INTRODUCTION
Listeriosis is a serious foodborne infection with a mortality rate of about 10–44% in foodborne outbreak cases [Reference Farber and Peterkin1]. Cases occur predominantly in the elderly, immunocompromised, and pregnant women and their neonates. Neonatal infection follows maternal sepsis and chorioamnionitis and can result in abortion, stillbirth, premature delivery or neonatal meningitis in those developing illness in the weeks after delivery. Common manifestations of listeriosis in adults include meningoencephalitis and a wide range of focal infections in organ systems and prostheses [Reference Schlech2].
In Australia, the incidence of listeriosis is low with about 2·5–3·6 cases per million population per annum. Doctors and laboratories notifiy about 50–60 non-perinatal and 5–10 materno-fetal infections to health departments annually [3]. Multiple risk factors and high-risk foods have been identified from outbreak investigations, case-control studies, and risk assessments overseas. High-risk foods include ready-to-eat foods, which are often of animal origin, and ‘delicatessen style’ processed foods such as processed meats, soft cheeses and smoked seafood, and a wide range of fruit and vegetable products [4]. Food safety authorities worldwide use these findings to develop advice for populations particularly vulnerable to listeriosis, such as the immunocompromised, pregnant women, and the elderly, and to guide food safety programmes. Most cases of listeriosis in Australia appear to be sporadic and local evidence for risk factors for listeriosis are limited to investigations of six clusters over the last 30 years that have been associated with mussels, processed meats, sandwiches and fruit salad [Reference Dalton5, Reference Mitchell6]. The aim of this case-control study was to identify dietary, medical and behavioural risk factors for invasive listeriosis in Australia and describe the spectrum of illness in patients with listeriosis.
METHODS
From November 2001 to December 2004, Australian State and Territory health departments attempted to interview all notified cases of listeriosis within 1 month of collection of a specimen that was positive by culture for Listeria monocytogenes. Listeriosis is a laboratory notifiable condition in all states and territories of Australia based on the culture of L. monocytogenes from a normally sterile site. Perinatal cases were defined as illness in a pregnant woman, fetal loss, or illness in a baby aged <3 months with isolation of L. monocytogenes from at least one of the materno-fetal pair from any of the following sites: the placenta or products of conception, fetal gastrointestinal contents, or a normally sterile site from a pregnant woman, fetus or baby. Pregnant women were recruited to case status for the purpose of identifying risk factors for infection. Non-perinatal cases were defined as all other cases with L. monocytogenes isolated from blood, cerebrospinal fluid or other normally sterile site. Cases were excluded if they were: (1) asymptomatic, except for perinatal mothers, (2) part of an outbreak associated with an identified food vehicle, (3) unable to be contacted within 4 weeks of culture date, and (4) where the treating doctor or family member refused participation.
We attempted to recruit two controls per case, except in Victoria where only cases were recruited to the study. For non-perinatal cases, we attempted to recruit two age-matched controls per case from clinics specializing in the treatment of the case's primary underlying immune condition. Where there was no known immunocompromising condition, age-matched controls were selected from a general practitioner's clinic. Perinatal controls were recruited from hospital-based antenatal clinics and had gestational attainment within 4 weeks of the matched case at the time of the positive culture. All controls were recruited within 4 weeks of conducting the case interview.
Interviewers conducted telephone interviews with cases, or their surrogates, and controls using a standard questionnaire. Information on the case's major underlying immunocompromising condition was obtained from the treating doctors, while other risk factors were elicited from the patient or a surrogate. Participants were asked about consumption of 43 food items, considered to be high risk for listeriosis in previous studies, that were consumed in the 4 weeks prior to specimen collection date for cases and the corresponding 4 weeks for perinatal controls and the 4 weeks prior to interview date for non-perinatal controls. Information on the frequency of consumption (weekly, 2–3 times per month, or monthly), place of preparation, site of purchase or consumption, and, where appropriate, whether eaten raw or cooked were collected for each item. Prior hospitalization was defined as 7–28 days prior to specimen collection date for cases or interview date for controls.
Descriptive, univariate and multivariate logistic regression analyses were conducted using Stata Intercooled version 10 (Stata Corp., USA) for both perinatal and non-perinatal cases and controls. All case-control analyses were matched. Conditional logistic regression was used for non-perinatal data and all variables with P values ⩽0·25 were included in multivariate models for further assessment. Final multivariate models were derived using a forwards stepwise approach. Model specification was assessed using the link test. Exact (matched) logistic regression was used for the perinatal data.
