Introduction
Weed adaptation is an inevitable and damaging consequence of modern agricultural practices that involve high cultivation intensity of the same annual crops and overreliance on herbicides. The evolutionary adaptation of weeds has indirectly but largely been influenced by the process of crop domestication that has selected for weeds acclimatized to specific or diverse new ecosystems (Baucom Reference Baucom2019). The ecological fitness advantages of weeds, mainly driven by their genetic architecture, have enabled multiple adaptation strategies such as prolonged seed dormancy, rapid growth rates, ease of dispersal, stress tolerance, and herbicide resistance—collectively known as agricultural weed syndrome (Guo et al. Reference Guo, Qiu, Li, Lu, Olsen and Fan2018; Vigueira et al. Reference Vigueira, Olsen and Caicedo2013). Modern farming practices that heavily and persistently rely on herbicides under high cropping intensity have exerted selective forces on arable weeds, resulting in the aggravation of this syndrome and leading to widespread selection and evolution of herbicide-resistant individuals that can thrive in the managed environment (Beckie Reference Beckie2006; Murphy and Lemerle Reference Murphy and Lemerle2006; Owen et al. Reference Owen, Goggin and Powles2015a; Sun et al. Reference Sun, Ashworth, Flower, Vila-Aiub, Rocha and Beckie2021).
Apart from widespread herbicide resistance, multiple adaptive strategies have been reported to evolve in weed populations that are linked to various farming operations across agricultural systems. For example, hand weeding led to crop mimicry, reaping led to dwarf stature (reviewed in Barrett Reference Barrett1983), intensive cropping led to delayed germination (Kleemann and Gill Reference Kleemann and Gill2006), repeated mowing led to prostrate growth habit (Warwick and Briggs Reference Warwick and Briggs1979), and weed seed destruction may lead to early flowering and reduced podding height on the stem to evade capture at crop harvest (Ashworth et al. Reference Ashworth, Walsh, Flower, Vila-Aiub and Powles2016). Moreover, weed adaptation in response to repetitive herbicide exposure has emerged as an unparalleled global threat to crop production. In contrast, the ruderal (non-crop disturbed) or natural areas surrounding intensively cultivated fields exhibit limited selection and evolution for herbicide resistance and life cycle or trait adaptations, indicating the prominent role of recurrent agronomic practices in directing the evolutionary patterns in weed adaptive traits (Owen et al. Reference Owen, Goggin and Powles2015a).
Since the 1990s, a number of studies have reported differential fitness costs in agricultural weeds that are resistant to one or multiple herbicides such as the inhibitors of acetyl CoA carboxylase (ACCase), acetolactate synthase (ALS), and 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) (Maity et al. Reference Maity, Singh, Jessup and Bagavathiannan2021a, Reference Maity, Singh, Martins, Ferreira and Bagavathiannan2021b; Martinez-Ghersa et al. Reference Martinez-Ghersa, Ghersa, Benech-Arnold, Mac Donough and Sanchez2000; Mortimer Reference Mortimer1997). Various plant adaptive traits such as plant height, biomass, tiller number, leaf area index, regrowth rate, photosynthetic rate, and seed production potential have shown divergent responses in herbicide-resistant populations as opposed to their less intensively managed or non-cropland counterparts (Darmency et al. Reference Darmency, Colbach and Corre2017; Gundel et al. Reference Gundel, Martinez-Ghersa and Ghersa2008; Jasieniuk et al. Reference Jasieniuk, Brulé-Babel and Morrison1996; Watkinson and White Reference Watkinson and White1985). For example, Ghanizadeh and Harrington (Reference Ghanizadeh and Harrington2019) reported reduced plant height and plant biomass in triazine-resistant common lambsquarters (Chenopodium album L.) phenotypes as compared with a susceptible cohort. Henckes et al. (Reference Henckes, Cechin, Schmitz, Piasecki, Vargas and Agostinetto2019) reported that Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot] biotypes resistant to glyphosate, iodosulfuron, and pyroxsulam produced taller plants with increased shoot dry matter and absolute growth rate; these plants, however, had fewer tillers and reduced leaf area ratio when compared with the susceptible biotypes.
Seed size and dormancy are two important plant adaptive traits that have affected the colonization and persistence of weed species and plant evolution in general, especially under human-influenced plant domestication regimes (Rees Reference Rees1996). Unsurprisingly, both of these seed traits have coadapted to different weed management practices along with other adaptation strategies such as widespread evolution of herbicide resistance (Kleemann and Gill Reference Kleemann and Gill2006; Maity et al. Reference Maity, Singh, Jessup and Bagavathiannan2021a; Owen et al. Reference Owen, Goggin and Powles2015a). Some studies failed to establish an association between altered weed seed traits and herbicide resistance (Harris et al. Reference Harris, Gosset and Toler1995; Holt and Thill Reference Holt and Thill1994; Park et al. Reference Park, Mallory-Smith, Ball and Mueller-Warrant2004; Wiederholt and Stoltenberg Reference Wiederholt and Stoltenberg1996). However, a number of studies have found that weed populations resistant to various herbicides exhibit alterations in their seed morphophysiological traits such as size, speed of germination, seed dormancy, and seedling vigor (Darmency et al. Reference Darmency, Colbach and Corre2017; Ghersa et al. Reference Ghersa, Martınez-Ghersa, Brewer and Roush1994; Maity et al. Reference Maity, Singh, Jessup and Bagavathiannan2021a; Nandula et al. Reference Nandula, Poston and Reddy2009; Owen et al. Reference Owen, Goggin and Powles2015a, Reference Owen, Martinez and Powles2015b; Yanniccari et al. Reference Yanniccari, Vila-Aiub, Istilart, Acciaresi and Castro2016). These alterations may enhance the fitness of herbicide-resistant individuals. For example, altered seed dormancy can change the expected weed emergence patterns under field conditions, with cohorts evading control by soil-residual preemergence herbicides or being less controlled by postemergence herbicide applications.
