INTRODUCTION
The epidemiological pattern of infectious diseases has changed throughout history [1, Reference Garrett2]. Explanations for these changes are usually based on different alternatives such as the appearance of new aetiological pathogens, climate change, environmental circumstances, improper use of antibiotics and changes in society in relation to behavioural and socio-demographic characteristics [Reference Lafferty3]. One recent example of this evolution is the emergence of a new strain of influenza virus [A(H1N1)pdm09] in spring 2009, which caused the first pandemic of the 21st century [4, 5].
Socio-demographic factors are conditions that influence the behaviour of infectious diseases [Reference Larrañaga6, Reference Stone7]. It has been shown that social disparities influence the appearance of the disease and cause differences in its perception, progress and outcome. These differences can be explained, at least partially, by differences in social characteristics, which include many components such as education level, income, material possessions, livelihood means and occupation [Reference Charland8, Reference Woodward and Kawachi9]. For public health analyses, these factors are usually summarized in a single indicator variable, denoted social class [Reference Domingo-Salvany10, Reference Álvarez-Dardet11].
Social class based on occupation is an indicator of socioeconomic position and health-related behaviour. Occupation is a very useful individual feature for social theories which attempt to measure the social classes and is a better indicator than education in studies comparing variables such as mortality or lifestyle [Reference Domingo-Salvany and Marcos12]. A classification based on occupation is a way of identifying inherent factors of social structure of social models [Reference Coma, Martí and Fernandez13, Reference Domingo-Salvany14].
Following this argument, it is possible that occupation classification might differentiate between groups of individuals with similar clinical and behavioural risk factors of influenza infection, by severity of A(H1N1)pdm09 influenza infection and also to differentiate them according to utilization of health services [Reference González-Candelas15].
Risk factors that lead to hospitalization from influenza can be very different from those associated with the risk of infection by influenza at the community level. Therefore, these two outcomes should be studied separately and, consequently, case-control studies should use two types of controls, influenza community cases or community non-influenza controls, respectively [Reference Baker16].
The aim of this study was to investigate the association between social class (categorized by type of occupation) and the occurrence of A(H1N1)pmd09 infection and hospitalization for two seasons (2009–2010 and 2010–2011).
METHODS
A multicentre study with matched cases and controls was conducted in 36 hospitals and 22 primary-care centres from seven Spanish regions (Andalusia, Basque Country, Catalonia, Castile & Leon, Madrid, Navarra, Valencian Community) [Reference Domínguez17]. Cases and controls were recruited between July 2009 and February 2011, and were aged >18 years at the date of inclusion. The minimum number of pairs needed to detect an odds ratio (OR) of 1·5 and assuming a prevalence of the investigated factors in controls of 0·30, a bilateral significance level alpha = 0·05 and a power of beta = 0·80 was 425 using the criteria proposed by Schlesselman [18]. To calculate the risk of infection, ambulatory cases were matched with ambulatory controls and to calculate the risk of hospitalization, hospitalized cases were matched with ambulatory cases.
Selection and matching of ambulatory cases, ambulatory controls and hospitalized cases
An ambulatory case of A(H1N1)pdm09 was considered if reverse transcription–polymerase chain reaction (RT–PCR) was positive for this virus in a suspected case visiting a primary-care centre. Ambulatory controls were people attending a primary-care centre for any reason other than influenza-like illness. Ambulatory cases were matched with ambulatory controls taking into account age (±5 years), date of diagnosis of the case (±10 days) and residential province of the case. When several potential controls were available, the selection was made by using a table of random numbers. If an adequate control was not found, the range of the diagnosis date was expanded. A hospitalized case was defined as a patient admitted to hospital for ⩾24 h with RT–PCR confirmed infection by influenza A(H1N1)pdm09 virus. Hospitalized cases excluded nosocomial infections (determined by onset of symptoms ⩾48 h after admission to the hospital). Ambulatory cases were matched to hospitalized cases by age (±5 years), residential province and date of admission to the hospital (±10 days).
Socio-demographic and pre-existing behavioural and medical variables
For all the subjects included in the study the following demographic and medical variables were obtained: age, marital status, tobacco and alcohol use, pregnancy (for women aged 15–49 years), chronic obstructive pulmonary disease (COPD), chronic respiratory failure, cardiovascular disease, diabetes, chronic liver disease, obesity type III (body mass index >40) [19], and treatment with systemic corticosteroids.
