In addition to its endocrine role, vitamin D has been demonstrated to have potent immunomodulatory activities( Reference Helming, Bose and Ehrchen 1 , Reference Van and Mathieu 2 ). It has been shown to be associated with an increased incidence of infections directly or indirectly( Reference James, Paolo and Ralph 3 ). Vitamin D deficiency is common in the normal population( Reference Goswami, Gupta and Goswami 4 , Reference Singh, Prakash and Tiwari 5 ) and patients with diabetes( Reference Mattila, Knekt and Mannisto 6 ). Diabetic foot infection reflects the altered immune state of the host due to changes in the mediators of the immune function. Cytokines are major mediators of the host's response to infection and thus play an important role in the differentiation of macrophages, eradication of infection and progression of wound-healing process( Reference Iacopino 7 , Reference Oncul, Yildiz and Gurer 8 ). An infection triggers inflammatory responses via the release of inflammatory cytokines such as IL-1β, IL-6, interferon-γ (IFN-γ) and TNF-α or chemokines such as IL-8 in the host, which are regulated by counter-regulatory mechanisms such as the production of anti-inflammatory cytokines such as IL-10 to avert a hyperinflammatory state necessary for infection control and effective wound healing.
Several known physiological factors such as decreased growth factor production( Reference Galkowska, Wojewodzka and Olszewski 9 – Reference Falanga 11 ), angiogenic response( Reference Geerlings and Hoepelman 12 ), and macrophage function( Reference Kazuichi, Jun and Masaaki 13 ) and impaired cytokine production( Reference Galiano, Tepper and Pelo 14 , Reference Peleg, Weerarathna and McCarthy 15 ) contribute to wound-healing abnormalities in individuals with diabetes. Moreover, underlying pathological conditions such as hyperglycaemia also disrupt the normal process of cytokine production( Reference Naguib, Al-Mashat and Desta 16 – Reference Brem and Tomic-Canic 19 ). This could further lead to the development of chronic wound where persistent and elevated inflammatory cell activities are considered to be critical( Reference Agren, Eaglstein and Ferguson 20 ).
There is a paucity of data on the role of systemic inflammation in diabetic foot infection patients in association with vitamin D status responsible for delayed wound healing. It has been proposed that severe vitamin D deficiency in addition to hyperglycaemia is a risk factor( Reference Tiwari, Pratyush and Gupta 21 ) for diabetic foot infection probably via immune dysregulation.
The present study aimed to evaluate the circulating concentrations of IL-1β, TNF-α, IFN-γ and IL-6 in patients with diabetic foot infection and assess the influence of vitamin D deficiency on the above-mentioned cytokines.
Subjects and methods
Subjects
Patients with diabetic foot infection visiting the diabetic foot clinic and endocrine outpatient unit of the University hospital located at coordinates 25°20′0″ North and 83°0′0″ East were enrolled as cases and diabetic patients without any evidence of infection served as controls in the present study. A detailed clinical history including age, sex, duration of diabetes, and concomitant and anti-diabetic medication usage was recorded in a preset pro forma. Infection was clinically diagnosed by culture positivity and/or higher total leukocyte counts in the presence of other clinical evidences such as fever and signs of inflammation. Bacterial culture and identification were done as described previously( Reference Tiwari, Pratyush and Dwivedi 22 ). The Wagner Diabetic Foot Ulcer Classification System (grade 0: no ulcer, but high-risk foot (e.g. deformities, callus and insensitivity); grade 1: superficial, full-thickness ulcer; grade 2: deeper ulcer, penetrating tendons, without bone involvement; grade 3: deeper ulcer with bone involvement and osteitis; grade 4: partial gangrene (e.g. toes and forefoot); and grade 5: gangrene of the whole foot) was used to grade diabetic foot infection.
Patients exhibiting clinical evidence of vascular insufficiency or taking immune-suppressants, multivitamins and Ca supplements were excluded from the study. A total of forty healthy subjects also participated voluntarily in the present study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Ethics Committee. Written informed consent was obtained from all the participants.
Sample collection
Blood samples were collected into a plain vial and an EDTA-containing vial. Sampling was uniform throughout the year without any seasonal variation in the study groups. Coagulated blood was centrifuged at 1800 g for 10 min. Serum was separated and aliquots of 300 μl were stored frozen at − 80°C until analysis. Blood samples with the anti-coagulant were used for estimating glycosylated Hb (HbA1C) concentration using the DS5 system (Drew Scientific, Inc.) based on ion-exchange chromatography.
