Sleepwalking is relatively common with an estimated lifetime prevalence of 6.9% (95% CI 4.6–10.3).Reference Stallman and Kohler 1 Although sleepwalking is usually a benign condition, it can occasionally result in injury to the sleepwalker or violence towards others. Violence during sleepwalking, however, is considered relatively uncommon, although no epidemiological studies have investigated the prevalence or incidence of violence during sleepwalking specifically, and most case study examples come from the forensic literature. The consequences for the victim of violence from a sleepwalker can range from disturbed sleep to death (e.g. PololskyReference Pololsky 2 ). Violence is defined here as the purposeful use of physical force against another person or property. Injury is defined as a bodily wound. Terminology that has been historically used in the literature, such as self-injury (harm inflicted on oneself) and parasuicide (non-fatal self-harm in which the person deliberately causes injury to him or herself), is not used here as there is little evidence of self-harm during sleepwalking.
Previous research on violence during sleepwalking has tended to group injuries to the self together with directed violence towards objects and people (e.g. Moldofsky et al Reference Moldofsky, Gilbert, Lue and MacLean 3 and Guilleminault et al Reference Guilleminault, Leger, Philip and Ohayon 4 ). To date, the differing aetiologies of injuries to the self or others have not been considered. Violence during sleepwalking occurs during an incidental encounter with, or when the sleepwalker is approached by another person,Reference Pressman 5 rather than the sleepwalker seeking out a specific person. Drawing from the literature on violence in general, violence during sleepwalking would meet the definition of impulsive, reactive type, rather than premeditated or proactive. Reactive aggression is characterised by high levels of autonomic arousal and precipitated by provocation and associated negative emotions, such as fear.Reference Reid Meloy 6 , Reference Stahl 7 Reactive aggression is impulsive and is initiated without regard for any future goal. The fight or flight reactions most frequently described by sleepwalkers to misperceived stimuli (e.g. PaiReference Pai 8 and JacksonReference Jackson 9 ) would theoretically have the potential to precipitate reactive, impulsive violence by some sleepwalkers, much as it might when people are awake. Injuries to the self that result from moving around with impaired perception and judgement during sleepwalking (e.g. The Port Macquarie News and Hastings River Advocate, 10 The Tenterfield Intercolonial Courier and Fairfield and Wallangarra Advocate, 11 Barrier Miner 12 and The Advertiser 13 ) are therefore by definition different to impulsive reactions to misperceived stimuli resulting in injuries to others or property and should therefore not be considered together.
To date, there have been no theoretically driven hypotheses about the aetiology of violence during sleepwalking and conclusions have been based solely on correlational research of sleepwalkers (e.g. OhayonReference Ohayon 14 ). Early research hypothesised that mental illness could explain both sleepwalking and violence during sleepwalking – no evidence has been found to support either hypothesis. Similar to the prevalence of violence while awake, relatively few sleepwalkers ever harm others during episodes of sleepwalking, suggesting that it is not a characteristic of sleepwalking per se but perhaps a characteristic of the person who is sleepwalking. No attention has yet been given to the relationship between reactive violence in sleepwalking and reactive violence or risk of violence while awake. A biopsychosocial model of violence during sleepwalking may provide an integrated model that incorporates biological and psychological empirical data on violence and sleepwalking that can inform assessment and treatment of violence involved in sleepwalking. For the purposes of this article, we exclude sleepwalking that is secondary to other conditions, for example, neurological diseases such as Parkinson's disease or dementia. First, we will review the biological basis for impulsive behaviour and compare that with the biological features of sleepwalking. Second, we will compare the psychosocial risk factors for impulsive behaviour with those mentioned in reports of violence during sleepwalking. Third, we will reconceptualise violence during sleepwalking in a model that takes into account the known biopsychosocial risk factors of impulsive violence. Finally, we will discuss implications for assessment and treatment and further research.