Australian cropping systems, particularly in Western Australia (WA), have high adoption of no or minimum tillage (Llewellyn et al. Reference Llewellyn, D’Emden and Kuehne2012). Although conservation tillage has provided enormous benefits in improving soil properties, elimination of tillage as a weed control measure has resulted in overreliance on herbicides, leading to changes in weed dynamics and adaptation strategies (Chauhan et al. Reference Chauhan, Gill and Preston2006; Owen et al. Reference Owen, Goggin and Powles2015a). Rigid ryegrass (Lolium rigidum Gaudin) infests most southern Australian cropping systems; brome grass (Bromus spp.), hare and smooth barley [Hordeum leporinum Link; syn. Hordeum murinum L. ssp. leporinum (Link) Arcang.; and Hordeum glaucum Steud.; syn. Hordeum murinum L. ssp. glaucum (Steud.) Tzvelev, respectively], and wild oat (Avena fatua L.) are also abundant and cause significant crop yield loss (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clarke2016). For example, Poole and Gill (Reference Poole and Gill1987) reported a 30% yield loss in wheat (Triticum aestivum L.) from Bromus spp. at densities of 100 plants m−2 in southern Australia; Dastheib et al. (Reference Dastheib, Rolston and Archie2003) reported a 25% to 30% yield loss in wheat and barley (Hordeum vulgare L.) in New Zealand.
In general, seeds of Bromus spp. can remain substantially dormant for more than 2 mo (Kleeman and Gill Reference Kleemann and Gill2006), seeds of H. leporinum for 3 mo (Bolger et al. Reference Bolger, Chapman and Le Coultre1999), and seeds of A. fatua for 3 to 4 mo (Paterson Reference Paterson1976) when stored at ambient room temperature after maturity. A number of studies in WA and other regions have confirmed the presence of field populations of these species resistant to one or multiple herbicides, and an association of seed traits, especially dormancy, with herbicide resistance (Owen and Powles Reference Owen and Powles2016; Owen et al. Reference Owen, Michael, Renton, Steadman and Powles2011, Reference Owen, Martinez and Powles2015b). We hypothesize that intensive cropping practices have divergently influenced field populations of these species for seed trait expression and response to commonly used herbicides as compared with adjacent ruderal populations. In-crop weed populations with larger seed size and enhanced seed dormancy may have a fitness advantage (e.g., greater emergence, growth, fecundity) relative to their nonmanaged ruderal counterparts, which could exacerbate management efficacy. Therefore, the aim of this study was to investigate whether field and adjacent ruderal populations of B. diandrus, H. leporinum, and A. fatua differ in seed size and dormancy in relation to herbicide susceptibility.
Materials and Methods
Seed Collection
Weed populations were collected from different farms (two populations per farm: cropland and ruderal habitat) randomly selected across a variety of farming systems (continuous, diverse, and pasture) in different agroclimatic cropping zones within the WA grainbelt (Table 1). The continuous farming system comprised wheat and barley crops, whereas the diverse system comprised crop sequences of cereals (wheat, barley, and oat [Avena sativa L.]), canola (Brassica napus L.), and lupin (Lupinus angustifolius L.). Multi-year pastures (subterranean clover [Trifolium subterraneum L.]) in rotation with cereal, oilseed, and pulse crops listed above constituted the pasture farming system. Glyphosate and paraquat were applied as burndown herbicides before seeding. Soil-residual herbicides such as triallate, trifluralin, and prosulfocarb were frequently applied preemergence before seeding or at the time of seeding. Both ACCase- and ALS-inhibiting herbicides were commonly applied postemergence in the annual crops and pasture establishment year. For H. leporinum, 12 populations were from continuous (6 in-crop and 6 ruderal) and 36 from pasture (18 in-crop and 18 ruderal) farming systems. For B. diandrus, 30 populations were from diverse (15 in-crop and 15 ruderal) and 12 from pasture (6 in-crop and 6 ruderal) systems. For A. fatua, 6 populations each from continuous and pasture (3 in-crop and 3 ruderal) and 12 populations from diverse (6 in-crop and 6 ruderal) systems were collected. Samples from mature seed heads for each population were collected from 40 to 50 plants randomly selected at each location (50 to 200 ha field area) during June 2018 before crop harvest. Collected seeds were threshed and cleaned from the chaff by aspiration, sieving, and forced-air separation.
