Introduction
Transmission of potential pathogens from contaminated environmental surfaces and patient care equipment in ambulatory settings is a concern for people with cystic fibrosis (pwCF). Thus, the 2013 infection prevention and control (IP&C) guidelines for CF included several recommendations for cleaning and disinfection of environmental surfaces and patient care equipment in CF clinics. Reference Saiman, Siegel and LiPuma1 However, assessments of the effectiveness of cleaning and disinfection in CF clinics have not been previously described.
Reported measures of cleaning effectiveness can include manual inspection, contact agar plate-based microbiologic sampling, fluorescent assays, and adenosine triphosphate (ATP) bioluminescence assays. The latter assays measure ATP produced by living microorganisms (and likely dead organisms) as well as organic material and have been used in the food industry and healthcare settings to assess bacterial contamination. Reference Boyce, Havill and Dumigan2,Reference Nante, Ceriale and Messina3 The bioluminescence reading, provided as relative light units (RLU), has correlated (although sometimes poorly) with aerobic bacterial counts from those surfaces. Reference Salsgiver, Bernstein and Simon4,Reference Deshpande, Dunn and Fox5 Generally, a reading of <250 RLU is used to define ‘passing’ in healthcare settings. Reference Lewis, Griffith and Gallo6 The portable ATP bioluminescence assay provides results within minutes, which can facilitate real-time assessment and feedback.
The objectives of this study were [1] to determine the feasibility of using an ATP assay to evaluate the cleanliness of commonly touched surfaces in clinics providing care to pwCF, [2] to assess if certain surfaces were more likely to ‘pass’ ATP testing after cleaning and standardized feedback to clinic staff, and [3] to compare pass rates in adult versus pediatric clinics.
Methods
Study design and participating CF centers
In this multi-center prospective study, eight commonly used CF clinic surfaces were tested at each study site for contamination using an ATP bioluminescence assay (3M™ Clean-Trace™, Saint Paul MN, USA). 7 From April to July, 2022, 19 (10 pediatric and 9 adult) clinics at 11 U.S. CF care centers implemented a standardized sampling and testing protocol. During the study period, all sites reported that they had resumed normal clinical activities after restrictions imposed by the COVID-19 pandemic. Cleaning was performed between patients by clinic staff (e.g., nursing personnel and respiratory therapy). All sites used single use wipes from sealed containers. Most clinics used alcohol-based wipes, while one clinic used 0.5% peroxide and one used a bleach- based agent. Fifteen clinics had exclusive clinic sessions dedicated to pwCF while two pediatric and two adult clinics had concomitant CF and non-CF patients during a given session. The care centers were located in the following U.S. regions: two in the Northeast, and three each in the West, Midwest, and South. All sites received local IRB approval to perform this study. The study had a waiver of written informed consent but required informing clinic staff prior to initiation of the sampling procedures.
Standardized sampling of selected surfaces and ATP testing
CF center directors and research coordinators at each site participated in a video call and received written instructions to facilitate standardized implementation of sampling, the testing protocol, and feedback. The eight surfaces tested were common to clinics providing care to pwCF and considered by the study team to be frequently touched by pwCF and/or clinic staff. Tested surfaces included: patient-facing side of the front desk at registration, blood pressure (BP) equipment (screen and on/off button for automated [16 clinics] or the bulb on manual BP measurement equipment [3 clinics]), table in the team workroom, patient chair for pulmonary function testing (PFT) or surface of portable PFT machines if testing was performed in the exam room, PFT computer keyboard space bar and mouse, exam room computer keyboard space bar and mouse, head of the exam table, and patient/visitor chair in the exam room. The arm rests of chairs were sampled; if no armrest was present, the seat was sampled.
