CHD is the most common neonatal congenital defect, impacting approximately 9 per 1000 live births, with about 3 per 1000 infants requiring surgical intervention in the neonatal period. Reference Marino, Lipkin and Newburger1,Reference Panigrahy, Schmithorst and Wisnowski2 Surgical care along with medical and nursing care advances in the field of CHD have led to increased survival rates among this population. As children with CHD are now surviving into adulthood, they have been identified as at risk for neurodevelopmental delays and disabilities. Thus, there has been a shift in their needs to include a greater focus on neurodevelopmental outcomes. Reference Bellinger, Wypij and DuPlessis3,Reference Goff, Shera and Tang4 Tied to neurodevelopmental outcomes, growth failure, and malnutrition are common consequences of CHD, and children with univentricular and cyanotic heart lesions are at highest risk for both acute and chronic growth failure. Reference Medoff-Cooper and Ravishankar5,Reference Ravishankar, Zak and Williams6 Although the exact aetiology of growth failure in infants with CHD remains overall unknown, poor oral feeding has been implicated as an important early contributor to the extensive long-term feeding challenges seen within this population. Reference Medoff-Cooper and Ravishankar5
The act of sucking requires normal function of the oromotor system and coordination of the central nervous system. Thus, dysfunction or injury to any of these components can lead to impaired ability to generate an adequate suck and result in poor oral feeding. Reference Medoff-Cooper and Ravishankar5,Reference Kogon, Ramaswamy and Todd7–Reference Natarajan, Reddy Anne and Aggarwal9 Although the coordination of sucking, swallowing, and breathing is crucial for adequate oral feeding, feeding is also an important neurodevelopmental milestone. Many neonates with complex CHD, defined as those that require cardiac intervention in the neonatal period for survival, often require urgent medical attention at birth with surgery in the first month of life. They therefore do not have the opportunity to meet this important neurodevelopmental milestone within the typical time frame. This lack of oral feeding early in the neonatal period potentially influences their long-term feeding abilities and overall growth trajectories. Reference Ravishankar, Zak and Williams6,Reference Medoff-Cooper and Irving10 The challenge to understanding the exact aetiology of poor oral feeding during the neonatal hospitalisation and long-term growth failure in children with CHD has been attributed to concurrent congenital problems, such as underlying genetic syndromes and acquired neurologic problems (e.g., structurally abnormal brain). Reference Wernovsky and Licht11
Typically, successful pre-operative oral feeding does not predict efficacious post-operative oral feeding in neonates with complex CHD. Reference Medoff-Cooper and Ravishankar5 Therefore, further vulnerabilities acquired from the time of surgery through the post-operative period, such as brain injury and vocal cord paralysis, may play a role in successful post-operative oral feeding. Given that many of these neonates have normal oral feeding mechanisms pre-operatively, the cause of the decline in their oral feeding abilities requires further inquiry. Reference Natarajan, Reddy Anne and Aggarwal9 As over 50% of neonates and infants with complex CHD are discharged home with tube feeding assistance Reference Medoff-Cooper and Ravishankar5 , further investigation into potential causative factors influencing poor post-operative oral feeding in neonates after cardiopulmonary bypass surgery may provide insight for potential interventions to support oral feeding success in survivors of neonatal cardiac surgery.
Although studies document poor oral feeding as a challenge in neonates with complex CHD, this evidence is representative of single studies and, to date, there has not been a comprehensive assessment and synthesis of published and unpublished single studies. Therefore, the purpose of this scoping review is to map the extent, range, and nature of the available research evidence on factors associated with oral feeding in full-term neonates or infants 6 months of age or younger with CHD. Mapping of the available research evidence will identify which factors have been studied, where the evidence lends itself to undertaking a future systematic review, and current gaps in the literature.
Materials and methods
This scoping review was conducted utilising the framework of Arksey and O’Malley (2005) that is guided by the requirement of identifying all relevant literature on a topic of interest regardless of the study design. The framework (2005) follows five steps: identification of the research question, identification of relevant studies, study selection, charting of the data, and collation, summarisation, and report of the results. It is important to note that congruent with this framework for scoping reviews, the quality of the included studies was not critically appraised. This scoping review was not registered; however, the original protocol (to which no amendments were made) can be obtained from the corresponding authors.
Inclusion criteria
Participants
This scoping review focused on neonates and infants less than or equal to 6 months of age with CHD; participants were all full-term, defined as greater than or equal to 36 weeks gestational age. The decision was made to include neonates and infants less than 6 months of age to ensure inclusion of all currently published literature on oral feeding in neonates and young infants with CHD. Studies that included both premature and full-term neonates were only included in this scoping review if subanalyses were performed to separate the cohorts, as prematurity can be associated with specific neurodevelopmental challenges that are different from full-term neonates. Studies that included infants older than 6 months of age were only included if subanalyses were performed to separate the results of the infants 6 months of age or younger from the older cohort.
