Up to 80% of individuals being treated with antipsychotics suffer from medication-induced weight gain. Reference Green, Patel, Goisman, Allison and Blackburn1 The magnitude of this weight gain may be substantially higher than usually reported. Reference Álvarez-Jiménez, González-Blanch, Crespo-Facorro, Hetrick, Rodriguez-Sanchez, Perez-Iglesias and Vázquez-Barquero2 Young people experiencing a first episode of psychosis are particularly susceptible to rapid and pronounced weight gain. Reference Zipursky, Gu, Green, Perkins, Tohen, McEvoy, Strakowski, Sharma, Kahn, Gur, Tollefson and Lieberman3 Weight gain has become a major concern in the treatment of psychosis because it may adversely affect treatment adherence and clinical outcomes and is associated with reduced quality of life, social stigma, and greater morbidity and mortality. Reference Wirshing4
As a result, there has been a growing interest in developing treatment alternatives to control or attenuate weight gain. A recent review of interventions to reduce weight gain in schizophrenia concluded that there was insufficient evidence to support the general use of adjunctive pharmacological interventions. Reference Faulkner, Cohn and Remington5 Therefore, the present study aimed to undertake a systematic review and meta-analysis of all relevant randomised controlled trials (RCTs) of non-pharmacological interventions to control antipsychotic-induced weight gain in patients with first-episode or chronic schizophrenia.
Method
Search strategy
Systematic bibliographic searches were performed to find relevant English and non-English language trials from the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), Medline, EMBASE, PsycINFO, CINAHL, UMI Proquest Digital Dissertations, Information Science Citation Index Expanded (SCI–EXPANDED), Information Social Sciences Citation Index (SSCI), Information Arts and Humanities Citation Index (A&HCI) and registers of ongoing clinical trials, with each database being searched from inception to May 2007. We additionally searched conference abstracts from ISI Science and Technology proceedings, and ISI Information Social Science and Humanities proceedings. The abstracts, titles and index terms of studies were searched using the following keywords: ‘weight gain’, ‘weight loss’, ‘weight change’ and ‘body weight’ in conjunction with ‘exercise’, ‘psychoeducation’, ‘intervention’, ‘diet’, ‘behavioural therapy’, ‘cognitive therapy’, ‘physical therapy’, ‘group intervention’, ‘management’, and ‘schizophrenia’ or ‘psychosis’. Further papers were found by hand-searching the references of all retrieved articles and previous reviews. We also screened hand-searched copies of the following journals (from January 2000): British Journal of Psychiatry, Journal of Clinical Psychiatry, American Journal of Psychiatry, Schizophrenia Bulletin, Schizophrenia Research and Journal of Clinical Psychopharmacology.
Study selection
Considered for inclusion were RCTs of a specific non-pharmacological adjunctive intervention aimed at preventing or controlling antipsychotic-induced weight gain, with at least 75% of participants diagnosed with schizophrenia-spectrum disorders using either DSM or ICD criteria. Comparison interventions could include either standard care or an active comparator intervention. Participants could be both young adults with recent-onset psychosis and adults with chronic schizophrenia, hospitalised or out-patients, during treatment with first- or second-generation antipsychotics. The primary outcome was considered to be mean change in body weight and body mass index (BMI) by the end of intervention, with secondary outcome measures including mean change in both body weight and BMI by follow-up. Additional secondary outcome measures comprised mean change on ratings of quality of life, medication adherence and relapse rates.
Two reviewers (M.A.-J. and C.G.-B.) independently assessed all potentially relevant articles for inclusion. Any disagreements were resolved through discussion.
Data extraction
Two reviewers (S.H. and M.A.-J.) independently extracted relevant data from included trials, including treatment approach (prevention of weight gain v. weight loss), the nature of the intervention (cognitive–behavioural therapy, CBT) v. nutritional counselling (psychoeducation, diet and exercise), treatment format (group v. individual), intervention provider, length of intervention, participants' characteristics, comparison intervention, antipsychotic type and dosage. Additional extracted information included measures of quality of life, medication adherence and relapse rates. Any discrepancies were resolved by consensus. Authors were contacted for the provision of missing data if necessary for the meta-analysis and to determine the eligibility of several studies.