Serum 25-hydroxyvitamin D assay
The serum concentration of 25-hydroxyvitamin D was determined by RIA using a commercial kit (Diasorin) in duplicate according to the manufacturer's protocol. Intra-assay and inter-assay variations (% CV) were 11·7 and 12·5 respectively.
Cytokine concentration estimation
The serum concentrations of IL-1β, IL-6, TNF-α (Diaclone) and IFN-γ (BD Biosciences) were estimated by ELISA using commercial kits according to the manufacturer's instructions. One aliquot was used for the estimation of each cytokine in duplicates. Samples with a test value below the detection limit were excluded from the analysis. The intra-assay and inter-assay CV (%) were 5·7 and 7·3 for IL-1β, 4·2 and 7·7 for IL-6, 3·3 and 9·0 for TNF-α, and 3·7 and 6·6 for IFN-γ, respectively.
Staging of vitamin D deficiency
Different cut-off values of vitamin D (25-hydroxyvitamin D) were chosen, i.e. < 25 nmol/l, < 50 nmol/l and < 75 nmol/l, to evaluate its effect, if any, on the cytokine profiles of the study participants. These cut-off values were similar to those described for Ca homoeostasis( Reference Holick, Binckley and Bischoff-Ferrari 23 ).
Statistical analysis
Data are presented as means and standard deviations or as means with their standard errors unless otherwise indicated. Continuous variables that were not normally distributed were logarithmically transformed before analysis. Statistical analysis was conducted using SPSS 16.0 (SPSS, Inc.). Parametric and non-parametric tests were used wherever applicable. Correlation analysis (Spearman) was carried out to determine the association between these inflammatory cytokines and 25-hydroxyvitamin D status in the study population.
Results
A total of 219 individuals (112 diabetic patients with foot infection (cases) and 107 diabetic patients without infection (controls)) participated in the study. The duration of diabetic foot infection varied from 1 month to 1 year, and the cases enrolled had infection of Wagner's grades 2–4. The mean total leukocyte count in cases was 14 971 (se 7556). Bacterial culture revealed the presence of microbial isolates varying from a minimum of one to a maximum of three per case. Escherichia coli was the most common bacterium isolated. The age, duration of diabetes, HbA1C concentration and BMI of the study participants are summarised in Table 1. No statistically significant difference was observed in mean age, duration of diabetes, HbA1C concentration and BMI between cases and controls. The mean concentrations of the inflammatory cytokines IL-1β (P= 0·02), TNF-α (P= 0·006) and IL-6 (P= 0·001) were significantly higher and that of 25-hydroxyvitamin D (P= 0·06) was comparatively lower in cases than in controls. The concentrations of IL-1β, IL-6, TNF-α and IFN-γ were below the detection limit of the assay, i.e. < 7, < 8, < 2 and 4·7 pg/ml, respectively, in 95 % of the healthy volunteers and thus data obtained from them could not be included in the analysis.
Table 1 Comparison of clinical parameters, inflammatory cytokine profiles and 25-hydroxyvitamin D status between cases and controls (Mean values with their standard errors)
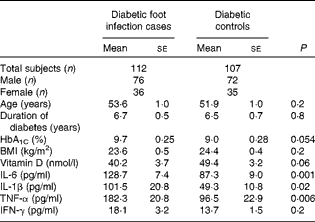
HbA1C, glycated Hb; IFN-γ, interferon-γ.
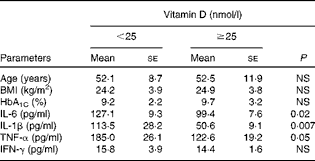
Vitamin D deficiency (25-hydroxyvitamin D concentration < 50 nmol/l) was found in 71·4 % of the diabetic foot infection cases, 61·6 % of the diabetic controls and 48·6 % of the healthy volunteers, but severe deficiency (25-hydroxyvitamin D concentration < 25 nmol/l) was most common in diabetic foot infection cases than in diabetic controls and healthy volunteers (48·2, 20·5 and 7·6 %, respectively) (Fig. 1). In both cases and controls, 25-hydroxyvitamin D concentration was found to be significantly negatively correlated with IL-1β (r − 0·323; P≤ 0·001) (Fig. 2(a)) as well as IL-6 (r − 0·154; P= 0·04) concentrations (Fig. 2(b)) and moderately correlated with TNF-α concentration (r − 0·102; P= 0·07) (Fig. 2(c)), but not with IFN-γ concentration (r − 0·009; P= 0·9). Independent correlation analysis revealed a non-significant negative correlation between vitamin D and cytokine concentrations in the study participants, except for IL-1β, which exhibited a significant correlation independently in cases and controls (Table 2). Patients with 25-hydroxyvitamin D concentration < 25 nmol/l had significantly higher concentrations of IL-1β and IL-6 than those with 25-hydroxyvitamin D concentration ≥ 25 nmol/l. Despite remarkable elevation in the corresponding concentration of TNF-α (185·0 (se 26·1) v. 122·6 (se 19·2); P= 0·05), the difference did not reach statistical significance in these subgroups (Table 3). There was no difference in IFN-γ concentration. No significant correlation was observed between cytokine concentrations and 25-hydroxyvitamin D cut-off value of 50 nmol/l.