Functional neuroanatomy
Functionally, the sleepwalking brain is characterised by a dissociated state which presents electrical and metabolic signatures of both wake and sleep. Electroencephalographic recordings have highlighted increased activation of motor and cingulate cortices in patients with sleep arousal disorders.Reference Januszko, Niemcewicz, Gajda, Wolynczyk-Gmaj, Piotrowska and Gmaj 15 , Reference Castelnovo, Riedner, Smith, Tononi, Boly and Benca 16 These recent studies confirm previous single-photon emission computed tomography (SPECT) investigations that described decreased cerebral blood flow in fronto- and temporoparietal association cortices and increased activation of thalamo-cingulate circuits.Reference Dang-Vu, Zadra, Labelle, Petit, Soucy and Montplaisir 17 , Reference Bassetti, Vella, Donati, Wielepp and Weder 18 The characteristic pattern of brain activity during sleepwalking episodes may explain patients’ skilled motor behaviour in the absence of awareness and cognitive control, especially during episodes of reactive violence. Moreover, the failure to activate heteromodal association cortical areas may be responsible for the cognitive and sensory distortions that accompany episodes of violence during sleepwalking. In fact, patients usually do not recognise the victims of their violent attacks even if it is a very close family member and instead direct their aggression to what they think is an intruder.
Impulsive aggression occurs within the context of provocation and resultant emotional arousal. Brain circuitry and neuromodulators are involved in the initiation and suppression of aggressive behaviour.Reference Siever 19 Two areas of the brain have been implicated in the aetiology of impulsive violence – the frontal lobe and the limbic system. Frontal lesions particularly in the orbitofrontal cortex increase the risk of impulsive violence.Reference Brower and Price 20 Limbic structures such as the amygdala and the cingulate cortex can be activated excessively to trigger violence. The orbital frontal cortex and anterior cingulate cortex calibrate behaviour to social cues and predict expectancies of reward or punishment,Reference Wager, Waugh, Lindquist, Noll, Fredrickson and Taylor 21 , Reference Ochsner, Ray, Cooper, Robertson, Chopra and Gabrieli 22 modulating or suppressing aggressive behaviour with potential negative consequences and excessive emotional triggers signalled by the limbic regions such as the amygdala and insula.Reference Siever 19 , Reference Cunningham, Raye and Johnson 23 Siever described a model of violence where emotions deriving from the limbic system, prominently the amygdala, cannot be inhibited by prefrontal structures such as the orbitofrontal and anterior cingulate cortices.Reference Siever 19 Figure 1 shows an adaptation of this model to explain violent behaviour during sleepwalking. An emotionally provocative stimulus during sleepwalking – for example, one that triggers a fight response – has the potential to result in violence because of reduced activation of inhibitory mechanisms during sleep in at-risk individuals. The auditory and visual impairments and distortions characteristic of sleepwalking may be further disrupted by drugs, alcohol or metabolic disturbances caused by illness. During wakefulness, appraisal of the threatening stimulus will occur in visual and auditory integration parts of the brain and in higher association areas such as the prefrontal, temporal and parietal cortexes.Reference Siever 19 The amygdala and related limbic areas process the stimulus in relation to previous conditioning influenced by social and cultural experiences. The hypoactivity of the orbital frontal cortex and cingulate gyrus during non-rapid eye movement sleep and sleepwalkingReference Pressman 5 and the corresponding negative emotions experienced may increase the likelihood of violence during sleepwalking in susceptible individuals because of an absence of modulation of the emotional trigger.Reference Davidson, Putnam and Larson 24
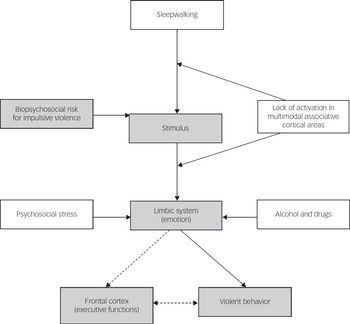
Fig. 1 Initiation and modulation of impulsive violence during sleepwalking. Shaded variables showed the pathway for impulsive violence while awake. Unshaded variables are related to sleepwalking violence. Dashed arrows are normal pathways that modulate violence not available during sleepwalking.