Within each jurisdiction, ethics approval was gained through the relevant State or regional ethics committees.
RESULTS
We recruited 136 cases from 1 November 2001 to 31 December 2004. Cases were recruited in all States and Territories with 113 (83%) recruited cases resident in NSW (53), Victoria (34), Western Australia (26). Cases in other jurisdictions were as follows: Queensland (14), South Australia (5), ACT (2), Northern Territory (1) and Tasmania (1). Jurisdictions began recruiting at different times; however, we recruited 136 (71%) of the 193 incident cases that were notified once jurisdictions had commenced the study. When all jurisdictions were participating 52/69 (75%) cases notified nationally in 2003 and 47/67 (70%) cases notified in 2004 were recruited. No outbreaks were detected during the study period.
There were 19 (14%) perinatal cases of which L. monocytogenes was isolated from the materno-fetal pair in 10 cases, from the mother only in two cases and from the fetus only in seven cases. Median fetal gestational age at diagnosis was 35 weeks (range 18–40) with a total of four deaths between 18 and 32 weeks for a fetal case-fatality rate of 21%.
Of the 117 non-perinatal cases, 55 (47%) were female, 87 (74%) were aged ⩾60 years and 47 (40%) were aged ⩾75 years. The site of culture was blood for 92 (79%), cerebrospinal fluid for 18 (15%), and other sites for seven (6%) cases. Twenty-two cases died giving a non-perinatal case-fatality rate of 19%.
Fever, chills, and headache were reported more frequently in pregnant women than non-perinatal cases, and diarrhoea was more often reported by non-perinatal cases (Fig. 1).
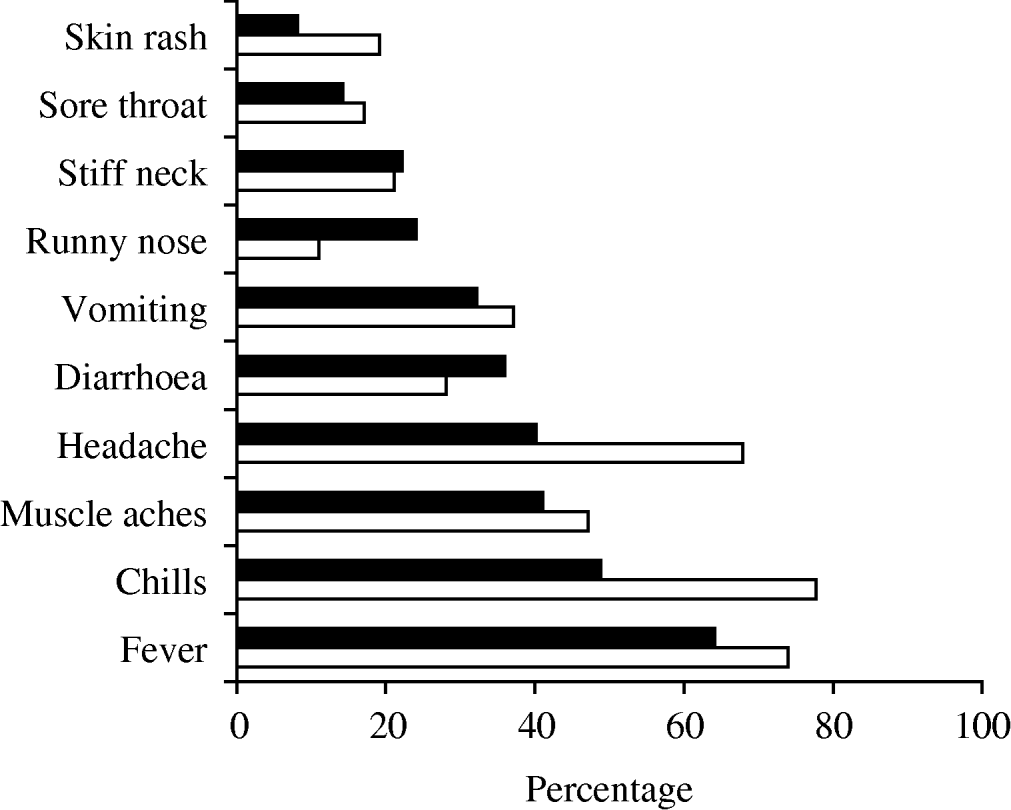
Fig. 1. Prevalence of symptoms in non-perinatal (▪) and perinatal (□) cases in the 4 weeks before specimen collection, Australia 2001–2004.