Assessment of methodological quality
Trials were assessed against the following quality criteria: random sequence generation, allocation concealment, masked assessment of outcomes, number of withdrawals, intention-to-treat analysis and manual-based intervention. A maximum credit of five points was given if random allocation and allocation concealment were adequate, outcome was assessed by masked raters, data were assessed according to the intention-to-treat principle and the intervention was manualised.
Statistical analyses
Outcomes were pooled using MetaView, meta-analytic standard software used by the Cochrane Collaboration (RevMan 4.2.9 (PC version), Cochrane Collaboration, Oxford, England). Given that weight and BMI are continuous outcome measures, the weighted mean difference (WMD) was estimated using a fixed-effect meta-analysis with 95% confidence intervals for both end-of-treatment and follow-up time points. We conducted one primary comparison (non-pharmacological interventions v. treatment as usual) and three subgroup comparisons (preventive v. weight loss interventions; individual v. group therapy; CBT v. nutritional counselling). We further examined treatment effects according to sample characteristics (recent-onset psychosis v. chronic schizophrenia). To investigate treatment effects in different subgroups the overlap of the confidence intervals of the summary estimates was considered. In addition, the significant differences between subgroups were explored following the method of Deeks et al. Reference Deeks, Altman, Bradburn, Egger, Smith and Altman6 This method is based on the chi-squared statistic test for heterogeneity. The statistic estimated is compared with a chi-squared distribution to test the significant difference between subgroups.
We assessed heterogeneity of intervention estimates by visually inspecting the overlap of confidence intervals on the forest plots and by the I-squared statistic. The I Reference Álvarez-Jiménez, González-Blanch, Crespo-Facorro, Hetrick, Rodriguez-Sanchez, Perez-Iglesias and Vázquez-Barquero2 -test of heterogeneity describes the proportion of total variation in study estimates that is due to heterogeneity. Reference Higgins, Thompson, Deeks and Altman7 If there was evidence of inconsistency of estimates across trials, a random-effects meta-analysis was fitted. Reference DerSimonian and Laird8 Random effects are, in general, more conservative than fixed-effects models because they take heterogeneity among studies into account. With decreasing heterogeneity the random-effects approach moves asymptotically towards a fixed-effects model. Additionally, data from included trials were entered into a funnel graph (trial effect v. trial size) in order to investigate the likelihood of overt publication bias. Reference Egger, Smith, Schneider and Minder9 In the absence of bias, the plot should resemble a symmetrical inverted funnel. Reference Light and Pillemer10 If publication bias exists it is expected that, of published studies, the largest ones will report the smallest effects. Reference Egger, Smith and Phillips11
Sensitivity analyses were performed to further assess the robustness of the findings to the choice of statistical method (fixed- or random-effects model), the exclusion of the lowest-quality trials (trials with a quality score lower than 1) and the exclusion of the smallest trials (trials with a sample size of less than 40 participants).
Results
Of 28 studies retrieved, 10 were eligible for inclusion. We excluded 5 studies that did not include comparison groups; Reference Centorrino, Wurtman, Duca, Fellman, Fogarty, Berry, Guay, Romeling, Kidwell, Cincotta and Baldessarini12–Reference Knox16 6 studies that were non-randomised; Reference Ball, Coons and Buchanan17–Reference Vreeland, Minsky, Menza, Rigassio Radler, Roemheld-Hamm and Stern22 2 RCTs that did not fully describe the sample characteristics and further information could not be obtained; Reference Harmatz and Lapuc23,Reference Rotatori, Fox and Wicks24 1 RCT after the authors confirmed that less than 75% of the sample had a diagnosis of schizophrenia-spectrum disorders; Reference Brown, Goetz, Van Sciver, Sullivan and Hamera25 1 RCT that reported 90% withdrawal rates and did not provide comparison group data; Reference Archie, Wilson, Osborne, Hobbs and McNiven26 1 RCT that only measured eating habits and did not provide body weight or BMI changes; Reference McCreadie, Kelly, Connolly, Williams, Baxter, Lean and Paterson27 and 1 which did not provide data in a usable format and we were unable to obtain further information. Reference Beebe, Tian, Morris, Goodwin, Allen and Kuldau28
