risk of CVD for other reasons. Only with such results will it be possible to assess definitively whether or not cocoa and chocolate, which was originally only a decadent indulgence, can affect public health.
We would like to suggest this checklist for future planning of cocoa and chocolate trials, although it is not exhaustive and designed only to help in future studies.
1. Where possible, conduct randomised, controlled, cross-over, multi-dose trials.
2. Use well-defined cocoa or chocolate (if possible, for industry to allow similar cocoa/chocolate to be available for independent researchers for future studies/repeating work).
3. Ensure bioavailability of the active component from its matrix.
4. Use an appropriate control of no-polyphenol chocolate.
5. Recruit volunteers with at least one non-optimal biomarker or disease risk factor.
6. Use a dose of cocoa or chocolate that can readily be incorporated into the daily diet, giving appropriate dietary advice to volunteers on balancing energy.
7. Measure composition including the polyphenol profile of the cocoa or chocolate before and after the trial (check for stability on storage or batch variations).
8. Ensure the final publication contains the analytical results along with the appropriate description of analytical methodology.
9. Carefully assess the biological relevance of the chosen biomarker, with special attention to antioxidant biomarkers.
10. Strive for transparency by registering human trials before they start with a recognised database, e.g. www.clinicaltrials.gov.
11. Attempt to publish null or negative results to enable balancing of the literature and preventing needless duplication of work. Challenge journals if papers are rejected on this basis.
Acknowledgements
The idea for this article was generated by Gary Williamson. All authors contributed equally for intellectual input and writing of the manuscript. G. Williamson and K. Cooper are employed by the Nestlé Research Center. J. Donovan receives a research grant from the Nestlé Research Center. A. Waterhouse declares no conflict of interest. There was no specific funding for this work.