To test selected surfaces the ATP assay was performed as previously described. Reference Boyce, Havill and Dumigan2,Reference Lewis, Griffith and Gallo6 In brief, a 10 x 10 cm area was sampled using a swab provided by the manufacturer and placed in a tube containing a solution with the enzyme luciferase. Swabs were then agitated for 20–30 seconds and inserted into the ATP bioluminescence reaction tube containing D-luciferin. The amount of light created by the reaction of luciferase and D-luciferin catalyzed by ATP was measured and reported as RLUs. Following the manufacturer’s recommendations, surface cleanliness passed if the RLUs were <250. 7
Schedule for sampling and staff feedback
Sampling and scheduled feedback occurred during a six-week period (Figure 1). Clinic staff received an informational flyer provided by the study leadership team explaining ATP testing and were notified that 5 rounds would be completed but sampling dates were not specified. Testing Round 1 (R1) and Round 2 (R2) were done before routine cleaning of surfaces had been performed: these results were considered baseline cleanliness. The following week, Round 3 (R3) testing was performed after routine cleaning had been completed. Clinic staff then received feedback on the results from R1-R3 testing by showing data from these three rounds for each surface. Round 4 (R4) testing was performed one week after the first scheduled feedback was provided and after routine cleaning had been performed. Staff then received the second scheduled feedback on the results from R1-R4 testing. Two weeks after R4, Round 5 (R5) testing was performed after routine cleaning.
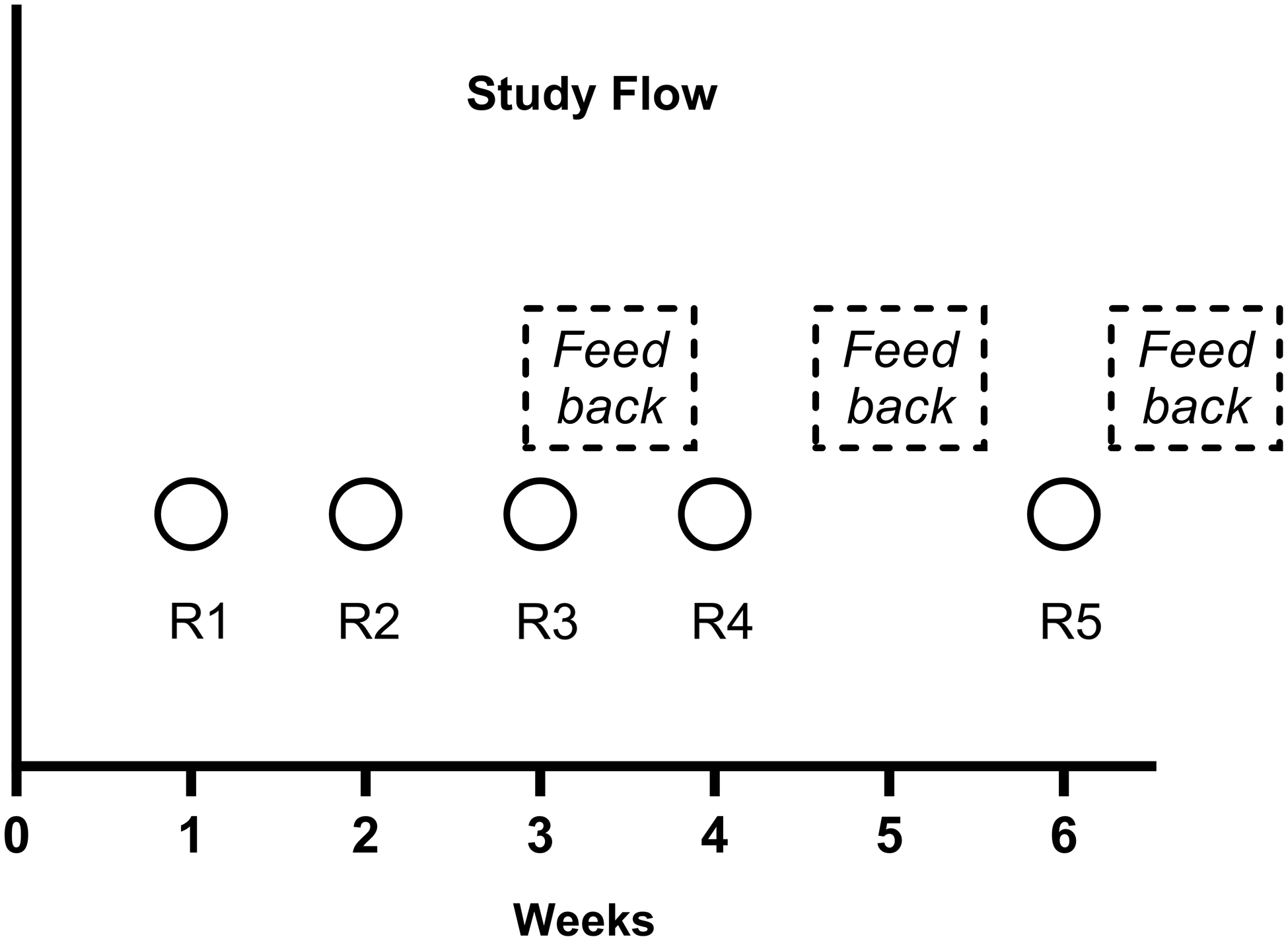
Figure 1. Time line for testing rounds and feedback during the 6-week study period. Circles with R indicate testing rounds. The mean of Rounds 1 and 2 testing were considered the baseline pass rate. Round 3 testing was performed after cleaning without feedback. Rounds 4 and 5 were performed after cleaning and feedback.