Six of the included trials investigated cognitive–behavioural intervention strategies; Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29–Reference Weber and Wyne34 three nutritional counselling interventions; Reference Evans, Newton and Higgins35–Reference Scocco, Longo and Caon37 and one combined nutritional and exercise interventions. Reference Wu, Wang, Bai, Huang and Lee38 Five trials tested group intervention formats Reference Brar, Ganguli, Pandina, Turkoz, Berry and Mahmoud30,Reference Khazaal, Fresard, Rabia, Chatton, Rothen, Pomini, Grasset, Borgeat and Zullino31,Reference McKibbin, Patterson, Norman, Patrick, Jin, Roesch, Mudaliar, Barrio, O'Hanlon, Griver, Sirkin and Jeste33,Reference Weber and Wyne34,Reference Littrell, Hilligoss, Kirshner, Petty and Johnson36 and five examined individual interventions. Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29,Reference Kwon, Choi, Bahk, Yoon Kim, Hyung Kim, Chul Shin, Park and Geun Oh32,Reference Evans, Newton and Higgins35,Reference Scocco, Longo and Caon37,Reference Wu, Wang, Bai, Huang and Lee38 Four studies aimed to prevent antipsychotic-induced weight gain Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29,Reference Evans, Newton and Higgins35–Reference Scocco, Longo and Caon37 and six aimed to reduce body weight in those who had already experienced weight increase. Reference Brar, Ganguli, Pandina, Turkoz, Berry and Mahmoud30–Reference Weber and Wyne34,Reference Wu, Wang, Bai, Huang and Lee38 Data could be extracted and pooled in meta-analyses from seven of the ten eligible studies. In three studies we were able to pool relevant data with the help of the authors. Reference Khazaal, Fresard, Rabia, Chatton, Rothen, Pomini, Grasset, Borgeat and Zullino31,Reference McKibbin, Patterson, Norman, Patrick, Jin, Roesch, Mudaliar, Barrio, O'Hanlon, Griver, Sirkin and Jeste33,Reference Scocco, Longo and Caon37
Interventions lasted between 8 weeks and 6 months with efficacy measures taken at the completion of the trial intervention. Three studies reported follow-up periods ranging from 2 to 3 months after the end of the intervention. Reference Khazaal, Fresard, Rabia, Chatton, Rothen, Pomini, Grasset, Borgeat and Zullino31,Reference Evans, Newton and Higgins35,Reference Littrell, Hilligoss, Kirshner, Petty and Johnson36 With one exception, Reference Wu, Wang, Bai, Huang and Lee38 all trials were carried out in out-patient settings. Only one trial utilised a sample of patients with recent-onset psychosis. Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29 Trials were conducted in Europe, Asia, the USA and Australia. Study medications included a broad range of first- and second-generation antipsychotics. Other characteristics of the included trials are outlined in the online Table DS1.
Results for all non-pharmacological interventions
Ten trials involving 482 patients compared non-pharmacological interventions with treatment as usual. There was a statistically significant reduction in mean body weight for those in the non-pharmacological intervention groups compared with those on treatment as usual (WMD=–2.56 kg, 95% CI −3.20 to −1.92 kg, P<0.001) (Fig. 1). There was no evidence of statistical heterogeneity (I 2=28.9%).
Pooling treatment effects of mean BMI change across all interventions yielded similar significant results in favour of the non-pharmacological interventions (WMD=–0.91 kg/m2, 95% CI −1.13 to −0.68 kg/m2, P<0.001), with no evidence of statistical heterogeneity (I 2=13.8%).
Follow-up outcomes
Three trials incorporated follow-up measures ranging from 2 months Reference Littrell, Hilligoss, Kirshner, Petty and Johnson36 to 3 months. Reference Khazaal, Fresard, Rabia, Chatton, Rothen, Pomini, Grasset, Borgeat and Zullino31,Reference Evans, Newton and Higgins35 Pooling treatment effects of mean change in body weight and in BMI demonstrated that the statistically significant advantages of non-pharmacological interventions were maintained at follow-up (WMD=–4.14 kg, 95% CI −5.80 to −2.49 kg, P<0.001). Although one trial Reference Evans, Newton and Higgins35 with high discontinuation rates at follow-up (n=31; 61%) reported results only for those who completed follow-up assessment, exclusion of this trial resulted in equivalent treatment effects.

Fig. 1 Efficacy of weight-management interventions aimed at preventing weight gain and those designed to reduce body weight v. treatment as usual (TAU). WMD, weighted mean difference.