Table 1. Farming systems and habitats investigated in this study.

Seed Size and Dormancy Evaluation
Immediately following collection, harvested seeds were placed in permeable paper bags and stored at ambient conditions (25 C in the dark). For each population, three replicate samples each comprising 100 seeds were weighed 12 mo after collection to assess relative seed size (mass), expressed as 100-seed weight (mg). A periodic evaluation of monthly (every 4 wk) seed dormancy release for 9 consecutive months after collection was performed using a standard test to assess germination. For each population, three replicates of 50 seeds each were subsampled every month. The subsampled seeds were sterilized in a 1:16 ratio of 12.5% sodium hypochlorite solution and then placed in 750-ml rectangular plastic trays (50 seeds per tray) containing 1% (10 g L−1) agar dissolved in deionized water. Trays were immediately transferred to a plant growth cabinet (Conviron® A1000; Conviron Australasia, Grovedale, Victoria 3216, Australia) that was set at environmental conditions conducive for seed germination: 12-h day at 20 C with white fluorescent light (30 to 60 μmol m−2s−1)/12-h night (dark) cycle at 11 C. Seed germination was evaluated daily for 4 wk after subsampling, with the criterion for germination being visible radicle protrusion (>1 mm) from the seed (Figure 1). Germinated seed was removed from the tray after each evaluation. At the end of the germination test, the remaining nongerminated seeds were assessed for viability by making a transverse section to expose the endosperm and incubating in 1% (10 g L−1) solution of 2,3,5-triphenyltetrazolium chloride for 24 h at 30 C. Evidence of pink staining of the embryo scored a seed as being viable; seeds with unstained embryos were considered nonviable (Verma and Majee Reference Verma and Majee2013). Nonviable seeds were not included in the calculation of total seed count. Dormancy was calculated by the following formula (Equation 1):

Figure 1. Difference in seed germination of in-crop (a) and ruderal samples (b) collected from a single farming system of Hordeum leporinum (A), Bromus diandrus (B), and Avena fatua (C).
where t(x) is the total viable seed count and g(x) is the total germinated seed count at evaluation time x, that is, after 4 wk from the start of a test.
Herbicide-Resistance Screening
Herbicide-resistance screening for B. diandrus and H. leporinum was conducted between June and August during the normal growing season in the Southern Hemisphere. Seeds of each population were germinated in 1% (10 g L−1) agar–water, as described earlier before being transplanted into plastic trays (40 cm by 30 cm by 10 cm) containing potting mix (50% composted pine bark, 25% peat, and 25% river sand). Plants were maintained outside with adequate light and soil moisture at the University of Western Australia until the 2- to 3-leaf stage. Fifty plants per herbicide for each population were sprayed with the upper range of the recommended dose of the herbicides as listed in Table 2, using a custom-built dual-nozzle (TeeJet® XR11001 flat fan, Springfield, IL, USA) cabinet sprayer (for details of spray application, see Owen et al. Reference Owen, Martinez and Powles2015b). Known herbicide-susceptible (100% mortality) and herbicide-resistant (<10% mortality) populations of each species were included as negative and positive controls, respectively, and the experiment was repeated. Herbicide efficacy was assessed by determining seedling mortality at 21 d after herbicide treatment (dead or alive). Flamprop-methyl was applied at the 4-leaf stage and evaluated for mortality at 28 d after application. The proportion of survivors for each population for a particular herbicide was determined based on the number of plants that survived the herbicide application out of the total number of plants sprayed. The preemergence triallate treatment was applied to the soil surface, and 10 mm of untreated soil was immediately placed on the soil surface to prevent volatilization; mortality was assessed at 21 d after treatment. Although a single discriminating herbicide dose, based on previous dose–response experiments, is commonly used to classify weed populations collected in field surveys as herbicide resistant when both positive and negative controls are included (e.g., Owen et al. Reference Owen, Goggin and Powles2015a, Reference Owen, Martinez and Powles2015b), survivorship in this study denotes “developing herbicide resistance” (DHR) to conservatively indicate reduced sensitivity or potentially herbicide resistant.
Table 2. Herbicides and rates used for resistance screening of Hordeum leporinum, Bromus diandrus, and Avena fatua populations.

a Abbreviations: ACCase, acetyl Co-A carboxylase; ALS, acetolactate synthase; EPSPS, 5-enolpyruvylshikimate-3-phosphate synthase.
b A dash (—) indicates specific herbicide was not used for screening resistance in the species.