Occupational variables
The occupation of the studied population was registered literally and, in order to develop the analyses, it was coded using the indicators of occupational social class postulated by the Working Group of Social Determinants of the Spanish Society of Epidemiology for use in the research and practice of public health in Spain. This classification was based on the National Classification of Occupations of 2011 [20] and a neo-Weberian perspective designated CSO-SEE12 (Clase Social Ocupacional–Sociedad Española de Epidemiología 2012) [Reference Domingo-Salvany14]. The classification and categories are: (1) Directors and managers of businesses with ⩾10 employees and professions traditionally associated with a university grade; (2) Directors and managers of businesses with <10 workers and professions traditionally associated with a university diploma and other as professional technical support, athletes and artists; (3) Middle occupations (administrative employees and professional support for administrative and other management services); (4) Self-employed; (5) Supervisors and workers in skilled technical occupations; (6) Workers qualified at the primary sector and other workers semi-skilled; and (7) Unskilled workers. These seven categories can be aggregated into six-, five-, three- and two-category classifications [Reference Domingo-Salvany14]. The two-category scheme aggregates categories (1)–(3) in a new category called non-manual workers, and categories (4)–(7) in a manual workers category [Reference Domingo-Salvany14].
This two-category classification was used for the main analyses presented here in order to maximize the statistical power of the study. To perform the classification, the occupation declared by all cases and controls was first assigned to one of the seven categories of the CSO-SEE12 classification and subsequently classified in the manual or non-manual class. Students, housewives and unemployed or handicapped individuals who did not report their last occupation were considered as unclassifiable and were excluded from the analyses.
Vaccination status for cases and controls was assessed by immunization records with an influenza pandemic vaccine, a monovalent vaccine with the A/California/04/2009 (H1N1) strain, in the 2009–2010 season and with a trivalent influenza vaccine that included the A/California/04/2009 (H1N1) strain in the 2010–2011 season.
Medical variables were obtained from hospital clinical records and primary healthcare centre registers. In hospitalized cases, vaccination status was obtained from hospital records or vaccination cards. If none of these was available, primary healthcare centre registers were consulted. For ambulatory cases and controls, vaccination status was obtained from primary healthcare centre registers or vaccination cards. Behavioural and occupational information was obtained by direct or phone interviews with the patient.
Ethical considerations
All information collected was treated as confidential, in strict compliance with the Spanish legislation on observational studies. The study was approved by Ethics Committees of the hospitals involved. Written informed consent was obtained from all patients included in the study.
Statistical analysis
Once the field occupation was completed, each epidemic wave database was checked, correcting any detected inconsistencies.
The association of the dependent variable, infection or hospitalization, with the primary independent variable (occupational social class) was estimated by calculating the odds ratio (OR) and 95% confidence intervals (CI). Multivariate analysis was performed using conditional logistic regression adjusting for age and influenza vaccination as well as for variables that are predictive of infection or hospitalization in a model constructed by selection procedure of variables, with a cut-off point of P < 0·2.
RESULTS
A total of 996 confirmed influenza A(H1N1)pdm09 ambulatory and 720 hospitalized cases were initially available for study. The ambulatory controls were 1062. After filtering, 715 ambulatory cases, 715 ambulatory controls and 406 hospitalized cases were retained for analysis, as shown in Figure 1.
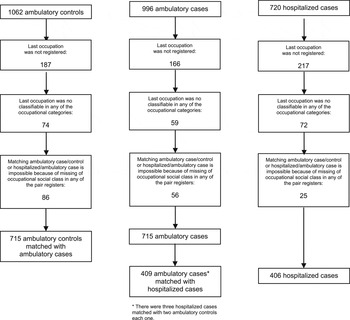
Fig. 1. Number of ambulatory controls, ambulatory cases and hospitalized cases included in the study.
No statistically significant differences were found in the proportion of manual and non-manual workers between ambulatory cases and ambulatory controls (Table 1). The occupation variable showed no association with A(H1N1)pmd09 infection (OR 0·83, 95% CI 0·68–1·02).
Table 1. Distribution of ambulatory cases and controls according to demographic, behavioural factors, medical conditions and occupational class

OR, Odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Despite the matching process, some differences between ambulatory cases and ambulatory controls were observed. The proportion of ambulatory cases aged >60 years was 12·8% while the proportion of ambulatory controls aged >60 years was 26·6%.