Fig. 1 Distribution of study participants (%) with respect to vitamin D status. ![]() , Diabetic foot infection;
, Diabetic foot infection; ![]() , diabetic control; ■, healthy control.
, diabetic control; ■, healthy control.

Fig. 2 Correlation between 25-hydroxyvitamin D (25(OH)vitamin D) and cytokines: (a) IL-1β (r − 0·323, P≤ 0·001), (b) IL-6 (r − 0·154, P= 0·04) and (c) TNF-α (r − 0·102, P= 0·07). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Table 2 Correlation between vitamin D and cytokine concentrations in cases, controls and total population

DFI, diabetic foot infection; DC, diabetic control; IFN-γ, interferon-γ.
Table 3 Comparison of inflammatory cytokine concentrations in subjects with and without severe vitamin D deficiency (Mean values with their standard errors)
Discussion
In the present study, patients with diabetic foot infection served as a model for significant immunological defects owing to the pathological combination of hyperglycaemia and infection. The concentrations of inflammatory cytokines were significantly elevated in patients with diabetic foot infection than in diabetic controls. Our findings were similar to those reported by Weigelt et al. ( Reference Weigelt, Rose and Poschen 24 ), who showed non-random up-regulation of several acute-phase proteins, cytokines and chemokines such as high-sensitivity C-reactive protein and IL-6 in patients with diabetic foot infection. Such perturbations were not observed for IFN-γ, the concentration of which increases in response to intracellular invasion of bacteria such as Mycobacterium tuberculosis and/or during autoimmune destruction conditions such as type 1 diabetes mellitus.
The variables known to influence cytokine production such as age and BMI( Reference Himmerich, Fulda and Linseisen 25 ), duration of diabetes( 26 ) and glycaemic status( Reference Maedler, Sergeev and Ris 27 ) were similar between the two groups in the present study. Interestingly, there was a negative correlation between cytokine and vitamin D concentrations. Similarly, findings from another study have shown that 1,25-dihydroxyvitamin D3 down-regulates the expression of the inflammatory cytokines TNF-α, IL-6 and IL-1β( Reference Giulietti, Etten and Overbergh 28 ) and suppresses IFN-γ-mediated macrophage activation( Reference Helming, Bose and Ehrchen 1 ). In a double-blind, randomised, placebo-controlled trial in congestive heart failure (CHF) patients, vitamin D supplementation was found to reduce the inflammatory cytokine milieu( Reference Schleithoff, Zittermann and Tenderich 29 ). However, findings reported by Clende et al. ( Reference Clendenen, Koenig and Arslan 30 ) in healthy women with variable cut-off values of 25-hydroxyvitamin D were in contrast to the above findings and failed to demonstrate any relationship with inflammatory cytokines. The concentrations of cytokines are influenced by several physiological factors, and we proposed that vitamin D could be one of them. There is no definite cut-off value defined for cytokines that could help differentiating healthy and diseased states or indicating the severity of the disease; thus, to study the association between vitamin D status and cytokine concentrations, a correlation analysis using Spearman's correlation was carried out in the present study.
Vitamin D deficiency and its various implications have been studied in patients with diabetes( Reference Mattila, Knekt and Mannisto 6 ). In the present study, severe vitamin D deficiency was more common in patients with diabetic foot infection than in diabetic controls. The photoconversion of 7-dehydrocholesterol to pre-vitamin D3 and its photoproducts is maximal between 11.00 and 14.00 hours throughout the year in India( Reference Harinarayan, Holick and Prasad 31 ). Rural sick patients spend more time in the morning for their hygiene-related care such as outdoor bath and oil massage, which is a common practice in the rural Indian population. Despite ample exposure to sun, patients with diabetic foot infection were found to be severely vitamin D deficient compared with controls in the present study. Although controls worked indoors, we speculate that these patients may have had higher non-occupational sun exposure than their diabetic foot infection counterparts during the time periods of the day when cutaneous vitamin D synthesis is greatest, which is after noon. Other factors that could be responsible for the difference in vitamin D status between controls and diabetic foot infection patients include skin pigmentation, clothing pattern and sunscreen usage( Reference McCarty 32 ). We did not assess these variables in the present study as we aimed to assess the association or effect of vitamin D deficiency with/on the immune function of such patients irrespective of their aetiology.