Data Analysis
Experiments were conducted under controlled growth chamber (germination test) or net house conditions (herbicide screening) in a factorial randomized complete block design, with main factors being farming system (continuous, diverse, or pasture), weed population habitat (in-crop vs. ruderal), and weed species. Each farm was considered a replicated experimental site. Before statistical tests were conducted, data were checked for normality using the Shapiro-Wilk W test. No transformations were required. ANOVA (see Table 3 for response variables) and Pearson nonparametric correlation (ordinal variable) were performed using JMP v. 15.0 (SAS Institute, Cary, NC). Interactions of interest are detailed in Tables 4 and 5 and Figures 2 and 3 (significance denoted at P < 0.05, P < 0.01, or P < 0.001). In the few cases where standard errors were zero for a population (Table 5), that is, all replicates were equal to the population mean, no specific statistical tests could be conducted; the arithmetic difference between means was considered consistent and reportable in the absence of dispersion of the sample means. Statistical graphs were created using SigmaPlot v. 14.0 (Systat Software, San Jose, CA).
Table 3. Significance of the experimental factors on various traits of the Hordeum leporinum, Bromus diandrus, and Avena fatua populations.

a Level of significance: *P < 0.05; **P < 0.01; ***P < 0.001.
b D50, time (months) required for 50% dormancy release.
Table 4. Mean time (months; SE in parentheses) required for 50% dormancy release (D50) of the Hordeum leporinum (HL), B. diandrus (BD), and Avena fatua (AF) seeds collected from three farming systems and two habitats across Western Australia. a

a Symbols: ∞, seed dormancy did not release during the study period; —, the study did not include any population of the weed species from the specific farming system or habitat (n = no. of populations). Capital letters A and B indicate significant difference (P < 0.05) between weed habitats (in-crop vs. ruderal) for each weed species within a farming system; lowercase letters indicate significant difference for in-crop populations among farming systems (a, b, and c) and for ruderal populations (x and y).
Table 5. Effect of farming system (continuous, diverse, or pasture) and weed population habitat (in-crop vs. ruderal) on survival (SE in parentheses) of Hordeum leporinum, Bromus diandrus, and Avena fatua in response to herbicide treatment. a
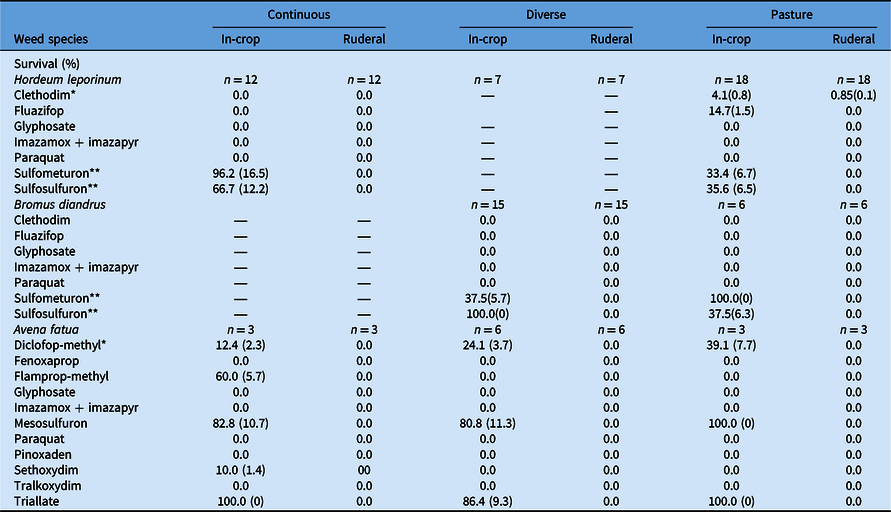
a A dash (—) indicates the study did not include any population of the weed species from the specific farming system or habitat. Significant difference between weed population habitat among farming systems at *P < 0.05 or **P < 0.01. Where SE is 0, arithmetic difference between habitats and/or farming systems was considered consistent and valid.

Figure 2. Effect of farming system (continuous, diverse, or pasture) and weed population habitat (in-crop vs. ruderal) on monthly seed dormancy (means ± SE) release after harvest of (A) Hordeum leporinum, (B) Bromus diandrus, and (C) Avena fatua. Asterisks denote significant difference between means of weed population habitat within a specific farming system: *P < 0.05; **P < 0.01; ***P < 0.001.

Figure 3. Effect of farming system (continuous, diverse, or pasture) and weed population habitat (in-crop vs. ruderal) on 100-seed weight of (A) Hordeum leporinum, (B) Bromus diandrus, and (C) Avena fatua. Different letters indicate significant differences between habitat among the farming systems at P < 0.05 (bars indicate SE).
Time (months) required for 50% dormancy release (D50) across the populations was estimated using a three-parameter sigmoidal model (Equation 2) on cumulative seed germination data collected over a 4-wk period. The model was constructed using SigmaPlot v. 14.0 and is described by Equation 2:
where y is cumulative germination (%) on the xth day, a is maximum germination (%), x 0 is days to 50% germination, and b = slope at x 0. Pearson correlation analysis was conducted in JMP PRO 14 to examine correlations between DHR to individual herbicides and seed size and dormancy. For this purpose, data on percent survival for each population in response to the different herbicides and percent dormancy (observed in the first-month evaluation) were used. Seed dormancy release rate can be influenced by numerous factors, including storage conditions. Percent seed dormancy represents a relatively consistent measurement of a population and hence is preferable to dormancy release rate for this correlation analysis. Due to the significant main effect of weed species on seed dormancy, seed size, and DHR, data are presented by species.