Subgroup analyses
Prevention of antipsychotic-induced weight gain v. weight loss
Trials were analysed according to whether they aimed to prevent antipsychotic-induced weight gain or whether they were designed to reduce weight in patients who were already overweight or obese (Fig. 1). Although there was evidence of some statistical heterogeneity among trials that intended to reduce weight gain (I 2=51.0% v. I 2=0.0% among those aimed to prevent weight gain) treatment effects were similar. Furthermore, when a random-effects model was fitted there was little change on the subgroup overall estimates (WMD=–2.32 kg, 95% CI −3.10 to −1.54 kg, P<0.001 v. WMD=–2.37 kg, 95% CI −3.54 to −1.21 kg, P<0.001 using a random-effects model). Trials that aimed to prevent weight gain appeared to show a slightly larger effect on mean body weight change than those designed to reduce weight (Fig. 1). However, the confidence intervals of the summary treatment estimates overlapped to an important degree. Subsequently, the approach described by Deeks et al Reference Deeks, Altman, Bradburn, Egger, Smith and Altman6 showed that there was no statistically significant difference between both subgroups (χ2=1.10, P=0.29).
Group v. individual therapy
The effect of intervention format was examined by analysing separately trials of group interventions and trials of individual approaches (Fig. 2). Although there was some evidence of inconsistency among group intervention trials (I 2=56.8% v. I 2=0.0% among individual intervention trials), estimates were similar. In addition, when a random-effects meta-analysis was fitted there was little effect on the subgroup overall estimates (WMD=–2.09 kg, 95% CI −3.05 to −1.13 kg, P<0.001 v. WMD=–2.30 kg, 95% CI −3.82 to −0.78 kg, P<0.001 fitting the random-effects model). Studies evaluating individual interventions seemed to show more benefit than the group intervention studies (Fig. 2). Again, visual examination of the confidence interval of the summary estimates indicated some degree of overlapping which was further confirmed by the lack of significant difference between subgroups (χ2=1.67, P=0.20).
Cognitive–behavioural therapy v. nutritional counselling interventions
Trials were analysed by type of non-pharmacological intervention: CBT v. nutritional counselling (Fig. 3). Although CBT trials appeared to show a smaller effect compared with nutritional counselling intervention trials (WMD=–2.14 kg, 95% CI −2.98 to −1.30 kg, P<0.001 v. WMD=–3.12 kg, 95% CI −4.10 to −2.14 kg, P<0.001 respectively), the confidence interval of the summary effects overlapped and there was no statistically significant difference between the subgroups (χ2=2.22, P=0.14).
Recent-onset psychosis v. chronic schizophrenia
Finally, trials were examined according to the characteristics of the sample: recent-onset psychosis v. chronic schizophrenia (Fig. 4). The only trial that evaluated an early intervention in young patients with recent-onset psychosis found that weight gain could be significantly attenuated (WMD=–2.80 kg, 95% CI −4.93 to −0.67 kg, P<0.01). Similar treatment effects were obtained in trials with participants with chronic schizophrenia (WMD=–2.54 kg, 95% CI −3.20 to −1.87 kg, P<0.001).
Additional outcome measures
Only two trials provided data regarding the impact on quality of life of these interventions. Know et al Reference Kwon, Choi, Bahk, Yoon Kim, Hyung Kim, Chul Shin, Park and Geun Oh32 did not find differences between the groups in terms of quality of life (only a trend towards statistical difference in the physical score changes), but Evans et al Reference Evans, Newton and Higgins35 reported significant differences in favour of the treatment group in subjective improvement in quality of life.
Finally, no trials reported data regarding the influence of weight-management interventions on medication adherence.

Fig. 2 Efficacy of individual and group weight-management interventions v. treatment as usual (TAU). WMD, weighted mean difference.
Assessment of risk of bias
A description of the conduct of the trials included in the metaanalysis and assessment of the risk of bias is presented in the online Table DS2. Few trials gave explicit reports of trial conduct; one described the generation of random sequences, Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29 only one fully disclosed allocation concealment, Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29 and a few provided explicit description of who was masked. The attrition rate for the 10 trials varied between 0 and 50% in the control groups, and 0 and 20.7% in the intervention groups. Only two trials Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29,Reference Littrell, Hilligoss, Kirshner, Petty and Johnson36 appeared to include all randomised patients in their analysis. Four trials were conducted using manual-based interventions.