Of all cases for whom information was available, 50/129 (39%) cases came from households where a Language Other Than English (LOTE) was spoken and 42/88 cases (48%) were born in countries other than Australia. The geographic region of birth for foreign-born cases was Europe (30 cases, predominantly Western and Southern Europe), Asia (eight cases), Middle East (one case) and Oceania (two cases) with missing data for one. The region of birth for 37 cases identified as living in households where a LOTE was spoken were Western Europe (15, 41%), Central Eastern Europe (5, 14%), Asia (6, 16%), Oceania (2, 5%) and Australia (9, 24%). Nine of 136 (7%) cases were from rural areas, and 15/131 (12%) were Aboriginal.
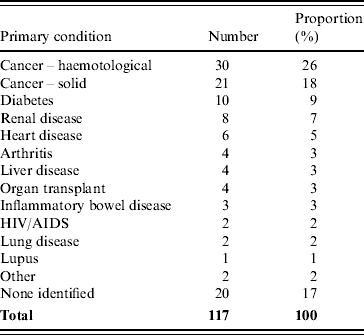
Treating doctors nominated the following conditions as the primary immunocompromising condition in the non-perinatal patients, haematological malignancy (26%), solid cancers (18%) diabetes (9%), and renal disease (7%) (Table 1).
Table 1. Primary underlying immunocompromising condition for non-perinatal listeriosis cases as reported by the case's treating doctor, Australia, 2001–2004
The most common immunocompromising medications used by non-perinatal cases in the 4 weeks prior to onset were antibiotics (37%), corticosteroids (36%), chemotherapy (26%), and gastric acid inhibitors (22%). Fifteen cases (13%) were residents of aged care institutions where meals were provided for them. Thirty-nine cases (33%) were admitted to hospital in the period 7–28 days prior to specimen collection.
Eighty-five controls were recruited for the 117 non-perinatal controls, 51 cases were matched to a control, 17 with one control each and 34 with two controls each. Twelve controls were recruited for the 19 perinatal cases, six cases were matched to two controls each. Controls for non-perinatal cases were recruited from NSW (55, 65%), Queensland (13, 15%), Western Australia (13, 15%), and South Australia (4, 5%). Controls for perinatal cases were recruited from NSW (8, 67%), and Western Australia (4, 33%). A higher percentage of non-perinatal cases (47%) than non-perinatal controls (38%) were female. A similar percentage of cases (74%) and controls (71%) were aged ⩾60 years.
In univariate analysis, risk factors for non-perinatal listeriosis cases included living in a household where a LOTE was spoken; prior hospitalization; and any one of antibiotic, prednisolone or gastric acid inhibitor administration in the 4 weeks prior to admission (Table 2), and consumption of liverwurst (Table 3). No food history was available for five of the non-perinatal cases.
Table 2. Case-specific risk factors for non-perinatal and perinatal listeriosis, Australia, 2001–2004
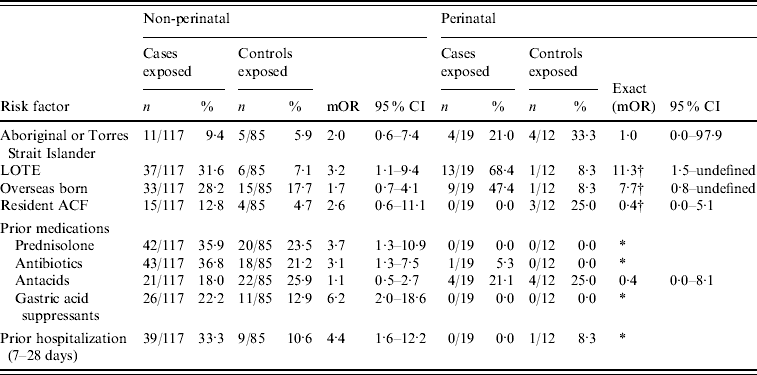
mOR, Matched odds ratio; CI, confidence interval; LOTE, language other than English; ACF, aged care facility.
* Unable to calculate as numerator or denominator=0 in matched analysis.
† Median unbiased estimates.
