Within the past decade, 3 novel β-lactam-β-lactamase inhibitor combinations with activity against carbapenem-resistant Enterobacterales (CRE) have received US Food and Drug Administration (FDA) approval: ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-cilastatin-relebactam.1–3 Furthermore, a novel siderophore-cephalosporin conjugate (ie, cefiderocol),4 aminoglycoside (ie, plazomicin),5 and tetracycline derivatives (ie, eravacycline and omadacycline)6,7 have also been introduced into the clinical arena in recent years. Understanding the comparative activity of these novel antibiotics is critical to avoiding unnecessary delays in effective therapy, particularly because CRE tend to infect vulnerable medical populations at high risk of mortality. Moreover, the introduction of rapid molecular diagnostics capable of identifying carbapenemase genes prior to antimicrobial susceptibility testing (AST) results further underscores the importance of understanding the likelihood of each novel agent’s activity against specific carbapenemase families.
Comprehensive investigations into the relative activity percentages of various novel agents against CRE are limited. Studies funded by pharmaceutical companies often limit evaluation to novel agents they have developed and marketed, and generally select traditional agents as comparators, rather than other novel ones. We evaluated the activity of 8 novel antibiotics against a cohort of consecutive CRE clinical isolates and investigated the incremental benefit in susceptibility percentage with the addition of a second agent (ie, aminoglycosides, fluoroquinolones, or polymyxins) to novel β-lactam agents to assist with empiric antibiotic decision making.
Methods
Description of isolates
From June 1, 2016, to June 30, 2021, a cohort of consecutive CRE clinical isolates was assembled. CRE were defined as isolates (1) exhibiting resistance to at least 1 carbapenem agent or (2) carrying at least 1 carbapenemase gene.8 CRE isolates were obtained from clinical specimens collected from patients receiving medical care at The Johns Hopkins Hospital, Bayview Medical Center, and Howard County General Hospital, all located in Maryland. Only the first isolate was included for patients who had multiple cultures growing the same species (eg, carbapenem-resistant Escherichia coli recovered in multiple specimens from the same patient). However, if different carbapenem-resistant species were recovered from the same patient (eg, E. coli and Klebsiella pneumoniae), the first isolate of each species was included.
Bacterial genus and species were identified using matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (Bruker Daltonics, Billerica, MA). Antimicrobial susceptibility testing (AST) results were determined using the BD Phoenix Automated System (BD Diagnostics, Sparks, MD) and interpreted following Clinical Laboratory and Standards Institute (CLSI) guidelines.9 All CRE isolates were stored at −80°C in glycerol until further testing was performed.
Antimicrobial susceptibility testing
Frozen isolates were subcultured twice to tryptic soy agar with 5% sheep blood. AST was performed using lyophilized Sensititer broth microdilution (BMD) GN7F and MDRGNX2F panels (Thermo Fisher Scientific, Waltham, MA). For all AST studies, quality control organisms were prepared each day of testing, as recommended by the manufacturer. Susceptibility criteria were interpreted using CLSI or FDA criteria, if CLSI criteria were not available. The following susceptibility criteria were applied to the results: ceftazidime-avibactam, ≤8/4 µg/mL (CLSI); meropenem-vaborbactam, ≤4/8 µg/mL (CLSI); imipenem-relebactam, ≤1/4 µg/mL (FDA); cefiderocol, ≤4 µg/mL (CLSI); tigecycline, ≤2 µg/mL (FDA); minocycline, ≤4 µg/mL (CLSI); eravacycline, ≤0.5 µg/mL (FDA); omadacycline, ≤4 µg/mL (FDA); gentamicin, ≤4 µg/mL (CLSI); tobramycin, ≤4 µg/mL (CLSI); amikacin, ≤16 µg/mL (CLSI); plazomicin, ≤2 µg/mL (FDA); ciprofloxacin, ≤0.25 µg/mL (CLSI); levofloxacin, ≤0.5 µg/mL (CLSI); and colistin intermediate, ≤2 µg/mL (CLSI).9,10
Whole-genome sequencing
CRE isolates underwent whole-genome sequencing (WGS) using short-read Illumina sequencing (MiSeq or HiSeq). Carbapenemase-producing CRE (CP-CRE) underwent additional sequencing using long-read nanopore sequencing. Genomic DNA was extracted from pure cultures using the DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany). WGS and analyses were performed as previously described.Reference Tamma, Fan and Bergman11 Assemblies were deposited to the National Institutes of Health Sequence Read Archive (PRJNA496461 and PRJNA686978).