Results and Discussion
Seed dormancy of all three weed species differed among the two habitats assessed in this study (Table 3; Figure 2). Populations collected from within weed-managed fields (in-crop) exhibited greater seed dormancy compared with the corresponding population collected from an unmanaged ruderal location (Figure 2). At 7 mo after harvest, H. leporinum in-crop populations exhibited 52% to 100% seed dormancy compared with 17% to 24% for populations collected from the corresponding ruderal locations. After the same 7-mo duration, B. diandrus populations collected from in-crop locations had a dormancy range of 13% to 61% compared with 0% to 14% in the populations collected from ruderal locations. The A. fatua in-crop populations exhibited 5% to 49% seed dormancy compared with 4% to 17% dormancy in ruderal populations (P < 0.05). Results indicate that there was also an effect of farming system on seed dormancy (Table 3; Figure 2). Populations of H. leporinum, B. diandrus, and A. fatua from the farming systems with annual crops, whether continuous or diverse, exhibited greater seed dormancy compared with the pasture-intensive farming system.
Rate of seed dormancy release was greatly impacted by both farming system and habitat (Table 4; Figure 2). In-crop populations released seed dormancy at a significantly slower rate than their ruderal counterparts, except for B. diandrus from the diverse system and H. leporinum from the pasture system. The time required for 50% dormancy release (D50) for H. leporinum was 3.1 mo or more for in-crop populations compared with 2.8 to 3.3 mo for ruderal populations. For B. diandrus, D50 ranged from 2.6 to 4.7 mo for in-crop populations as compared with 1.5 to 2.3 mo for ruderal populations; D50 of A. fatua ranged from 3.3 to 5.9 mo for in-crop populations and 2.7 to 3.4 mo for ruderal populations (Table 4). There was no consistent effect of farming system on dormancy release rate. Farming system had a significant influence on D50 (Table 3), particularly for in-crop populations (Table 4). The A. fatua in-crop populations from the pasture system released seed dormancy faster (D50 of 3.3 mo) than the continuous (D50 of 4.3 mo) and diverse systems (D50 of 5.9 mo; P < 0.05). In contrast, B. diandrus in-crop populations from the diverse system released seed dormancy faster than in-crop populations from the pasture system (Table 4).
Seed size as inferred from 100-seed weight of the in-crop populations differed from their ruderal counterparts across farming systems. This study also found that farming system had a substantial influence on seed size (Table 3). In-crop B. diandrus populations had greater 100-seed weight than ruderal populations from the pasture and diverse systems (Figure 3). Additionally, the 100-seed weight of in-crop H. leporinum populations from the pasture system was 70% greater than for ruderal populations. The trend was consistent for B. diandrus, with 26% greater seed size for in-crop compared with ruderal populations from the same system. However, H. leporinum from the continuous system and A. fatua from all three systems showed the opposite trend. Under the continuous system, in-crop populations had lighter seeds than the ruderal populations for H. leporinum and A. fatua. Avena fatua from the pasture system showed a similar trend (P < 0.05; Figure 3). However, B. diandrus in-crop populations had greater seed weight than ruderal populations in the diverse farming system, but A. fatua in-crop populations had lighter seeds than their ruderal counterparts from the same system.
Developing herbicide resistance, assessed as the survival of plants following herbicide exposure, varied significantly among species, farming systems, and habitats (Tables 3 and 5). Overall, all ruderal populations of the three species did not survive the field rate of these herbicides, except for <1% of plants of H. leporinum when treated with clethodim (Table 5). Hordeum leporinum in-crop populations from the continuous system had greater DHR to ALS inhibitors than populations from the pasture system: sulfometuron (96% vs. 33%, respectively) and sulfosulfuron (67% vs. 36%, respectively) (P < 0.05). Moreover, H. leporinum in-crop populations in the pasture system showed low levels of DHR to clethodim (4%) and fluazifop (15%).
Bromus diandrus in-crop populations collected from the diverse system showed greater DHR to sulfosulfuron compared with populations collected from the pasture system (100% vs. 38%, respectively; P < 0.05). However, the opposite trend was evident for sulfometuron (38% vs. 100%, respectively) (Table 5). Avena fatua in-crop populations from the continuous farming system had DHR to diclofop-methyl (ACCase inhibitor), sethoxydim (ACCase inhibitor), flamprop-methyl (mitosis inhibitor), mesosulfuron (ALS inhibitor), and triallate (lipid synthesis inhibitor) (Table 5). In-crop populations of this weed in the diverse and pasture systems showed a similar DHR profile, except to flamprop-methyl and sethoxydim (i.e., susceptible).
Seed size, expressed as 100-seed weight, exhibited strong positive correlation with seed dormancy, irrespective of the farming system and habitat (Table 6). The correlation (r) was 0.79 for H. leporinum (P < 0.0001) and 0.85 for B. diandrus (P < 0.05). Avena fatua showed a low yet significant correlation (r = 0.37: P < 0.01) between seed size and dormancy. The seed size of B. diandrus was significantly correlated with sulfometuron DHR (r=0.75; P < 0.05) (Table 6); however, this correlation was not evident for any other herbicides tested in this study (data not shown).