Neurotransmitters have been implicated in the functioning of the cortical and limbic brain areas that may be associated with increased risk of violence.Reference Siever 19 In the cortical areas, these include reduced serotonin and increased catecholamines, dopamine and norepinephrine. In the limbic areas, they include reduced gamma-aminobutyric acid (GABA), enhanced glutamine and enhanced acetylcholine. It has long been hypothesised that serotonin may inhibit and dopamine may increase violent behaviour.Reference Linnoila and Virkkunen 25 , Reference Coccaro 26
A substantial proportion of impulsive aggressive behaviour is inherited, as it is the predisposition to sleepwalking.Reference Abe and Shimakawa 27 , Reference Bakwin 28 A study of common functional polymorphism in monoamine oxidase A (MAOA) on brain structure and function showed that low expression variant compared with the high expression allele was associated with the risk of violent behaviour.Reference Meyer-Lindenberg, Buckholtz, Kolachana, Hariri, Pezawas and Blasi 29 This predicted pronounced limbic volume reductions and hyperresponsive amygdalae during emotional arousal with diminished reactivity of regulatory prefrontal region. In men, this expression was also associated with changes in orbitofrontal volume, amygdala and hippocampus hyperreactivity during aversive recall, and impaired cingulate activation during cognitive inhibition.
The impaired regulation of forebrain areas that causes emotional hyperexcitability is thought to derive from a faulty modulation of these brain regions by neurotransmitter systems such as serotonin and noradrenaline, which are preferentially oxidised by the MAOA enzyme. Violent offenders have low cerebrospinal fluid (CSF) concentrations of 5-hydroxyindoleacetic acid (5-HIAA) – a marker of serotonin turnover – related to irritability, impaired impulse control and psychasthenia (found in case studies of violent sleepwalkers). These results are supported by several studies that have found a strong link between serotonergic dysregulation and impulsive aggression (reviewed in Bortolato et al Reference Bortolato, Pivac, Muck Seler, Nikolac Perkovic, Pessia and Di Giovanni 30 and Coccaro et al Reference Coccaro, Fanning, Phan and Lee 31 ). In general, low brain levels of serotonin have been linked to aggressive behaviourReference Higley and Linnoila 32 , Reference Linnoila, Virkkunen and Roy 33 and to enhanced sensitivity to negative eventsReference Bari, Theobald, Caprioli, Mar, Aidoo-Micah and Dalley 34 , Reference Evers, Cools, Clark, van der Veen, Jolles and Sahakian 35 in both humans and laboratory animals. However, chronic high levels of extracellular serotonin – such as in cases of diminished activity of catalytic enzymes (e.g. MAOA) – may cause a downregulation of serotonergic receptors and other compensatory mechanisms leading to maladaptive responses to stress and repeated episodes of violent and antisocial behaviour.
On the contrary, functional variations in the activity of genes involved in the regulation of the noradrenergic system have been mainly studied in the context of the individual's response to stress. Particularly stressful events are a known potential trigger for sleepwalking episodes in susceptible individuals. Moreover, chronic and acute imbalance in the activity of the noradrenergic system has been associated with impulsivity and diminished behavioural control.Reference Bari and Robbins 36 In children with attention deficit hyperactivity disorder, a polymorphism in the gene coding for dopamine beta hydroxylase – the enzyme responsible for the synthesis of noradrenaline – has been associated with increased impulsive aggression.Reference Hess, Reif, Strobel, Boreatti-Hummer, Heine and Lesch 37 As in the case of serotonin, several studies have highlighted gender differences in the effects of specific noradrenergic gene variants on aggressive behaviour. However, even when gender is taken into account, results are often ambiguous. One study conducted in women found a strong link between polymorphisms in the gene coding for catechol-o-methyltransferase (COMT) – an enzyme involved in the catalytic pathway of catecholamines – and physical aggression.Reference Kulikova, Maluchenko, Timofeeva, Shlepzova, Schegolkova and Sysoeva 38 This latter result challenges previous accounts describing a preferential effect of COMT gene alterations on aggressive behaviour mostly restricted to male individuals.
Psychosocial risk factors for impulsive violence
In sleepwalkers, impulsive violence and impulsivity in general may have multiple origins. It has been shown that sleepwalkers display impaired inhibitory control as a result of disrupted sleep patterns and sleep deprivation compared with healthy individuals.Reference Labelle, Dang-Vu, Petit, Desautels, Montplaisir and Zadra 39 The resulting impulsive behaviour during everyday life might increase the risk of engaging in dangerous activities such as drug use or behavioural addictions (e.g. gambling).Reference Bari, Robbins, Dalley and Olmstead 40 The emotional consequences of such activities can, in turn, increase the incidence of sleepwalking events in susceptible individuals as well as the risk of impulsive violence during these episodes.