Statistical analysis
The χ2 test was used to evaluate differences between susceptibility proportions across drug–organism combinations for each of the 4 novel β-lactams and 4 tetracycline derivatives, followed by post-hoc tests of pairwise comparisons between agents. Bonferroni corrections of P values were applied for the 4 β-lactams (group 1) and for the 4 tetracycline-derivatives (group 2), separately. The χ2 test was also used to compare susceptibilities between CP-CRE and non–carbapenemase-producing CRE (non-CP-CRE). Statistical analyses were performed using R version 4.1.1 software (R Foundation for Statistical Learning, Vienna, Austria).
Results
Overall results
In total, 603 consecutive CRE clinical isolates collected from sterile sources were identified. Isolates were collected from the following specimen sources: urine (n = 189), respiratory (n = 133), intra-abdominal fluid (n = 132), blood (n = 88), skin and soft tissue (n = 40), osteoarticular (n = 16), and biliary (n = 5). Table 1 lists the species recovered and the percent susceptibility to 8 last-resort antibiotic agents. The most frequently identified organisms were Klebsiella pneumoniae (n = 229, 38%), Enterobacter cloacae complex (n = 158, 26%), and Escherichia coli (n = 95, 16%). No association between year of isolate and susceptibility percentages were observed for any of the 8 antibiotic agents.
Table 1. Activity of 8 Last-Resort Antibiotics Against 603 Consecutive Carbapenem-Resistant Enterobacterales (CRE) Clinical Isolates Obtained From Unique Patients

Susceptibility to the novel β-lactam agents across the 603 CRE clinical isolates was as follows: ceftazidime-avibactam (95%), meropenem-vaborbactam (92%), imipenem-relebactam (84%), and cefiderocol (92%). Of note, as imipenem-relebactam susceptibility criteria do not apply to Morganella spp., Proteus spp., and Providencia spp. these organisms were not included in the imipenem-relebactam analysis. Pairwise comparisons indicated significant differences in overall susceptibilities between other novel β-lactam agents and imipenem-relebactam (P < .002).
The following susceptibility percentages to tetracycline derivatives were obtained: tigecycline (94%), minocycline (56%), eravacycline (74%), and omadacycline (69%). Pairwise comparisons indicated that there were significant differences in overall susceptibilities between tigecycline and all other agents (P < .001) as well as between all other agents and minocycline (P < .001).
CP-CRE and non–CP-CRE
Of the 603 CRE isolates, 276 (46%) and 327 (54%) were CP-CRE and non–CP-CRE, respectively. Of the 327 non–CP-CRE isolates, the most common identified resistance mechanisms included the presence of extended-spectrum β-lactamase (ESBL) genes and/or ampC genes in conjunction with porin mutations or loss (eg, ompK35 and ompK36), which were identified in 249 (76%) of non–CP-CRE isolates.
Table 1 describes the species recovered categorized by the presence of carbapenemase production and the percent susceptibility to 8 last-resort antibiotic agents. No significant differences were detected between susceptibilities in CP-CRE and non–CP-CRE across any of the 8 β-lactam or tetracycline-derivative antibiotics.
Activity against specific carbapenemase genes
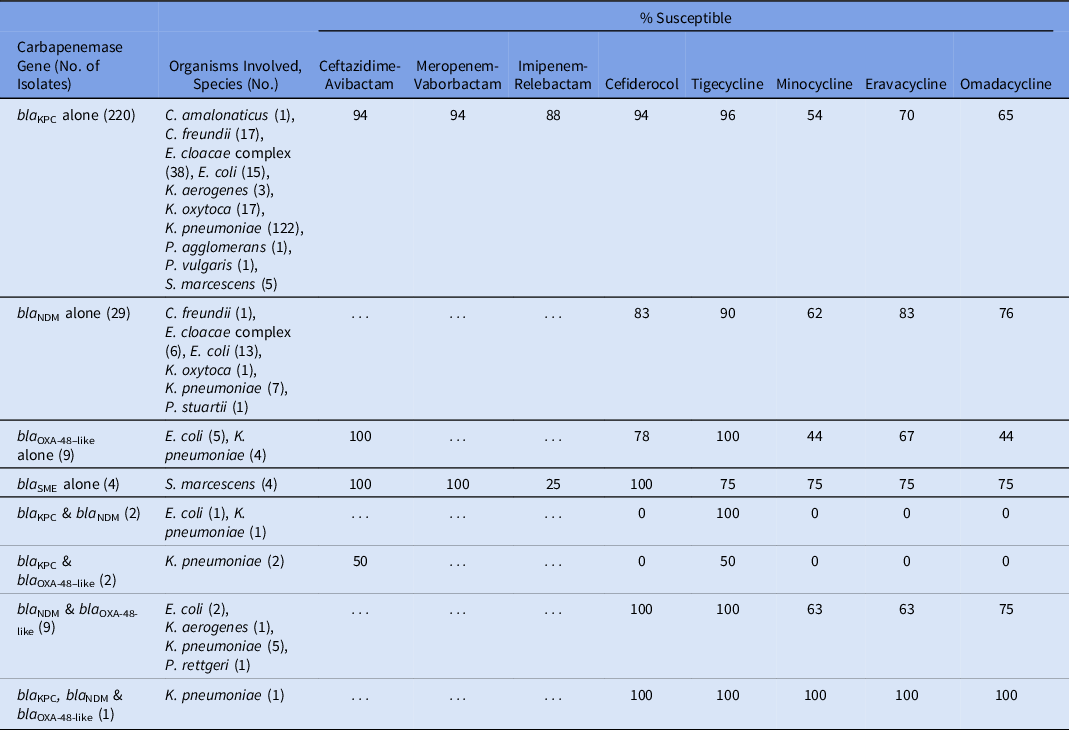
Table 2 lists the susceptibility of the 8 β-lactam and tetracycline antibiotics against specific carbapenemases. Of β-lactam agents, ceftazidime-avibactam, meropenem-vaborbactam, and cefiderocol had activity against KPC-producing isolates, all exhibiting 94% activity against the 220 KPC-producing CRE isolates (excluding those that contained additional carbapenemase genes). Imipenem-relebactam was active against 88% of the KPC-producing isolates. Cefiderocol was active against 83% of the 29 NDM-producing isolates. Ceftazidime-avibactam had 100% activity against the 9 isolates producing OXA-48–like carbapenemases. Tigecycline had the highest activity among the tetracycline-derivates against isolates producing KPC, NDM, or OXA-48–like enzymes. Isolates producing >1 carbapenemase enzyme generally had reduced activity to both β-lactam and tetracycline-derivative agents.