Setting
All studies analysing full-term neonates and infants less than or equal to 6 months of age with CHD regardless of setting were included in this scoping review. All studies that analysed the outcome variable of interest regardless of inpatient versus outpatient setting were included. The rationale for inclusion of studies regardless of setting was that the variable of oral feeding is a chronic challenge in the population of interest, and there is often longitudinal evaluation of this variable beginning in the neonatal hospitalisation period and continuing into the outpatient environment.
Study designs
The following study designs were included: randomised control trials, non-randomised controlled trials, cluster-randomised control trials, non-randomised cluster-controlled trials (including controlled before and after studies), interrupted time series, one group pre–post-test studies, cohort studies, case–control studies, and cross-sectional studies. Mixed methods studies were included only if they clearly defined a quantitative methodology consistent with one of the included quantitative study designs. Qualitative studies were excluded from this review.
Variables of interest
Studies were included if they evaluated full oral feeding, whether as a dependent or independent variable. For the purposes of this scoping review, oral feeding was defined as “nutrient intake via the oral route” to enable a comprehensive view of the currently published literature. Studies that evaluated dysphagia were included only if the aim of the study was to evaluate oral feeding. There were no restrictions on how and when oral was measured; however, this information was extracted from the included studies. Additional variables that were evaluated in association with full oral feeding were also considered to be of interest. In synthesising the evidence, these additional variables were categorised as either risk factors or health-related outcomes.
Search strategy
The search strategy included bibliographic electronic databases to identify both unpublished and published evidence relevant to the identified question. The search strategy and database inclusion were developed in collaboration with a team member (RJ) who has expertise in medical and nursing literature inquiry. The following electronic databases were queried from 1 January, 1975, through 31 May, 2021, using search terms appropriate for each database (see Supplementary Table 1): PubMed, Embase, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Web of Science (WoS), Scopus, Psychological Abstracts (PsycINFO), and ProQuest Dissertations and Abstracts. The year 1975 was chosen as a start date for the database search based on the history of CHD surgical strategies and high mortality rates of neonates and infants with critical CHD, as 1975 marked the first arterial switch operation performed for an infant with transposition of the great arteries. Reference Marathe and Talwar12
In addition to the electronic database search, a hand-search of the reference lists of included studies was conducted. To identify grey literature, experts in the field were contacted and the following online sites for relevant conference proceedings, abstracts, and reports were searched: The Children’s Hospital of Philadelphia (CHOP) Cardiology, Pediatric Cardiac Intensive Care Society (PCICS), Cardiac Neurodevelopmental Outcome Collaborative (CNOC), and Pediatric Academic Society (PAS).
Study selection
Two reviewers (MJ and JD) independently screened potential titles and abstracts and full text (see Supplementary Table 2) for inclusion in this scoping review using Distiller SR software. The screening forms, reflective of the inclusion criteria, for both levels were piloted with the reviewers (MJ and JD). During title and abstract screening, if one reviewer screened the citation as relevant based on the screening criteria (“yes”) or insufficient information to adequately evaluate the relevance (“unsure”), the reference was moved to full-text screening. For a citation to be excluded during title and abstract screening, both reviewers had to deem the citation as not being relevant to the inclusion criteria (“no”). Agreement was required between reviewers for full-text screening for inclusion (“yes”) or exclusion (“no”), with discrepancies able to be resolved via discussion between the reviewers. Reference Arksey and O’Malley13
Data extraction
One author (MJ) created a standardised data extraction form with guidance from team member (JY), who has extensive expertise in the conduct of evidence syntheses. The data extraction form was piloted by two reviewers (MJ and JD) prior to proceeding with data extraction. No changes were made to the data extraction form prior to implementation. Two reviewers (MJ and JD) extracted data independently using this form within Distiller SR software (see Supplementary Table 3) and resolved discrepancies through discussion.
Results
Reference retrieval
The electronic database search initially identified 2004 references relevant to the research question, with no references identified through additional searching mechanisms. After removal of duplicates, 1361 references underwent title and abstract screening (see Figure 1, Search Strategy Development). Of these, 269 were identified for full-text screening. Through full-text screening, 25 references relevant to the review question were identified and included. Reference Kogon, Ramaswamy and Todd7,Reference Averin and Uzark14–Reference Yi, Kim and Huh37 The primary reasons for exclusion of references at title and abstract and full-text screening are identified in Figure 1. See Supplementary Table 4 for characteristics of the included studies.