Table 3. Food-specific risk factors for non-perinatal and perinatal listeriosis
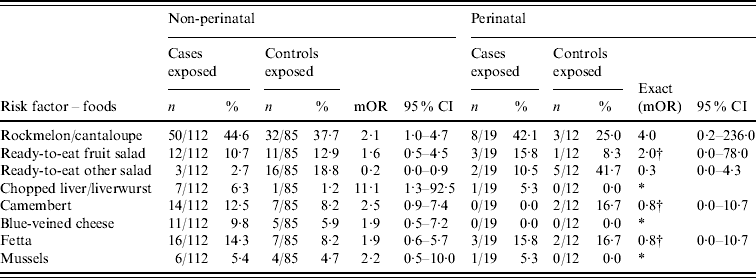
mOR, Matched odds ratio; CI, confidence interval.
* Unable to calculate as numerator or denominator=0 in matched analysis.
† Median unbiased estimates.
Multivariate analysis of non-perinatal cases identified the following risk factors for listeriosis: prior hospitalization (OR 4·3, 95% CI 1·0–18·3), use of gastric acid inhibitors (OR 9·4, 95% CI 2·4–37·4), and consumption of camembert (OR 4·7, 95% CI 1·1–20·6). Model specification was assessed using the link test and found to be acceptable. Analysis of risk factors for perinatal cases identified living in a household where a LOTE was spoken as the only risk factor associated with listeriosis (OR 11·3, 95% CI 1·5–undefined). No association was found between the frequency of consumption of any other individual foods and perinatal or non-perinatal listeriosis (data not shown). Many other case-specific and food-specific risk factors were examined but no meaningful associations with Listeria infection were identified (see Appendix Tables 1 and 2).
Of LOTE cases, only 1/8 (13%) perinatal cases and 1/19 (5%) non-perinatal cases reported that a healthcare worker had warned them to avoid specific foods to prevent Listeria prior to their illness with listeriosis. This compares with 3/5 (60%) perinatal cases and 10/61 (16%) non-perinatal cases in non-LOTE households.
Of the patients that were hospitalized or made clinic visits in the 2 months prior to their listeriosis diagnosis, the following high-risk foods were reportedly consumed during their visit, lettuce 11/39 patients (28%), diced chicken sandwiches 5/39 (13%), ham sandwiches 15/40 (38%), fresh fruit salad 8/39 (21%), soft cheese 4/39 (10%) rockmelon (cantaloupe) or strawberries 9/39 (23%). Eighteen of 45 (40%) cases consumed at least one of these high-risk foods during hospitalization. For these 18 cases, the following immunocompromising conditions were identified: age >75 years (1), haematological cancer (8), solid cancer (3), HIV/AIDS (1), heart disease (2), organ transplant (1), renal impairment (1), and mixed connective tissue disease (1).
DISCUSSION
This first national case-control study of listeriosis in Australia provides evidence to support existing listeriosis prevention policies but also highlights the need for an increased focus on preventing listeriosis in non-English speaking people and their families and increased attention to safety of food service in hospitals.
The associations between perinatal infections and living in a household where a LOTE was spoken and the tendency to report lower rates of being warned about the risks of listeriosis in this group suggest that a focus should be placed on preventing illness in non-English speaking pregnant mothers. Speaking a LOTE cannot, of itself, be a risk factor for listeriosis. It may be a surrogate for lack of information on how to prevent listeriosis infection or a cultural marker for the consumption of high-risk foods. The low number of controls reporting living in a household where a LOTE is spoken is probably due in part to bias introduced through lack of adherence to a systematic selection of controls leading to an artificially low rate in the controls. It is possible that clinics may have broken the protocol and preferentially approached potential controls with a good command of English leading to an overestimate of LOTE as a risk factor. Nevertheless, 68% of cases reported living in a household where a LOTE is spoken compared to only 21% of the community reporting speaking a LOTE in the 2006 Census which suggests a real association, perhaps through a surrogate pathway, between speaking a LOTE and listeriosis [7]. This supports the need for review of food safety warnings in languages other than English. The Food Standards Australia and New Zealand (FSANZ) website only provides a brochure on listeriosis and food in English [8]. Languages used in State and Territory brochures vary widely with some only including English while others provide up to 14 languages other than English. While the lower frequency of reporting prior Listeria prevention messages by LOTE cases compared to non-LOTE cases is not significant it is still a concerning finding and is consistent with lower availability of educational material in languages other than English.