Table 2. Activity of 8 Last-Resort Antibiotics Against 276 Consecutive Carbapenemase-Producing Enterobacterales Clinical Isolates Obtained From Unique Patients
Additive value of combination therapy
The incremental benefit of agents frequently combined as components of combination therapy (ie, aminoglycosides, fluoroquinolones, or polymyxins) when added to a novel β-lactam agent was investigated (Fig. 1). The calculations displayed in Figure 1 reflect isolates with in vitro susceptibility to either the β-lactam or the additive agent. Organisms known to be intrinsically resistant to the polymyxins were removed from the analysis, including Morganella spp, Proteus spp, Providencia spp, and Serratia spp. Generally, aminoglycosides and polymyxins provided greater incremental benefit as second agents compared to the fluoroquinolones. The percentages of susceptibility to ciprofloxacin and levofloxacin were identical, and neither agent provided substantial additive value to any of the β-lactam agents. Of aminoglycosides, plazomicin, and amikacin provided the greatest additive value, providing nearly identical incremental benefits ranging from an additional 4%–11% compared to β-lactam therapy alone (all P values <.001). The β-lactam that benefitted the most from the addition of a second agent was imipenem-relebactam.

Fig. 1. Additional percentage coverage provided by novel β-lactam agents in combination with aminoglycosides, fluoroquinolones, or polymyxins, compared to novel β-lactam monotherapy.
Discussion
Evaluating a cohort of 603 consecutive clinical CRE isolates, ceftazidime-avibactam and tigecycline were the β-lactam and tetracycline-derivatives, respectively, with the highest likelihood of activity, regardless of whether organisms were carbapenemase producing or not. When specific carbapenemase genes were identified, the following β-lactams had the highest activity: KPC-producing (ceftazidime-avibactam, meropenem-vaborbactam, and cefiderocol at 94%), NDM-producing (cefiderocol, 83%), and OXA-48-like–producing (ceftazidime-avibactam, 100%). These findings underscore the important role of carbapenemase gene identification in guiding antibiotic decision making.Reference Tamma, Aitken, Bonomo, Mathers, van Duin and Clancy12
Moreover, we investigated the incremental benefit of adding an aminoglycoside, fluoroquinolone, or polymyxin to each of the novel β-lactams to determine whether they substantively increased the likelihood of activity against CRE isolates for empiric antibiotic decision making. Clinical trial data comparing the outcomes of patients with CRE infections treated with combination therapy (eg, ceftazidime-avibactam and amikacin) versus β-lactam monotherapy (eg, ceftazidime-avibactam) are not available. An observational study comparing the outcomes of 577 patients receiving ceftazidime-avibactam or ceftazidime-avibactam plus a second agent for the treatment of KPC-producing infections did not identify a mortality benefit with this approach.Reference Tumbarello, Raffaelli and Giannella13 However, for ill-appearing patients known to be colonized with CRE or in regions of high CRE endemicity, the addition of a second agent to a novel β-lactam may still have a role to increase the likelihood that at least 1 active antibiotic agent is being administered while awaiting AST results. In our cohort, while the addition of a fluoroquinolone to a novel β-lactam was generally of limited additive value, the addition of an aminoglycoside to a β-lactam, particularly amikacin or plazomicin, increased the likelihood of activity across all β-lactams.