In keeping with the emotional sequelae of impulsive behaviour during wakefulness, studies have shown that even acute stressful events can cause an increased sensitivity of brain areas responsible for the individuals’ reaction to threatening stimuli. In particular, amygdala activation displays a diminished specificity for negative valence and increased responsivity to non-threatening events,Reference van Marle, Hermans, Qin and Fernandez 41 possibly leading to unmotivated violent reactions.
Substance use and impulsivityReference Tikkanen, Tiihonen, Rautiainen, Paunio, Bevilacqua and Panarsky 42 – Reference Virkkunen, Goldman, Nielsen and Linnoila 44 contribute to both cognitive distortions and disinhibition that precipitate impulsive violence.Reference Siever 19 Several classes of drugs of misuse are known to be risk factors for impulsive and aggressive behaviour, both during intoxication and withdrawal.Reference Hoaken and Stewart 45 However, as exemplified above, the relationship between substance use and violent aggression during sleepwalking should be considered not only as a possible precipitating factor but also as a consequence of impulsive traits already present in the perpetrating individual. In fact, impulsive personality traits are strongly linked to substance use and addiction.Reference Bari, Robbins, Dalley and Olmstead 40
Other psychosocial risks factors for violence when sleepwalking include subthreshold daytime risk factors, such as poor verbal skills or low educationReference Barratt, Stanford, Kent and Alan 46 – evidenced by shouting or temper tantrums – the experience of normalisation of aggression, such as with the military, or prior experience of violence, such as child maltreatment,Reference Byrd and Manuck 47 family conflictReference Connolly and Beaver 48 or cognitive schema.Reference Seager 49 These biopsychosocial risk factors are summarised in Appendix 1.
Prevention and safety
Safety must be the first consideration for people living with an individual at risk of violence during sleepwalking episodes. A number of steps can be taken to reduce the risk of harm from violence during sleepwalking. Sleeping in a separate room with a locked door would be important until the person is no longer deemed to be at risk of sleepwalking violence. All obvious weapons, such as guns, knives and bats, should be removed from the sleepwalker's bedroom and if possible from the house. Sleepwalkers with known risk factors for violence should avoid alcohol and drug use or at least limit their intake to reduce risk. Finally, exposure to stressful social situations and sleep deprivation should be minimised. When this is not possible, patients may counteract the negative emotional consequences of such events with pharmacological aids, under the guidance of a physician.
Future research priorities
The proposed model of the relationship between biopsychosocial risk factors for impulsive violence and violence during sleepwalking can be used to guide empirical research. Initial efforts should focus on the degree of biopsychosocial risk factors in violent sleepwalkers. Correlational studies that examine the degree of co-occurrence between violent episodes during sleepwalking and impulsive, reckless behaviour during wakefulness may shed light on the underlying causes of aggressive violence during sleepwalking. Although the model depicts biopsychosocial risk factors as the vulnerability to violence during sleepwalking, this has yet to be demonstrated empirically. We believe this is an important first step in understanding the relationship between biopsychosocial risk factors and sleepwalking violence. It will be important to test this model with clinical and community samples to avoid confounding secondary gains that may be present in forensic populations. This will also enable the model to be applied to the breadth of violent sleepwalking behaviour, rather than just the extreme, homicidal cases that appear in the forensic literature.
Assessment
Published case studies typically lack the rigor of a thorough biopsychosocial evaluation of violence in sleepwalking. Because the model infers that risk for violence while sleepwalking develops from the risks for impulsive behaviour while awake coupled with the vulnerabilities of sleepwalking, it is important that assessment includes all vulnerabilities and low-level impulse–control problem behaviours during waking – for example, temper tantrums, shouting when frustrated, difficulty negotiating with others – rather than just involving clinical disorders. It would be advantageous for the assessment to be conducted independently with multiple informants including people living in the same house and family. Components of a biopsychosocial model assessment are shown in Appendix 2.