The increased usage of gastric acid inhibitors in the month before illness in listeriosis cases compared to controls in our study is consistent with findings from other studies identifying acid suppression as a risk for bacterial enteric infection [Reference Neal9, Reference Leonard, Marshall and Moayyedi10]. While the association with gastric acid inhibitors may have been confounded by comorbid factors that were not appropriately controlled for in the matching process, at least one study found that current users, but not former users, of H2 antagonists were at increased risk for salmonellosis infection [Reference Neal9]. Additionally, antacid and cimetidine administration were associated with listeriosis infection in case-control studies that attempted to control for comorbid conditions [Reference Ho11]. Long-term H2 antagonist therapy has been associated with an increased risk of faecal carriage of L. monocytogenes and animal studies have confirmed that decreasing gastric acidity in rats increases the risk of L. monocytogenes infection [Reference Cobb12, Reference Schlech, Chase and Badley13].
Eighteen hospitalized patients with immunocompromising conditions consumed high-risk foods that Australian food safety agencies recommend should be avoided by persons at risk for listeriosis. Surveys of long-term care facilities in the USA have identified that high-risk foods are often served to residents aged >65 years [Reference Nelson14]. In October 2007, a new national food safety standard was gazetted that requires businesses and institutions that provide food service to vulnerable persons, such as in hospitals and aged care institutions, to implement a documented and audited food safety programme by October 2008 [15].
It is very difficult to provide robust food safety information to immunocompromised people, as they are not a homogeneous group and clinicians and patients cannot easily predict risk of infection in individuals. Pregnancy is an ideal time to provide counselling on the risk of Listeria infection and this complements counselling on healthy eating in pregnancy in antenatal clinics and classes. One study conducted in Australia found that only 13% of English-speaking pregnant women were able to identify all six high-risk foods in a survey and women from a non-English speaking background were 12 times more likely to select ‘don't know’ when asked about a range of high-risk foods [Reference Torvaldsen16].
Apart from the associations found with camembert and chopped liver/liverwurst consumption this study did not identify any other particular food as a substantial contributor to listeriosis. Identifying high-risk foods in a case-control study that spans multiple years is more challenging than in a single outbreak incident as the rate of contamination of even high-risk foods may vary widely over time. Subtyping of the Listeria isolates with comparison to food isolates may increase the chance of identifying high-risk foods with subtype concordance and efforts to uniformly subtype isolates from each jurisdiction are in progress. However, in Australia subtyping for listeriosis is usually only performed when outbreaks are identified. In future studies consideration should be given to the use of case-case comparison assisted by routine subtyping of all isolates – as used in the study by Varma et al. [Reference Varma17]. In the USA, PulseNet provides a national system for the uniform subtyping, comparison and archiving of L. monocytogenes strains [Reference Swaminathan18]. Such a system would assist the recognition and investigation of outbreaks and development of a standardized national database of clinical L. monocytogenes isolate subtypes in Australia should be a priority.
Our study may have had limited power to detect high-risk foods due to small numbers, particularly for perinatal cases, where we only recruited 19 cases and 12 controls.
The study conducted by Varma et al. [Reference Varma17] identified a significant association with melons consumed in a commercial establishment and hummus prepared in a commercial establishment. No link with acid-suppressing medication was identified. In this study an association with rockmelon or cantaloupe approached significance in the univariate analysis but not the multivariate analysis. Place of consumption was not collected for rockmelon, nor was hummus exposure.
There are many other limitations to this study. We had difficulty recruiting controls from busy clinics. Food recall over multiple weeks is difficult, and it is likely that this study examines usual food preferences rather than exact exposures. The use of proxies for deceased cases may have led to exposure misclassification.
Hospitals and aged care institutions should ensure that food safety programmes for food service to vulnerable persons comply with the new standard, particularly in regard to the exclusion of high-risk foods. Auditing of the programmes should pay special attention to exclusion of potentially hazardous foods where the facility may not have the capacity to identify, validate and monitor hazard control interventions for those foods. This study identified that listeriosis prevention messages need to be disseminated in multiple languages and primary-care practitioners should ensure that patients from households speaking a LOTE receive counselling on listeriosis prevention.
This study adds to the concern associated with acid suppression removing a barrier to bacterial infection. There may be a place for counselling of patients taking such medications or a warning on the product insert regarding the potential increased risk of bacterial enteric infection.
Listeriosis is a serious and preventable foodborne infection for which vulnerable populations and high-risk foods have been known for many decades. While surveillance can be further enhanced to develop food safety policy into the future, it is important that both the well established vulnerable populations and newly recognized LOTE groups are protected as soon as possible. The effectiveness of the implementation of the new food safety programmes for food service to vulnerable persons should be carefully evaluated to ensure optimal protection of this group.