Each site sent their results to the study team at the lead site (University of North Carolina) after each testing round. For feedback semi-standardized wording was provided to all sites by the study team. This consisted of sharing the site’s ATP testing results and emphasizing importance of cleaning (see Appendix).
Statistical analyses
The ATP testing results from all study sites were combined to analyze the rates of pass/fail and absolute RLUs measurements. Descriptive statistics were generated to report pass/fail rates based on <250 versus ≥250 RLU (primary outcome), respectively, for each surface and for each testing round. Odds ratios (ORs) and 95% confidence intervals (CI95) were calculated for each surface comparing baseline cleaning (mean of R1 and R2) to each subsequent round. Additionally, ORs comparing pediatric and adult clinics were calculated for each surface. The pass/fail rates in each round (averaged for all study sites) were modeled for each surface using a generalized linear mixed model (GLMM) with logit link for pediatric and adult clinics. An additional GLMM was fit for pediatric and adult clinics separately using data from all surfaces from all testing rounds. The distribution of pass/fail rates between adult and pediatric clinics were assessed using chi-squared analysis. Further analyses were conducted using absolute RLUs as continuous measures using a 2-sided t-test for paired and Friedman test for multiple comparisons.
Analyses were performed using R version 3.6.1 and Graph Prism Version 10.1.2.
Results
ATP testing pass rates
During the study period, 750 ATP tests were performed at 10 pediatric and 9 adult CF care clinics; 395 tests were performed in pediatric clinics and 355 in adult clinics. One CF center that included both an adult and a pediatric clinic, did not have a designated team workroom and thus could not sample the workroom table.
Combining results from all clinics, 72% of surfaces passed during baseline testing (mean of R1 and R2). The pass rate increased to 79%, 83%, and 85% of surfaces in R3, R4, and R5, respectively. In the GLMM that included all surfaces and rounds, the odds of passing compared to baseline were 1.57 (for R3 (CI95 0.94, 2.64); 2.16 times higher (CI95 1.25, 3.72, P = 0.006) for R4 and 2.57 times higher (CI95 1.47, 4.52, P = 0.001) for R5. No study site had a surface(s) that consistently failed in all rounds.
The overall pass-rate was significantly higher in adult compared to pediatric clinics (86% vs 71%; P < 0.001). In pediatric clinics, pass rates were 62% for baseline testing, 75% for R3, 73% for R4, and 82% for R5. In adult clinics, pass rates were 83% for baseline testing, 83% for R3, 93% for R4, and 87% for R5. The odds of surfaces passing in adult clinics compared to surfaces in pediatric clinics were 3.24 times higher (CI95 1.22, 8.63, P = 0.02). Pass rates for all clinics, adult clinics, and pediatric clinics for all surfaces and cycles are shown in Figure 2.