Table 6. Association of seed dormancy (%) with 100-seed weight and developing herbicide resistance status of the three weed species in response to weed population habitat across farming systems. a
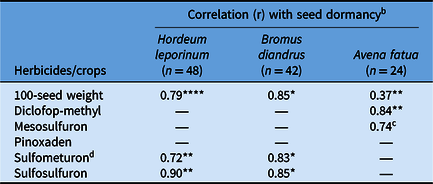
a A dash (—) indicates the study did not include any population or the populations did not show significant correlation for these specific scenarios (n = no. of populations).
b Significance level of the correlation between herbicide resistance or 100-seed weight and seed dormancy: *P < 0.05; **P < 0.01, ****P < 0.0001.
c P < 0.09.
d Correlation between B. diandrus survival (%) to sulfometuron and 100-seed weight was 0.75* (P < 0.05).
Populations with greater seed dormancy also exhibited greater DHR in the three weed species. This association was particularly evident for sulfometuron (r = 0.72, P < 0.01, and r = 0.83, P < 0.05, in H. leporinum and B. diandrus, respectively) and sulfosulfuron (r = 0.90, P < 0.01, and r = 0.85, P < 0.05, in H. leporinum and B. diandrus, respectively). This correlation was not significant for the other herbicides investigated for these two species (data not shown). A similar correlation between seed dormancy and DHR was identified in A. fatua populations for the ACCase inhibitor diclofop-methyl (r = 0.84, P < 0.01), but a weak correlation was seen with resistance to the ALS inhibitor mesosulfuron (r = 0.74, P < 0.09) (Table 6).
This study found that H. leporinum, B. diandrus, and A. fatua present in intensively managed situations such as continuously cropped fields exhibit a higher level of seed dormancy compared with their counterparts from surrounding ruderal areas. Moreover, the annual crop populations of H. leporinum and B. diandrus tended to have greater seed size compared with ruderal populations. Several factors related to plant genetics as well as cropping practices might contribute to these differences observed between populations in cropped and non-cropped areas. While genotype plays a critical role in determining seed size and dormancy across plant species (Baskin and Baskin Reference Baskin and Baskin2001; Fenner Reference Fenner1991), soil moisture stress and other stresses at any critical growth stage can alter or reduce seed size and can also reduce seed dormancy level, as determined in L. rigidum (Goggin et al. Reference Goggin, Emery, Powles and Steadman2010; Steadman et al. Reference Steadman, Ellery, Chapman, Moore and Turner2004). Intensive cropping in semiarid (nonirrigated) arable land often leads to faster uptake of soil moisture by successive, early-maturing crops compared with natural grassland situations (Liu et al. Reference Liu, Pan, Zhuang, Miralles, Teuling, Zhang, An, Dong, Zhang, He, Wang, Pan, Bai and Niyogi2015; Niu et al. Reference Niu, Musal and Liu2015), creating a relative drought condition for weed seed production. This situation may lead to increased seed dormancy or reduced seed germination, as reported by Cici (Reference Cici2017) in cowcockle [Vaccaria hispanica (Mill.) Rauschert]. Such a cropping practice–induced survival strategy may decrease the risk of reproductive failure in weeds and increase their persistence in arable lands, especially under temporal environmental uncertainty.
We report that larger seeds had significantly more dormancy in all three weed species in the study. Gardarin and Colbach (Reference Gardarin and Colbach2014) analyzed the seed size and dormancy in a number of weed species, including smooth pigweed (Amaranthus hybridus L.), and reported that larger seeds had higher levels of dormancy, in agreement with Grime et al. (Reference Grime, Mason, Curtis, Rodman, Band, Mowforth, Neil and Shaw1981), who analyzed more than 400 species collected in Sheffield, UK. Although Maity et al. (Reference Maity, Singh, Martins, Ferreira and Bagavathiannan2021b) did not observe any strong correlation between L. perenne ssp. multiflorum seed size and dormancy, Rubio de Casas et al. (Reference Rubio de Casas, Willis, Pearse, Baskin, Baskin and Cavender-Bares2017) and Chen et al. (Reference Chen, Poschlod, Antonelli, Liu and Dickie2020) noted that plant species with large seeds usually exhibit reduced dormancy and can thrive in relatively unfavorable growing conditions. Their latter conclusion regarding stressful growing conditions agrees with findings in the present study in that the high dormant populations (correlated with large seed size) showed increased survivability to herbicide application, an imposed plant stress.