To determine the influence of study quality on the overall estimates, we performed stratified analysis according to methodological quality. The four low-quality trials (0 points) Reference Kwon, Choi, Bahk, Yoon Kim, Hyung Kim, Chul Shin, Park and Geun Oh32,Reference Evans, Newton and Higgins35,Reference Scocco, Longo and Caon37,Reference Wu, Wang, Bai, Huang and Lee38 showed more benefit than the higher-quality trials (WMD=–2.96 kg, 95% CI −3.90 to −2.03 kg). Exclusion of these studies, however, affected the overall effect and the confidence intervals only marginally (WMD=–2.21 kg, 95% CI −3.08 to −1.33 kg).

Fig. 3 Efficacy of cognitive–behavioural and nutritional interventions v. treatment as usual (TAU). WMD, weighted mean difference. Statistical pooling used a fixed-effects statistical model for this outcome.
Publication bias
The funnel plot showed evidence of mild asymmetry (Fig. 5). The smallest studies (fewer than 40 participants included in the analysis) Reference Weber and Wyne34,Reference Evans, Newton and Higgins35,Reference Scocco, Longo and Caon37 showed slightly larger effects (WMD=–3.00 kg, 95% CI −4.53 to −1.46 kg). However, exclusion of the smallest studies had little effect on the overall estimate WMD=–2.47 kg, 95% CI −3.17 to −1.77 kg).
Discussion
Adjunctive non-pharmacological interventions are effective in reducing or attenuating antipsychotic-induced weight gain when compared with treatment as usual in patients with schizophrenia-spectrum disorders. These findings with regard to reduction in mean body weight were confirmed by similar reductions in BMI, which is considered to be a better indicator of obesity and being overweight. Furthermore, treatment effects may be maintained at follow-up.

Fig. 4 Efficacy of non-pharmacological interventions v. treatment as usual (TAU) in participants with recent-onset psychosis or with chronic schizophrenia. WMD, weighted mean difference.
Effects of intervention modality
Results from this study showed no statistically significant or practically important differences between therapeutic approaches, either individual compared with group interventions, or CBT compared with nutritional counselling. Conversely, there is evidence that suggests that adherence to weight-management programmes is positively correlated with further weight loss. Reference Pendlebury, Bushe, Wildgust and Holt39 The choice of therapeutic approach will depend, then, on those factors that are likely to engage patients in a therapeutic alliance in order to produce associated losses. It is plausible, however, that particular patient age groups have different needs (e.g. young people may have different developmental needs to those who develop psychosis later in life) with regard to engagement in psychological treatments. Reference Haddock, Lewis, Bentall, Dunn, Drake and Tarrier40 Adventure- and recreation-based interventions, for instance, have been shown to be acceptable for individuals with chronic schizophrenia and may increase treatment adherence and promote further occupational and social gains. Reference Voruganti, Whatham, Bard, Parker, Babbey, Ryan, Lee and MacCrimmon21 Similarly, preventive, multicomponent and flexible approaches that included exercise, diet and behavioural interventions have shown to be highly acceptable for young people with recent-onset psychosis. Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29 Thus, the tailored combination of weight-management techniques in a flexible and innovative manner which addresses individual needs and promotes therapeutic alliance is likely to produce best outcomes.
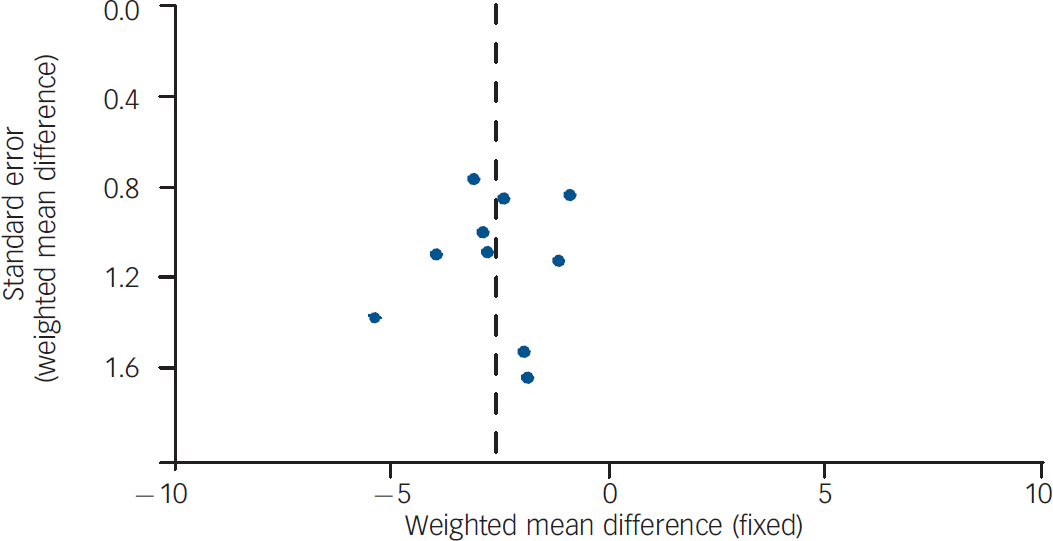
Fig. 5 Funnel plot of 10 randomised controlled trials of non-pharmacological weight-management intervention.