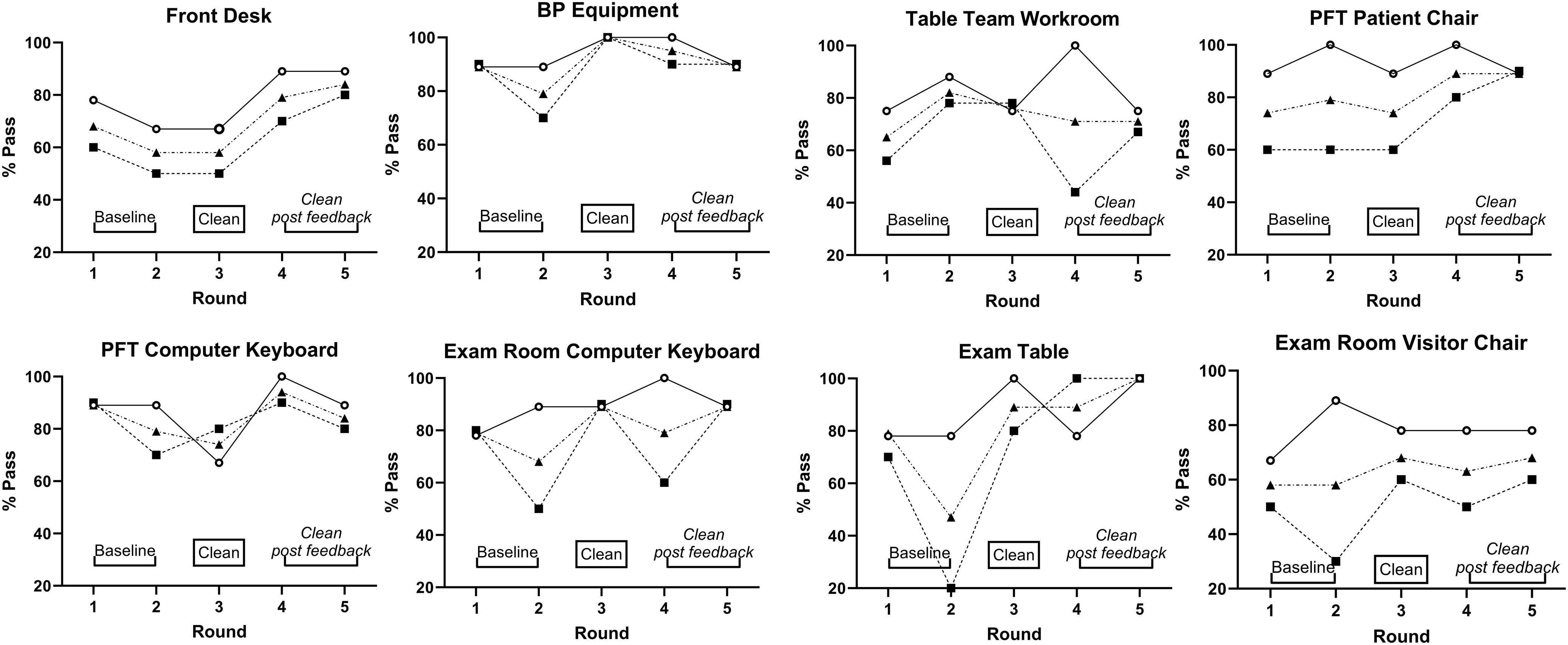
Figure 2. The testing pass rates (RLUs <250) of 8 tested surfaces in 19 clinics for Round 1 through Round 5. Pass rates for adult clinics (solid line, circles), pediatric clinics (dotted line, squares), and all clinics combined (dotted line, triangles) are shown.
Pass rates and RLU values for specific testing surfaces in pediatric versus adult clinics
In the GLMM for all surfaces in pediatric clinics, the odds of passing were 2.08-times (CI95 1.07, 4.04, P = 0.031) and 3.57-times higher (CI95 1.73, 7.40, P < 0.001) at R3 and R5, respectively, compared to baseline. Pass rates in R4 were similar to baseline; OR 1.92 (CI95 0.99, 3.70). Comparison of combined R4&R5 to R3 as clean baseline did not show further improvement in pass rates after feedback. When evaluating surfaces across all study sites, the BP equipment and PFT computer keyboard consistently passed, but the exam room patient/visitor chairs consistently failed in all rounds when averaged across study sites (Table 1).