In our previous study( Reference Tiwari, Pratyush and Gupta 21 ), we have shown vitamin D concentration of 10 ng/ml (25 nmol/l) to be a possible risk factor (OR = 4) for diabetic foot infection using logistic regression analysis with a proposition that it might result in immune dysregulation. In the present study, we chose this cut-off value to assess the difference in immune status defined by cytokine concentrations at this risk point and found that there was a significant difference in the concentrations of IL-1 and IL-6 in patients with severe vitamin D deficiency (serum 25-hydroxyvitamin D concentration < 25 nmol/l) in comparison with patients with serum 25-hydroxyvitamin D concentration ≥ 25 nmol/l, which substantiated our previous finding. The difference was also notable for TNF-α. Our finding was similar to that reported by another study, in which 25(OH) vitamin D concentration < 25 nmol/l was found to be associated with higher baseline concentrations of TNF-α, IL-6 and IL-8 in tuberculosis–immune reconstitution inflammatory syndrome patients( Reference Conesa-Botella, Meintjes and Coussens 33 ).
The results of the present study showed that elevated cytokine responses occur as a consequence of vitamin D deficiency in patients with diabetic foot infection. The results also served to redefine the cut-off value of 25-hydroxyvitamin D deficiency for healthy and diseased populations. Based on these results, we suggest a 25-hydroxyvitamin D concentration value < 25 nmol/l to be the cut-off for unfavourable immunological alterations. Studies including various other immunological parameters would provide evidence for this distinctive hypothesis and these results in the future.
Vitamin D and cytokines are intrinsic factors, whereas infection is an extrinsic factor that influences cytokine production in the host. We tried to identify vitamin D deficiency as an additional factor contributing to immune dysfunction in a particular group of diabetic patients, i.e. with foot infection having a glycaemic status similar to that of diabetic patients without foot infection. The negative association between vitamin D and cytokine concentrations in diabetic patients was found to be disrupted when cytokine responses escalated in response to foot infection. The cross-sectional design of the present study is a limitation and therefore the results need to be substantiated by an ex vivo experiment on immune cell responses to vitamin D stimulation and cytokine production.
In conclusion, diabetic foot infection represents a state where inflammatory responses occur in an uncontrolled manner owing to the pathological bad trio of hyperglycaemia, wound and infection. Vitamin D deficiency escalated inflammatory cytokine release in patients with diabetic foot infection, particularly when its serum concentrations were very low. Therefore, we suggest a 25-hydroxyvitamin D concentration value < 25 nmol/l as the ‘cut-off’ for unfavourable immunological alterations in patients with diabetes mellitus. Furthermore, we propose atypical interplay of inflammatory cytokines and vitamin D to have clinical implications for patients with diabetic foot infection.
Acknowledgements
The authors thank Mr Rammohan Rayicherela, former Biostatistician at Banaras Hindu University and Senior Associate Biostatistics at Cognizant Technology Solutions, for helping with the statistical analysis. They also thank Ms Spandana Birajdar, Sub-editor, Diabetes Health Magazine, Chellaram Diabetes Institute, Pune, India, for language corrections.
The present study was funded by the Indian Council of Medical Research (ICMR), New Delhi, India, in the form of a grant (IRIS ID no. ICMR/RHN/2008-04670) to S. K. S. for conducting the study. The ICMR has no role in the design and analysis of the study and in the writing of the article. S. T. and D. D. P. thank the University Grant Commission (UGC), New Delhi, India, for providing Research Fellowships.
The authors’ contributions are as follows: S. T. collected the data, carried out the experiments, analysed the data, and drafted the manuscript; D. D. P. collected and compiled the data and helped with the experiments and manuscript preparation; S. K. G. provided the samples and edited the manuscript; S. K. S. designed the study, gained support for conducting the study, provided the samples and resources for the study and edited the manuscript. Part of the study has been presented in ENDO 2011 meeting by S. K. S.
The authors declare no potential conflicts of interest.