Our study had several limitations. Our cohort consisted of isolates from patients in the mid-Atlantic United States and may not be reflective of other regions of the United States or other regions of the world. Region-specific antibiograms are necessary to understand local susceptibility data. Moreover, for regions with a high prevalence of CRE clinical isolates, the development of regional combination antibiograms specific for CRE organisms can provide data on the combinations of antibiotics associated with the highest likelihood of adequate coverage when novel agents need to be administered on an empiric basis.Reference Hsu, Carroll and Milstone14–16
We included the first CRE species isolated from unique patients. Therefore, our results do not reflect the potential emergence of resistance in subsequent isolates after exposure to novel agents. As an example, >90% of all KPC-producing isolates were susceptible to ceftazidime-avibactam and meropenem-vaborbactam. Estimates of the emergence of resistance after clinical exposure of CRE isolates to ceftazidime-avibactam and meropenem-vaborbactam have been described to be ∼20%Reference Tumbarello, Raffaelli and Giannella13,Reference Shields, Potoski and Haidar17–Reference Ackley, Roshdy and Meredith21 and 5%,Reference Ackley, Roshdy and Meredith21–Reference Shields, McCreary and Marini23 respectively. With the inclusion of subsequent isolates, susceptibility percentages would likely be lowered, particularly to ceftazidime-avibactam, in which acquired resistance due to amino acid substitutions in the KPC carbapenemase are not rare events.Reference Papp-Wallace, Mack, Taracila and Bonomo24 Notably, Sensititer MDRGNX2F panels were used to generate cefiderocol MICs for the current study. In early 2022, an investigation from the manufacturer found that these panels may produce lower cefiderocol MICs compared to reference BMD for E. coli and Klebsiella isolates.
Importantly, in vitro susceptibility does not necessarily translate into improved clinical outcomes. Factors such as adequate and sustained antibiotic penetration to the site of infection and drug-specific toxicities need to be considered when selecting amongst antibiotics. For example, colistin enhanced CRE coverage by 4%–9% across novel β-lactam agents in our cohort. However, colistin is administered as a prodrug, leading to unreliable plasma concentrations.Reference Garonzik, Li and Thamlikitkul25 Additionally, its associated nephrotoxicity often precludes its use for patients with existing renal disease.Reference Pogue, Lee and Marchaim26 As a second example, although tigecycline exhibited 94% activity against CRE isolates, tetracycline-derivatives achieve rapid tissue distribution following administration, resulting in limited concentrations in urine and poor serum concentrations,Reference Agwuh and MacGowan27 limiting their effectiveness for certain sites of infection.
In conclusion, selecting among novel agents can be challenging because it requires a nuanced understanding of the molecular epidemiology of gram-negative resistance mechanisms. This research provides insights into the comparative activity of novel β-lactam and tetracycline-derivate agents against CRE isolates and the additive value of a second agent as empiric therapy. However, in vitro activity is just one component of the complex decision-making process of selecting the most effective antibiotic or combination of antibiotic agents.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official view of the funding agency.
Financial support
This work was funded by the CDC’s Prevention Epicenter Program (grant no. U54CK000617-01-00).
Conflicts of interest
S.E.C. reports receiving personal fees from Basilea and Theravance, outside of the submitted work. P.J.S. reports receiving grants and personal fees from Accelerate Diagnostics, OpGen, and BD Diagnostics; grants from bioMerieux, Affinity Biosensors, and Hardy Diagnostics; and personal fees from Roche Diagnostics, Shionogi, GeneCapture, outside the submitted work. All other authors report no conflicts of interest relevant to this article.