Table 1. ATP testing results of environmental surfaces and patient-care equipment in pediatric and adult CF clinics
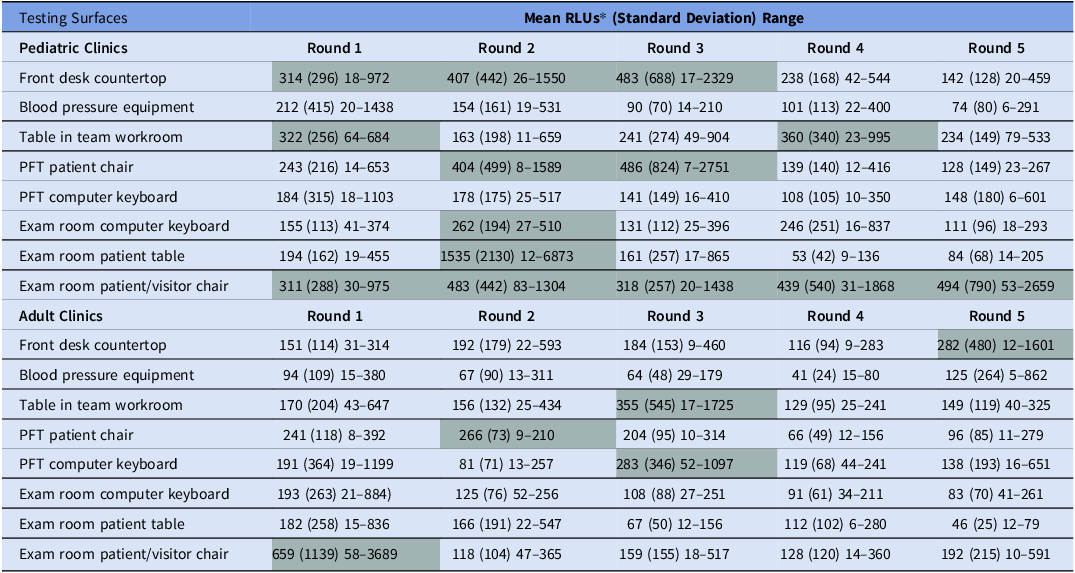
*Shaded cells indicate surfaces whose RLUs averaged across study sites were ≥250 and thus failed.
In the model assessing all surfaces in adult clinics, the odds of passing were 3.14 times higher (CI95 1.09, 9.08, P = 0.034) for R4 and were similar R3 (OR 1.00 [CI95 0.44, 2.28]) and R5 (OR1.50 [CI95 0.62, 3.62]), compared to baseline. Using R3 as baseline there was no difference in pass-rates to combined R4&R5 either. The BP equipment and, exam room keyboards, and exam room tables all passed in each round. Pass rates for manual BP equipment had similar pass rates compared to automatic BP equipment; 87% versus 93%, respectively, P = 0.61.
Variability for RLUs among sites and from round to round was more pronounced at pediatric than at adult clinics (Table 1). Pass-fail rates and RLUs were similar in clinics that used alcohol- based disinfectants versus clinics that used other cleaning agents. In pediatric clinics, evaluation of changes in RLU relative to baseline showed significant reductions for the exam table in R4 (P < 0.01) and-R5 (P = 0.01), and for the front desk area in R3. In adult clinics, changes in RLU relative to baseline showed significant reductions for the front desk at R4, but all values were <250 RLUs.
Discussion
This study showed [1] the feasibility of using an ATP bioluminescence assay in ambulatory settings to monitor cleanliness of environmental surfaces and equipment. All sites had excellent adherence to the study protocol as indicated by complete sampling rates. Overall, the mean pass rates for all tested surfaces, at all rounds, at all study sites were 75%–80%; [2] certain surfaces (BP equipment) were more likely to pass whereas the exam room chair in pediatric clinics had the lowest pass rates compared to other surfaces even after feedback. Further this study showed that [3] the pass rates were higher in adult clinics than in pediatric clinics. Based on improved pass rates with cleaning (R3) with further improvement in R5 for certain surfaces in the pediatric clinics (Figure 2), cleaning and feedback appeared to be helpful. Improvements also differed by surfaces; the highest pass-rates for the front desk, exam room table and chair occurred at R5.