Larger seed size can promote increased vigor of the emerging seedlings leading to more robust plants in general (Bean Reference Bean1972; Bebawi et al. Reference Bebawi, Eplee and Norris1984; Marcos-Filho Reference Marcos-Filho2015; Naylor and Jana Reference Naylor and Jana1976), which ultimately strengthens a weed’s ability to persist in adverse environments (Bu et al. Reference Bu, Chen, Xu, Liu, Jia and Du2007; Buckley et al. Reference Buckley, Downey, Fowler, Hill, Memmot, Norambuena, Pitcairn, Shaw, Sheppard, Winks, Wittenberg and Rees2003; Guillemin and Chauvel Reference Guillemin and Chauvel2011). Weeds with greater seed mass or weight also facilitate range expansion potential, as reported by Daws et al. (Reference Daws, Hall, Flynn and Pritchard2007) in a meta-analysis of 376 species and by Anderson (Reference Anderson1990) investigating narrowleaf hawksbeard (Crepis tectorum L). Nurse and DiTommaso (Reference Nurse and DiTommaso2005) observed fewer velvetleaf (Abutilon theophrasti Medik) seeds produced in-crop under high corn (Zea mays L.) competition as compared with the field edges. Conditions leading to a reduction in the number of seeds often results in the production of larger seeds due to higher resource allocation per seed that may aid population persistence (Gallagher et al. Reference Gallagher, Granger, Snyder, Pittmann and Fuerst2013; Sawhney and Naylor Reference Sawhney and Naylor1982). Larger seeds promote faster growth, which may facilitate plants to develop to an advanced stage of growth that is less than optimum for effective herbicidal control. Consequently, the larger plants likely receive a lower dose than required for control, which can gradually lead to quantitative herbicide resistance evolution (Manalil et al. Reference Manalil, Busi, Renton and Powles2011). Although larger seed size was not generally associated with high DHR in this study, sulfometuron DHR in B. diandrus showed considerable positive correlation with seed size. Maity et al. (Reference Maity, Singh, Jessup and Bagavathiannan2021a) reported that L. perenne ssp. multiflorum seeds with larger size exhibited significantly greater resistance to multiple herbicides. Conducting additional studies with globally important weed species will provide further insights into this association.
There is a substantial level of DHR to multiple herbicide chemistries in all three weed species in this study. Widespread evolution of herbicide-resistant weed populations is now common in Australia for the major arable weeds, including Avena spp., Bromus spp., and Hordeum spp. (Broster et al. Reference Broster, Koetz and Wu2011; Heap Reference Heap2021; Owen and Powles Reference Owen and Powles2009, Reference Owen and Powles2016). As ACCase and ALS inhibitors are the most frequently used herbicides to selectively control these weed species in a crop, resistance evolution to these high-risk herbicides is an inadvertent but expected outcome of their recurrent usage.
We found a direct and positive correlation between DHR (in-crop habitat) and seed dormancy of populations of the three species in this study. This finding substantiates a widespread view that weed populations in intensive cropping systems exhibit greater seed dormancy than those populations inhabiting pasture or non-cropped areas (Gundel et al. Reference Gundel, Martinez-Ghersa and Ghersa2008; Owen et al. Reference Owen, Michael, Renton, Steadman and Powles2011; Vila-Aiub et al. Reference Vila-Aiub, Neve and Powles2005). Owen et al. (Reference Owen, Goggin and Powles2015a, Reference Owen, Martinez and Powles2015b) reported a consistent trend of higher seed dormancy in L. rigidum and Bromus spp. populations from within intensively cropped fields that exhibited substantial resistance to different ACCase and ALS inhibitors. They postulated that the dormant cohort of a weed population escapes the pre-sowing weed management tools (preemergence herbicides or tillage) and thus augments the population with dormant individuals. These later-emerging individuals are exposed to repeated application of selective postemergence herbicides and eventually evolve resistance (Maity et al. Reference Maity, Singh, Jessup and Bagavathiannan2021a). Similar phenomena were observed in Hordeum spp. in response to ALS-inhibiting and bipyridyl herbicides in WA (Owen et al. Reference Owen, Goggin and Powles2012; Yu et al. Reference Yu, Nelson, Zheng, Jackson and Powles2007), ACCase-inhibiting herbicides in southern Australia (Shergill et al. Reference Shergill, Malone, Boutsalis, Preston and Gill2015), and ACCase-inhibiting and bipyridyl herbicides in eastern Australia (Heap Reference Heap2021; Tucker and Powles Reference Tucker and Powles1991), and in A. fatua in response to herbicides of multiple sites of action including ALS inhibitors in Montana, USA (Lehnhoff et al. Reference Lehnhoff, Keith, Dyer, Peterson and Menalled2013) and WA (Owen and Powles Reference Owen and Powles2016). However, these studies did not focus on quantifying the degree of correlation between the levels of seed dormancy and herbicide resistance.
The in-crop weed populations in this study were exposed to similar herbicide chemistries for at least 5 yr. Weed control measures are often applied repetitively at the same growth stage of the weeds, which may lead to a gradual but steady evolutionary shift to evade such measures (Sun et al. Reference Sun, Ashworth, Flower, Vila-Aiub, Rocha and Beckie2021). Co-occurrence of higher seed dormancy and DHR in the in-crop populations of H. leporinum, B. diandrus, and A. fatua tested in this study supports this assumption. However, coevolution or coexistence of herbicide resistance and seed dormancy observed in recent studies is a complex ecological phenomenon to decipher. Selection of resistance with recurrent herbicide use may not directly lead to an increase in seed dormancy (Gundel et al. Reference Gundel, Martinez-Ghersa and Ghersa2008) or vice versa.