Weight gain induced by antipsychotics and first-episode psychosis
To date, only one RCT has shown the effectiveness of preventive strategies in attenuating antipsychotic-induced weight gain in a young cohort with recent-onset psychosis. Reference Álvarez-Jiménez, González-Blanch, Vázquez-Barquero, Pérez-Iglesias, Martínez-García, Pérez-Pardal, Ramírez-Bonilla and Crespo-Facorro29 Although there are few studies, it seems apparent that there is great potential for interventions aimed at early stages, before weight gain takes place. Weight gain is arguably a greater problem for young people experiencing a first episode of psychosis. This group is considered to be especially susceptible to substantial weight gain, Reference Álvarez-Jiménez, González-Blanch, Crespo-Facorro, Hetrick, Rodriguez-Sanchez, Perez-Iglesias and Vázquez-Barquero2 which could interfere with the early recovery process. First, younger populations are already less disposed to adhering to medication regimes Reference Coldham, Addington and Addington41 and potential weight gain may exacerbate non-adherence. Second, the physical changes produced by weight gain may result in social discrimination and stigma as young patients are more sensitive to issues of body image and self-esteem than their older counterparts. Reference Gortmaker, Must, Perrin, Sobol and Dietz42 Early interventions could prevent or attenuate this medication side-effect as well as the adverse consequences derived from weight gain.
This is consistent with a clinical staging model where treatment effects are thought to be the greatest when delivered as early as possible. Reference McGorry, Hickie, Yung, Pantelis and Jackson43 Two fundamental assumptions underlie this model. First, patients in the earliest stages of schizophrenia have a better response to treatment and a better prognosis than those in later stages. Second, treatments offered in the early stages should be more benign as well as more effective. Given this background, preventive weight-management interventions have the potential to be more effective, acceptable, cost-efficient and beneficial.
Clinical implications
How clinically meaningful is a weight loss of 2.6 kg? Several authoritative bodies, such as the Institute of Medicine, 44 have implied that weight losses of as little as 5% in individuals at risk of metabolic syndromes can result in clinically meaningful reductions in morbidity and risk of early mortality. The majority of individuals with schizophrenia experience clinically significant weight gain, which is associated with greater risk of developing several diseases, including diabetes, hypertension and coronary heart disease. As a result, people with schizophrenia have a 20% shorter life expectancy than the population at large. Reference Marder, Essock, Miller, Buchanan, Casey, Davis, Kane, Lieberman, Schooler, Covell, Stroup, Weissman, Wirshing, Hall, Pogach, Pi-Sunyer, Bigger, Friedman, Kleinberg, Yevich, Davis and Shon45 In this review, the average baseline weight was approximately 80 kg (ranging from 66.5 to 101.3 kg). Therefore, even a weight loss of 1.9–3.2 kg represents a reduction of 2.5–4.0% of initial body weight in a significant number of patients. It may be plausible, then, to expect that these reductions in body weight could result in corresponding reductions in morbidity and early mortality.