Pregnancy was much more frequent in ambulatory cases than in ambulatory controls. The prevalence of diabetes in ambulatory controls was higher than in ambulatory cases. Ambulatory cases were treated with corticoids in a higher proportion than ambulatory controls. Influenza vaccination was three times more frequent in ambulatory controls (Table 1).
In hospitalized cases, the proportion of manual workers was higher than for ambulatory cases (Table 2). Hospitalized cases aged >60 years were almost double (24·7%) that of ambulatory cases (12·8%). In hospitalized cases pregnancy was slightly more frequent than in ambulatory cases. Major chronic conditions showed significant differences, with a higher prevalence in hospitalized cases in COPD, chronic respiratory failure, chronic liver disease, body mass index >40, and diabetes (Table 2).
Table 2. Distribution of hospitalized cases and ambulatory cases according to demographic, behavioural factors, medical conditions and occupational class

OR, Odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
In the adjusted comparison of ambulatory cases and controls using a conditional logistic regression model (Table 3), no significant relationship between A(H1N1)pmd09 infection and the main independent variable, occupational class, was observed (aOR 0·97, 95% CI 0·74–1·27). Older ages remained protective of the infection and so did the influenza pandemic vaccine. Pregnant women continued presenting a strong association with the infection.
Table 3. Risk factors associated with A(H1N1)pmd09 infection in a conditional logistic regression model

aOR, Adjusted odds ratio; CI, confidence interval.
* Statistical power: 25·9%.
In a similar analysis comparing hospitalized cases and ambulatory cases (Table 4), the relationship between occupational class (non-manual and manual workers) and hospitalization for A(H1N1)pmd09 resulted in statistical significance (aOR 1·53, 95% CI 1·01–2·31). Older age groups were highly predisposed to hospitalization, as was the case for diabetes, COPD and pregnancy.
Table 4. Risk factors associated with A(H1N1)pmd09 hospitalization in a conditional logistic regression model

aOR, Adjusted odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
* Statistical power: 84·3%
DISCUSSION
Manual workers had 53% more risk of being hospitalized once they had been infected with by influenza A(H1N1)pmd09 virus. Occupational social class was not associated with infection, but the statistical power to detect differences was low in the present study. Public health policies should take these results into account in order to emphasize and adapt messages about preventive recommendations to the manual worker population and their families.
The lack of difference in the risk of infection between manual and non-manual workers is consistent with studies that highlight more infection risk is only associated with occupations with more social exposition [Reference Suarthana21].
The higher risk of hospitalization in the lower social class is congruent with a recent meta-analysis by Tricco et al. [Reference Tricco22] that evidences more risk of hospitalization in socially vulnerable groups vs. non-vulnerable people. In this meta-analysis, risk of hospitalization considered conditions such as pregnancy and comorbidities such as chronic lung conditions, heart conditions, and diabetes with an adjusted OR of 2·26 (95% CI 1·53–3·32). Another study by Van Kerkhove et al. [Reference Van Kerkhove23] pooled data on risk factors for A(H1N1)pmd09 severe outcome and included data from governmental surveillance programmes across 19 countries, including Spain. In that study, vulnerable social groups had a higher risk of hospitalization considering the same demographic, behavioural factors and medical conditions that we have considered in the present study and the results about rates of hospitalization are close to ours [Reference Van Kerkhove23].
The existence of poorer life conditions expressed throughout the lifespan and also using occupational social class has been demonstrated in Van Raalte et al.'s [Reference Van Raalte, Martikainen and Myrskylä24] cross-sectional analyses of trends in adult lifespan variation over four decades (1971–2010) by occupational social class (manual, lower non-manual, upper non-manual) using Finnish register data. One of the main findings of their study was the existence of disparities in access to new medical treatments and campaigns to discourage self-deleterious behaviour, circumstance that would not apply in our study because during the study period the Spanish public health system was universal and free.
When focusing on the influence of socioeconomic status in the clinical evolution of acute infectious diseases, a Danish population-based cohort study on bacteraemia in working patients [Reference Koch25] revealed that people of lower socioeconomic status had higher mortality within 30 days after bacteraemia than those of higher status.