Darmency et al. (Reference Darmency, Colbach and Corre2017) surmised that the linkage between seed dormancy and herbicide resistance may arise through selection of both traits simultaneously (pleiotropy). Two traits can arise simultaneously via separate mechanisms: the production by a single gene of two or more apparently unrelated effects (Gundel et al. Reference Gundel, Martinez-Ghersa and Ghersa2008) or linkage between two separate genes conferring dormancy and resistance (Jordan Reference Jordan1999), though the latter is a rare process (Délye et al. Reference Délye, Menchari, Michel, Cadet and Le Corre2013b). Délye et al. (Reference Délye, Jasieniuk and Le Corre2013a) postulated that single-locus mutations (similar to target-site mutations) and complex multigenic stress responses (similar to non–target site mutations) in weeds may underlie the simultaneous evolution of multiple traits. In recent studies, single-locus resistance mutations have sometimes been linked to seed biochemical compositions that alter seed germination dynamics, such as fatty acids (Tremolières et al. Reference Tremolières, Darmency, Gasquez, Dron and Connan1988), abscisic acid (Topuz et al. Reference Topuz, Nemli, Fatima and Mattoo2015; Touraud et al. Reference Touraud, Leydecker and Darmency1987), and branched-chain amino acids (Eberlein et al. Reference Eberlein, Guttieri, Berger, Fellman, Mallory-Smith, Thill, Baerg and Belknap1999). However, ALS mutations are expected to promote faster germination because of lower abscisic acid content (Topuz et al. Reference Topuz, Nemli, Fatima and Mattoo2015), as abscisic acid is associated with higher dormancy (Kermode Reference Kermode2005). Yet evolution of weed populations with greater seed dormancy and tolerance to ALS-inhibiting herbicides, as evident from multiple recent studies including the present study, contradicts these assumptions. Much more research is required to understand the causal factors influencing this association. In general, epistatic interactions between alleles at different loci may impact the selection of weed traits that enhance fitness (Leon et al. Reference Leon, Dunne and Gould2021).
It has been clearly demonstrated that along with the widespread evolution of herbicide resistance, herbicide-dependent farming systems also select for weed life cycle traits that enable weeds to evade control practices. This study illustrates the ability of in-crop H. leporinum, B. diandrus, and A. fatua populations exposed to recurrent herbicide selection to escape early-season herbicide application through enhanced dormancy, as previously documented (Fleet and Gill Reference Fleet and Gill2012; Kleemann and Gill Reference Kleemann and Gill2006; Owen et al. Reference Owen, Michael, Renton, Steadman and Powles2011). The combination of increased seed dormancy and herbicide resistance poses a tremendous ecological and agronomic challenge as populations with greater seed dormancy are able to evade the use of preseeding tillage or effective preemergence herbicide applications. These attributes can lead to increased usage of postemergence herbicides and limit the diversity of weed control tactics applied later in the crop season, which may lead to an increased selection intensity for herbicide resistance. In Australia, the net effect of prolonged weed seed dormancy on crop and weed growth and seed yield will depend on the duration of preemergence herbicide bioactivity in soil (e.g., pyroxasulfone greater than trifluralin), competitiveness of the crop species (e.g., cereals more competitive than pulses), period of the growing season (time required for crop canopy closure, emergence time differential between crop and dormant weeds, duration of crop growth advantage as soils cool going into winter), and timing of precipitation events.
While this study demonstrates that simultaneous evolution of both weed seed traits and DHR is occurring, such widespread adaptations need not be inevitable. The application of sufficient weed control diversity involving cultural, physical/mechanical, and herbicidal weed control tools within a crop–weed competitive environment has been demonstrated to reduce weed seedbanks to low and manageable levels (Sun et al. Reference Sun, Ashworth, Flower, Vila-Aiub, Rocha and Beckie2021; Walsh et al. Reference Walsh, Broster, Schwartz-Lazaro, Norsworthy, Davis, Tidemann, Beckie, Lyon, Soni, Neve and Bagavathiannan2017). Weed management diversity should help mitigate unidirectional selection pressures driving weed-trait adaptations. By minimizing weed seedbanks through maximizing crop competition, preharvest nonselective herbicide application (spray topping) when required, and harvest weed seed control (destroying or removing weed seeds in the chaff fraction of harvested crop residue), growers can limit the number of weeds under selection and thereby help mitigate further shifts in resistance evolution in response to herbicide exposure and physiological or phenological adaptations to evade weed control practices.
Acknowledgments
The authors acknowledge the Endeavour Leadership Award by the Australian Government, the Netaji Subhash ICAR-International Scholarship by the Indian Council of Agricultural Research, and Tom Slick Graduate Research Fellowship from Texas A&M AgriLife Research granted to AM. We appreciate the technical assistance of Shane Baxter and others for sample collection, processing, and evaluation. No conflicts of interest have been declared.