Limitations of the study
This study has some limitations. First, most of the trials included short-term follow-up periods. As a result we could not draw conclusions on the long-term effectiveness of these interventions. Second, reporting on generation of random sequence, allocation concealment, intention-to-treat analyses and masking was poor, making assessment of the potential for biased estimates of treatment effect difficult. Reference Higgins, Thompson, Deeks and Altman7 Given the relationship between poor reporting and larger treatment effects, Reference Juni, Altman and Egger46 findings reported by these trials may have overestimated summary treatment effects. Third, it must be noted that subgroup analyses are observational in their nature and are not based on randomised comparisons. Moreover, some of these comparisons were limited by the sample size. Therefore, differences between treatment modalities need to be explored in adequately designed RCTs. Furthermore, there was evidence of skew in the data provided by several trials included in the present review. Meta-analytic techniques frequently face the problem of managing non-parametric data. Although there is not a clear consensus regarding the resolution of this statistical issue, we note the limitations of our analysis in accounting for skewed data. Another limitation relates to the generalisability of the findings to clinical practice. Therapists in clinical trials are highly motivated and skilled in the implementation of the intervention being tested, which may affect the generalisability of the results to the population of therapists. As a result, these findings need to be evaluated in pragmatic trials of intervention effectiveness in a range of clinical settings. Finally, as with all systematic reviews, publication bias is a potential source of error. Although there was some evidence of such bias, exclusion of the smallest studies only marginally affected the overall effect.
Strengths of the study
Although it is plausible that some studies assessing non-pharmacological interventions to manage antipsychotic-induced weight gain were not discovered by our literature search, our procedures kept this to a minimum. We conducted a thorough search of the electronic literature, including databases that contain unpublished literature, undertook hand-searches and made efforts to access grey literature. Another common problem in meta-analysis is incomplete reporting of consistent outcome data in primary articles. We minimised the impact of such incomplete reporting by contacting authors when feasible.
This review includes several trials not included in previous meta-analysis of weight-management interventions, Reference Faulkner, Cohn and Remington5 a focus on non-pharmacological approaches with careful evaluation of different treatment strategies and an assessment of trial conduct and potential risk of bias. Although previous systematic reviews have also suggested the effectiveness of healthy living interventions in patients with schizophrenia, Reference Bradshaw, Lovell and Harris47 they included a limited number of RCTs as well as quasi-experimental studies and did not perform meta-analytic techniques. Furthermore, we found a notable consistency across all study estimates, which was reflected in the robustness of the findings across analytic methods and when the smallest and lowest-quality studies were excluded.
Implications for future research
Although the results from this study suggest that non-pharmacological interventions may be effective in reducing antipsychotic-induced weight gain, further research needs to address several salient issues. Given the adverse impact of weight gain on medication adherence and relapse rates, Reference Weiden, Mackell and McDonnell48 quality of life, Reference Allison, Mackell and McDonnell49 social stigma and discrimination Reference Aronne50 as well as self-esteem, Reference Crisp, Gelder, Rix, Meltzer and Rowlands51 interventions to prevent weight gain have the potential to reduce these negative effects. Even though these outcomes were not consistently reported or measured, there is some evidence that nutritional counselling improves quality of life, overall health and body image. Reference Evans, Newton and Higgins35 Further, CBT may promote client satisfaction Reference Brar, Ganguli, Pandina, Turkoz, Berry and Mahmoud30 and physical well-being. Reference Kwon, Choi, Bahk, Yoon Kim, Hyung Kim, Chul Shin, Park and Geun Oh32 Moreover, we are aware of no data that would allow precise quantification of the impact of weight-management interventions on adherence to medication regimens, subsequent relapse rates and other salient aspects such as perception of social stigma and social isolation. Further research should investigate these issues in order to fully elucidate all the potential benefits of these interventions.
Well-designed trials are required, including further comparison studies of one type of treatment against another. These trials should also address fundamental questions such as the effects of longer interventions and booster sessions, long-term maintenance of outcomes, intervention effects on clinical morbidity and physical health, as well as their cost-effectiveness. In addition, the development and evolution of preventive treatment strategies is critical. Future interventions should be innovative and encourage engagement with therapy by promoting well-being and global recovery.
Acknowledgements
The present study has been funded by a grant from the Marqués de Valdecilla Public Foundation – Marqués de Valdecilla Research Institute (FMV–IFIMAV), Santander, Spain. The authors acknowledge Sara Gook of ORYGEN Research Centre, University of Melbourne, for helpful comments on an earlier draft of this paper. We also thank authors who provided additional information, including Dr Yasser Khazaal, Dr Christine McKibbin, Dr Paolo Scocco and Dr Steve Brown.








eLetters
No eLetters have been published for this article.