Our study confirms that risk factors for influenza hospitalization are different from those of influenza infection. Age is probably the variable that shows this difference more clearly. While hospitalization risk markedly increased with age, as previously shown by other authors [Reference Anderson26], older age groups are associated with a markedly lower risk of infection. This result is in line with the report by Hoschler et al. [Reference Hoschler27] about the large increase in antibody levels after the 2010–2011 winter in persons aged >75 years. Clinical surveillance data suggest that elderly persons (>65 years) were relatively protected from infection with A(H1N1)pdm09 virus.
The meta-analysis by Mertz et al. [Reference Mertz28] on infection and hospitalization during pregnancy concluded, in accord with our study, that pregnancy is a major risk factor for hospitalization in infected pregnant women, but not for other outcomes (community-acquired pneumonia, mortality, admission to an intensive care unit or need for ventilatory support). Their study also confirmed the importance of three chronic diseases (COPD, cardiovascular disease, diabetes) for the risk of hospitalization by influenza infection, the same as in our study with the distinction that in the Mertz et al. study diabetes also increased the risk of death in A(H1N1)pdm09 patients.
The results of our study show that vaccination can prevent 59% (95% CI 23–77) of influenza infections. This value is similar to that in a European multi-centre case-control study by Kissling et al. [Reference Kissling29] that found in 2010–2011 a vaccine effectiveness (VE) of 41·3% (95% CI 2·6–66·4) in people aged 15–59 years and of 59·9% (95% CI 16·7–80·7) in people aged ⩾60 years. In a case-control study including all ages that was performed in the UK in 2010–2011, Pebody et al. [Reference Pebody30] found a VE of 57% (95% CI 42–68). In a case-control study in England and Scotland performed in 2009–2010, Hardelid et al. [Reference Hardelid31] found a VE of 72% (95% CI 21–90), a point estimate higher than ours but with confidence intervals similar to those we have obtained.
This study has several strengths. It is a multicentre study that involves centres throughout Spain. All ambulatory and hospitalized cases were confirmed by RT–PCR and the vaccination and medical conditions of the participants were directly obtained from medical records. To ensure the observed association between occupational class, all co-associated variables described in other studies about the risk of infection or hospitalization by A(H1N1)pmd09 virus have been included in the analysis.
Nevertheless, the study has some limitations related to the indicator chosen to measure social class, in our case, occupation. As mentioned above, this is a highly valid indicator to measure social class, but it would have been useful to combine it with additional indicators such as home-ownership [Reference Jackson, Jones and Mishra32]. In our study, the register of the occupation has been made by encoding the last occupation declared by each individual included in the study into the occupational social-class categories. Following the recommendations of Domingo-Salvany [Reference Domingo-Salvany14] there are two ways of enquiring about occupation class, the one we have used in this study, i.e. a single open question about the actual or former occupation, and the same question with six multiple choice answers. Therefore, it was possible to encode more than the 90% of literally registered last occupation reports.
CONCLUSION
This study adds to previous findings that occupation as an individual characteristic representing social class, is associated with poorer results in health.
Our study confirms that manual workers have a higher risk of hospitalization when they are infected by influenza A(H1N1)pdm09 virus than other occupations but they do not have a different probability of being infected by the virus. Vaccination prevents infection in 59% of vaccinated individuals and therefore vaccination programmes should be reinforced.