ACKNOWLEDGEMENTS
We thank Dot Little for data management and Katerina Lewandowski for data quality and analysis work, Joy Gregory for assistance with review of the methodology and in initiating and conducting the study, and the many interviewers, patients and carers who contributed to the study. This study was conducted under the OzFoodNet programme of work and was funded by the Australian Government Department of Health & Ageing and NSW Health through the Hunter Medical Research Institute.
DECLARATION OF INTEREST
None.
Appendix Table 1. Additional case-specific risk factors for non-perinatal and perinatal listeriosis, Australia, 2001–2004
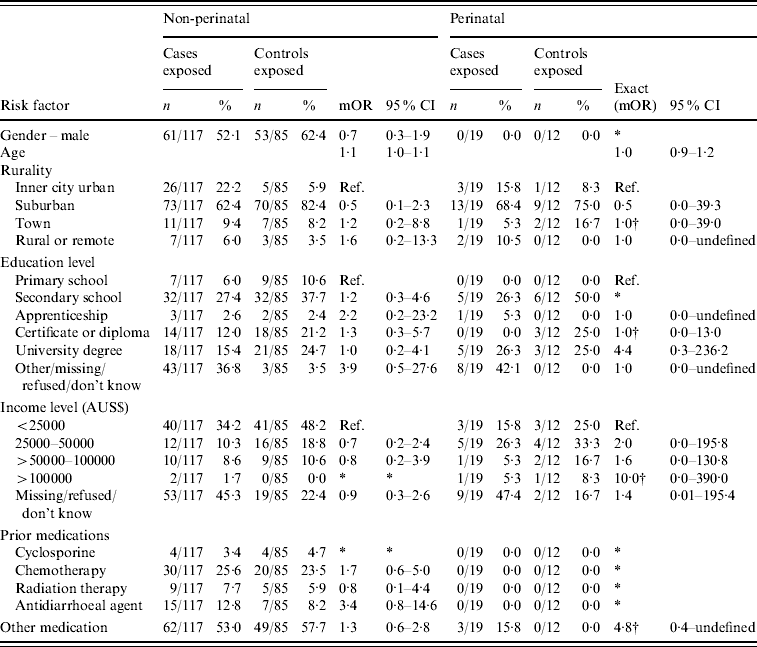
mOR, Matched odds ratio; CI, confidence interval.
* Unable to calculate as numerator or denominator=0 in matched analysis.
† Median unbiased estimates.
Appendix Table 2. Additional food-specific risk factors for non-perinatal and perinatal listeriosis
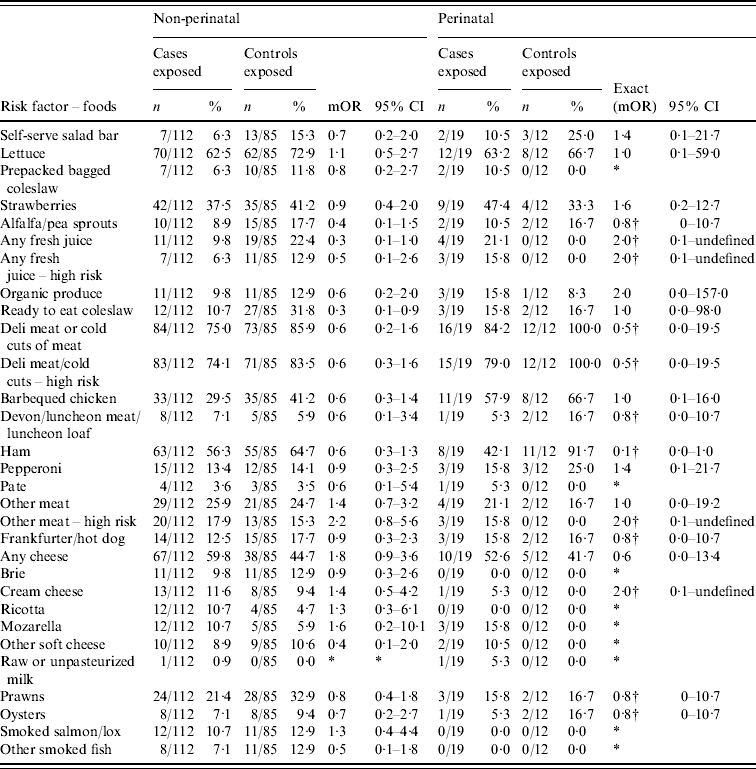
mOR, Matched odds ratio; CI, confidence interval.
* Unable to calculate as numerator or denominator=0 in matched analysis.
† Median unbiased estimates.