The higher rate of contamination in pediatric clinics may be explained by more frequent touching of surroundings and less frequent hand hygiene performed by young patients and potentially their accompanying siblings. Besides clinic spaces passing more often in the adult compared to pediatric clinics there were differences by surface that we can only speculative on. The BP equipment was “cleanest” which may be due to it’s easy to clean flat surface and that the person who is in direct patient contact wipes it down immediately after use. On the other hand, key-boards in the exam room may be more difficult to remember after a visit and in pediatric clinics children often play with it. Interestingly, the Front desk/Check in surface showed marked improvement after feedback which may be due to this surface not receiving attention at baseline.
Contaminated surfaces can serve as reservoirs for pathogens causing healthcare-associated infections in both CF and non-CF populations as supported by epidemiologic studies and experimental data. Reference Hota8–Reference Kuczewski, Henaff and Regard12 In CF clinics, potential pathogens can survive on sinks, tables, toys, and chairs for extended periods. Reference Festini, Buzzetti and Bassi13,Reference Zimakoff, Hoiby and Rosendal14 Transmission of Pseudomonas aeruginosa within CF care centers has been documented in many countries, Reference Kidd, Ramsay and Hu15,Reference Stapleton, Izydorcyzk and Clark16 with fewer shared strains after implementation of stricter IP&C measures. Reference Hoiby and Pedersen17,Reference Wiehlmann, Cramer and Ulrich18 However, we could only find one prior study that addressed strategies to reduce the potential risk posed by contaminated surfaces in CF clinics. Using agar contact plates to quantify bacterial burden before cleaning, the highest colony forming units (CFUs) were noted in and around sinks, and on exam room chairs and desks. Reference Allen, Jadkauskaite and Shafi19 Following manual cleaning, CFUs were significantly reduced for most surfaces. While the study found that ultraviolet (UV) lights further reduced CFUs on all surfaces, UV disinfection can adversely affect clinic flow due to the time required, UV lights are costly to maintain, and are not currently recommended for CF care settings. Reference Saiman, Siegel and LiPuma1
ATP testing of cleaning/disinfection of environmental surfaces and patient-care equipment facilitates feedback as results are available within a minute and easily comprehensible. We provided standardized feedback on the study’s results to staff responsible for cleaning to reiterate the importance and effectiveness of their efforts. Feedback compared to cleaning alone did not enhance pass rates in the combined results for all surfaces although changes were seen for some surfaces, e.g., front desk, exam room table (Figure 2). Feedback has been shown to enhance cleaning effectiveness in the setting of terminal cleaning of hospital rooms. Reference Branch-Elliman, Robillard and McCarthy20,Reference Martin, Salsgiver and Bernstein21 A recent multicenter randomized trial evaluated effectiveness of different methods of cleanliness monitoring compared to ultraviolet/ fluorescent marker detection and feedback, ATP monitoring and feedback was associated with a significant reduction in cleaning failures and in the incidence rate of multi-drug resistant organism (MDRO) infection and colonization in ICUs. Reference Ziegler, Babcock and Welbel22
Concerns about ATP technology exist. Reference Whiteley, Derry and Glasbey23 Sciortino et al. compared different commercial ATP luminometers to assess repeatability, linear range, and reproducibility using defined, experimental bacterial inocula placed on stainless steel surfaces. Reference Sciortino and Giles24 In their model, the instrument used in our study detected ATP for as long as 10 days and provided good linearity of results between 102 to 107 CFU for three test organisms (S. aureus. Acinetobacter baumannii, and Candida albicans). However, the instrument showed higher inter-operator differences than other luminometers suggesting that training for the sampling method is important. Reference Sciortino and Giles24 To facilitate training, we provided direct communication via audio-visual meetings, written instructions, and additionally an instructional video to all study personnel to standardize surface sampling. Surface testing was done by the same person at each clinic (except two testing rounds in one clinic)—and in the 9 centers that had pediatric and adult clinics, the same person sampled the surfaces which served to reduce inter-person variations. Several cleaning agents can lead to quenching of ATP. Reference Omidbakhsh, Ahmadpour and Kenny25,Reference Moore, Smyth and Singleton26 , which can potentially lead to higher pass-rates. Thus, comparisons with baseline measures are recommended. Baseline pass-rates here were high compared to other publications that were done in inpatient settings or ICUs. Reference Boyce, Havill and Dumigan2,Reference Moore, Smyth and Singleton26 Possible explanations are the short stay in outpatient clinics compared to inpatient care and sampling areas next to the patient in ICUs. Potentially high attention to cleaning by staff working in the clinics who are instructed to clean immediately after the patient contact also contribute. The variations of RLU within the same study site indicate that failures do occur and that ongoing education is helpful.