APPENDIX
Other members of the CIBERESP Cases and Controls in Pandemic Influenza Working Group
Andalusia: E. Azor, J. Carrillo, R. Moyano, J. A. Navarro, M. Vázquez, F. Zafra (Médico Centinela), M. A. Bueno, M. Delgado, M. L. Gómez, M. Mariscal, B. Martínez, J. P. Quesada, M. Sillero (Compl. Hosp. Jaén), M. Carnero, J. Fernández-Crehuet, J. del Diego Salas (Hosp. Virgen de la Victoria), V. Fuentes (Hosp. Costa del Sol), V. Gallardo, E. Pérez (Servicio de Epidemiología), R. López (Hosp. Infanta Elena de Huelva), J. R. Maldonado (Hosp. Torrecárdenas), A. Morillo (Hosp. Virgen del Rocío), I. Pedrosa Corral, M. F. Bautista, J. M. Navarro, M. Pérez (Lab. Referencia Gripe), S. Oña (Hosp. Carlos Haya), M. J. Pérez (Hosp. Virgen de Valme), M. C. Ubago (Hosp. Virgen de las Nieves), M. Zarzuela (Hosp. Puerta del Mar). Castile and Leon: P. Sanz (Universidad de León), D. Carriedo, F. Díez, I. Fernández, S. Fernández, M. P. Sanz (Compl. Asist. Universitario, León), J. J. Castrodeza, A. Pérez, R. Ortiz de Lejarazu (Centro Nacional de Gripe, Valladolid), J. Ortiz (Hosp. El Bierzo), A. Pueyo, J. L. Viejo, A. Seco (Compl. Asist. Burgos), P. Redondo (Serv. Territorial de Sanidad y Bienestar Social, León), T. Fernandez, A. Molina (Inst. Biomedicina, Universidad de León). Catalonia: A. Agustí, A. Torres, A. Trilla, A. Vilella (Hosp. Clínic); F. Barbé (Hosp. Arnau de Vilanova); L. Blanch, G. Navarro (Hosp. Sabadell); X. Bonfill, J. López-Contreras, V. Pomar, M. T. Puig (Hosp. Sant Pau); E. Borràs, A. Martínez, N. Torner; C. Bravo, F. Moraga (Hosp. Vall d'Hebrón); F. Calafell (Universitat Pompeu Fabra); J. Caylà, C. Tortajada (Agencia de Salud Publica de Barcelona); I. Garcia, J. Ruiz (Hosp. Germans Trias i Pujol); J. J. García (Hosp. Sant Joan de Deu); M. Baricot, O. Garín (CIBERESP); J. Alonso (IMIM-Hosp. del Mar), J. Gea, J. P. Horcajada (Universitat Pompeu Fabra (CIBER Enfermedades Respiratorias); T. Pumarola (Red Esp. Inv. en Patología Infecciosa); N. Hayes (Hosp. Clínic_CRESIB); A. Rosell, J. Dorca (Hosp. de Bellvitge), M. Saez (Universidad de Girona). Madrid: A. Castro (CIBER Enfermedades Respiratorias); C. Álvarez, M. Enríquez, A. Hernández Voth, F. Pozo (Hosp. 12 de Octubre), F. Baquero, R. Cantón, J. C. Galán, A. Robustillo, M. Valdeón (Hosp. Universitario Ramón y Cajal); E. Córdoba, F. Domínguez, M. García Barquero, J. García, R Génova, E. Gil, S. Jiménez, M. A. Lopaz, J. López, F. Martín, M. L. Martínez, M. Ordobás, E. Rodriguez, S. Sánchez, C. Valdés (Área de Epidemiología, Comunidad de Madrid), J. R. Paño, M. Romero (Hosp. Universitario La Paz). Navarre: A. Martínez, L. Martínez (Inst. de Salud Pública), M. Ruiz, P. Fanlo, F. Gil, V. Martínez-Artola (Compl. Hosp. Navarra), M. E. Ursua, M. Sota, M. T. Virto, J. Gamboa, F. Pérez-Afonso (Médico Centinelas). The Basque Country: U. Aguirre, A. Caspelastegui, P. P. España, J. M. Antoñana, I. Astigarraga, J. I. Pijoan, I. Pocheville, M. Santiago, J. I. Villate (Hosp. Cruces), J. Arístegui, A. Escobar, M. I. Garrote (Hosp. Basurto), A. Bilbao, C. Garaizar (Fundación Vasca de Innovación e Investigación Sanitarias), G. Cilla, J. Korta, E. Pérez-Trallero, C. Sarasqueta (Hosp. Donostia), F. Aizpuru, J. L. Lobo, C. Salado (Hosp. Txagorritxu), J. Alustiza (Hosp. Mendaro), F. J. Troya (Hosp. de Santiago). Valencia Community: J. Blanquer (Hosp. Clínico), M. Morales (Hosp. Doctor Peset).
ACKNOWLEDGEMENTS
We thank Mr Josep Ramon Marsal Mora and the Lleida Suport to Research Unit of IDIAP-Jordi Gol Institute for statistical advice and Dr Eudald Magrí Carles for preparing the software necessary to perform the final elaboration of this article.
This study was supported by the Ministry of Science and Innovation, Institute of Health Carlos III, Programme of Research on Influenza A/H1N1 (Grant GR09/0030), the Catalan Agency for the Management of Grants for University Research (AGAUR, Grant no. 2014/SGR1403) and CIBER Epidemiología y Salud Pública (CIBERESP). The funders had no role in the study design, data collection, analysis, the decision to publish or the preparation of the manuscript.
DECLARATION OF INTEREST
None.