Treatment
Although there are no evidence-based interventions for sleepwalking,Reference Stallman and Kohler 51 there are a range of psychological and pharmacological interventions that may address the risk factors for violence during sleepwalking. Parent training, based on social learning theory, is effective for paediatric temper tantrums and conduct problems 52 and may be an important early intervention for sleepwalkers with impulsivity problems to prevent violent incidents in adulthood. Cognitive–behavioural therapy is useful for impulse–control, interpersonal difficulties and antisocial behaviour. 53 Motivational interviewing is useful for enhancing intrinsic motivation to reduce substance use by exploring and resolving ambivalence and may therefore be useful for related substance useReference Carrolla, Balla, Nicha, Martinoa, Frankfortera and Farentinosb 54 and may be particularly helpful for sleepwalkers who may not have a substance use disorder but substance use increases the likelihood of them being violent while sleepwalking. Non-emotional training that has been shown to induce changes in amygdala reactivity to aversive information and alter amygdala–prefrontal connectivityReference Cohen, Margulies, Ashkenazi, Schaefer, Taubert and Henik 55 may also be a promising intervention. There are a range of pharmacological interventions that modulate neurotransmitters, for example selective serotonin reuptake inhibitors (SSRIs) to increase serotonin availability in prefrontal regions, inhibiting the subcortical regions,Reference New, Buchsbaum, Hazlett, Goodman, Koenigsberg and Lo 56 mood stabilisers and anticonvulsants that alter glutamatergic or GABAergic balance, reducing irritability and impulsivity.Reference Hollander, Tracy, Swann, Coccaro, McElroy and Wozniak 57 Pharmacological interventions should be used with caution until there is an evidence base for their effectiveness with violent sleepwalkers, as they may increase the likelihood of sleepwalking (see Stallman et al Reference Stallman, Kohler and White 58 for a review) or other sleep problems.
Summary
Sleepwalking can occasionally include violent behaviour towards others. The aetiology of this is not well understood. The aim of this paper was to integrate the biological, neurological and psychological literature on reactive violence and sleepwalking to propose a biopsychosocial model of violence during sleepwalking. The model suggests that vulnerable sleepwalkers may be at risk for violence during sleepwalking because of impaired auditory and visual perception that occurs in both sleepwalking and reactive violence. The inactivation of brain areas responsible for higher cognitive functions such as impulse control and the hyperreactivity of lower-level systems involved in aggressive and defensive behaviour may underlie the manifestation of violent episodes during sleepwalking. Genetically determined or acquired imbalances in neurotransmitters and neuromodulatory systems that play an important role both in the transition between sleep and wakefulness and in the regulation of emotions and impulses are also hypothesised to contribute to the emergence of aggressive violence in sleepwalkers. Although these pre-existing conditions represent biological risk factors, episodes of violence during sleepwalking are usually described during periods of intense psychological stress which may act as a trigger for negative emotions and violent reactions to perceived threat. This theoretical model may also account for conflicting thoughts around the role of substance use in sleepwalking. Research is needed to test the model in both community and forensic populations. It has significant implications and opportunities for treatment options if it is proven.
Appendix 1
Summary of biopsychosocial risk factors for impulsive violence
Biological
-
• Genetics: male
-
• Brain structure
-
• Neurotransmitters
-
• <2% stage 4 sleep
-
• Age <50 years
Psychological
-
• Poor impulse control
-
• Poor verbal skills/poor education
-
• Substance use
-
• Psychiatric disorders
-
• Schizophrenia
-
• Antisocial personality disorder
-
• Borderline personality disorder
Social
-
• Parenting
-
• Family history of violence
-
• Availability of weapons
-
• Cultural norms
-
• Gender roles
Appendix 2
Biopsychosiocial assessment of violent sleepwalkers
Presenting problem
-
• Presenting event, memory of event, time of night
-
• Substance use prior to the event
-
• Relationship history with the victim
-
• Psychosocial stressors
-
• Access to weapons
Medical history
-
• History of sleepwalking
-
• History of violence during sleepwalking
-
• Substance use history
-
• Presence of other sleep problems
-
• Presence of neurodevelopmental or neurological conditions
-
• Presence of psychiatric conditions
Developmental history
-
• Education history
-
• Employment history
-
• Social and cultural history including -quality of parenting, how conflict was demonstrated and resolved by each family member, abuse to self or others
-
• Language and communication skills
Forensic history
Testing
-
• Sleep deprivation polysomnography
-
• Impulse control questionnaireReference Bari, Kellermann, Studer, Absher and Cloutier 50
-
• Executive functioning





eLetters
No eLetters have been published for this article.