Studies have also shown that correlation between RLU values and CFU ranged from significant to very poor, which supports using set cut-off benchmarks rather than actual values. Reference Nante, Ceriale and Messina3,Reference Lewis, Griffith and Gallo6 The lack of correlation may be explained by technical aspects; i.e.; other organic material (e.g., blood cells, sloughed skin cells) reacting with the assay reagents. As such, RLU to CFU comparisons were much better in studies using defined bacterial solutions rather than surfaces. Reference Sciortino and Giles24 Several studies have evaluated different RLU cut-off values to consider a surface “clean” – ranging between 100 and 500 RLU. A recent metanalysis showed a cut-off of 250 RLU to be most common across studies, yet the cut-off should be based on the recommendations of the specific ATP system used. Reference Nante, Ceriale and Messina3 We chose a value of < 250 RLU as the passing cut-off based on the manufacturer’s recommendations and our prior work, which showed this cut off had acceptable sensitivity and specificity when compared to CFU counts. Reference Salsgiver, Bernstein and Simon4,Reference Mulvey, Redding and Robertson27
Limitations of our study include lack of generalizability as participating sites were large CF care centers. Furthermore, our sample size was relatively small compared to the ∼110 accredited CF centers in the U.S. We selected frequently touched surfaces based on the investigators’ experience and prior observations of surfaces frequently touched by staff, e.g., computer keyboards and computer mouse. Reference Huslage, Rutala and Sickbert-Bennett28,Reference Weinstein29 However we did not measure touching frequency at the study sites. Prior studies testing the impact of improved cleaning on infection rates were conducted for inpatients who have a longer exposure to the hospital environment than those in clinic. Reference Ziegler, Babcock and Welbel22,Reference Moore, Smyth and Singleton26 Therefore, we cannot extrapolate our findings to reduced infections for outpatient clinics. The Hawthorne effect may have influenced results as staff had been informed about the study although staff did not know exactly when ATP testing would occur. Although this study was conducted after the height of the SARS-CoV-2 pandemic, there may have been increased attention to IP&C practices. However, study sites confirmed that patient volumes and clinic flow had returned to pre-pandemic levels. Reference Muhlebach, Cunningham and LiPuma30 . Thus, we expected opportunities for contamination of environmental surfaces and patient care equipment to be similar to that experienced prior to the pandemic. Finally, we do not know the long-term effectiveness of the feedback or sustainability of this intervention.
In summary our study findings demonstrated the feasibility of using an ATP bioluminescence assay to measure cleanliness of environmental surfaces and patient care equipment in ambulatory settings caring for pwCF. Differences found between the cleanliness of pediatric versus adult clinics can inform centers about the challenges cleaning clinics that care for younger patients and the possible sources of transmissible pathogens. Further larger quality improvement efforts focusing on surface and equipment cleaning in CF care settings are desirable as is determining sustainability.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2024.102.
Financial support
Funding for the study was from the U.S. Cystic Fibrosis Foundation in form of a grant MUHLEB19A0). We also thank all participating centers.
Competing interests
M.S. Muhlebach, Kushal Shah, T. Shields, M. Ansar, J.J. Zhou, and J.J. LiPuma, and L. Saiman have no conflicts to disclose in relation to this manuscript.