Figure 1. Search strategy development PRISMA flow diagram
Study designs
Of the 25 references included in the final narrative synthesis, study designs were as follows: 20 cohort studies Reference Kogon, Ramaswamy and Todd7,Reference Averin and Uzark14,Reference Chaves, Baker-Smith and Rosenthal15,Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference Dewan, Cephus, Owczarzak and Ocampo19–Reference Harrison21,Reference Hsieh, Tabbutt and Xu23,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25–Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pham, Connelly, Wei, Sykes and O’Brien31–Reference Yi, Kim and Huh37 , 2 cross-sectional studies Reference de Souza, Gigoski, Etges and Barbosa18,Reference Pereira, Firpo and Gasparin30 , 1 quasi-experimental with non-equivalent group design Reference Coker-Bolt, Jarrard, Woodard and Merrill16 , 1 case–control study Reference Harrison and Ferree22 , and 1 non-randomised control trial. Reference Indramohan, Pedigo and Rostoker24 Data collection methods were retrospective chart review (n = 17) Reference Kogon, Ramaswamy and Todd7,Reference Averin and Uzark14,Reference Chaves, Baker-Smith and Rosenthal15,Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Harrison and Ferree22,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25–Reference Kataria-Hale, Cognata and Hagan27,Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pham, Connelly, Wei, Sykes and O’Brien31–Reference Yi, Kim and Huh37 or prospective enrolment (n = 7). Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference de Souza, Gigoski, Etges and Barbosa18,Reference Harrison21,Reference Hsieh, Tabbutt and Xu23,Reference Indramohan, Pedigo and Rostoker24,Reference Lambert, Pike and Medoff-Cooper28,Reference Pereira, Firpo and Gasparin30 In one intervention study Reference Coker-Bolt, Jarrard, Woodard and Merrill16 , details of recruitment for the intervention group were not recorded, and historical controls were used as the comparison group. Recruitment of subjects was based on referred clinical populations in 96% (24/25) of the included studies.
Population
Geographic location of the 25 included studies varied, with the majority of studies from the United States (n = 21). Reference Kogon, Ramaswamy and Todd7,Reference Averin and Uzark14–Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference Dewan, Cephus, Owczarzak and Ocampo19–Reference Lambert, Pike and Medoff-Cooper28,Reference Pham, Connelly, Wei, Sykes and O’Brien31–Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley36 Other locations of included studies were Brazil (n = 2) Reference de Souza, Gigoski, Etges and Barbosa18,Reference Pereira, Firpo and Gasparin30 , Australia (n = 1) Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29 , and the Republic of Korea (n = 1). Reference Yi, Kim and Huh37 Sample sizes ranged from 15 to 2201 subjects, with the majority of the studies having sample sizes greater than 50 patients with CHD (n = 19). Reference Kogon, Ramaswamy and Todd7,Reference Averin and Uzark14,Reference Chaves, Baker-Smith and Rosenthal15,Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference Dewan, Cephus, Owczarzak and Ocampo19,Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25–Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pham, Connelly, Wei, Sykes and O’Brien31–Reference Yi, Kim and Huh37
Nine studies recruited only subjects with single-ventricle physiology Reference Averin and Uzark14–Reference Coker-Bolt, Jarrard, Woodard and Merrill16,Reference Hsieh, Tabbutt and Xu23,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25,Reference Lambert, Pike and Medoff-Cooper28,Reference Pham, Connelly, Wei, Sykes and O’Brien31,Reference Piggott, Babb and Yong33,Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley36 , 2 studies recruited only subjects with transposition of the great arteries Reference Harrison21,Reference Harrison and Ferree22 , and 14 studies recruited subjects with various critical congenital heart lesions. Reference Kogon, Ramaswamy and Todd7,Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17–Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Indramohan, Pedigo and Rostoker24,Reference Karsch, Irving, Aylward and Mahle26,Reference Kataria-Hale, Cognata and Hagan27,Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pereira, Firpo and Gasparin30,Reference Pierick, Pierick and Reinking32,Reference Pourmoghadam, DeCampli and Ruzmetov34,Reference Sables-Baus, Kaufman, Cook and Da Cruz35,Reference Yi, Kim and Huh37 Eight studies recruited only neonates with CHD that were full-term gestation (greater than 36 weeks gestation) Reference Kogon, Ramaswamy and Todd7,Reference Coker-Bolt, Jarrard, Woodard and Merrill16,Reference Harrison21–Reference Indramohan, Pedigo and Rostoker24,Reference Lambert, Pike and Medoff-Cooper28,Reference Yi, Kim and Huh37 , 10 studies included both premature and full-term neonates with CHD Reference Chaves, Baker-Smith and Rosenthal15,Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Karsch, Irving, Aylward and Mahle26,Reference Kataria-Hale, Cognata and Hagan27,Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pham, Connelly, Wei, Sykes and O’Brien31–Reference Piggott, Babb and Yong33,Reference Sables-Baus, Kaufman, Cook and Da Cruz35 , and 7 studies did not record the gestational age of the recruited subjects. Reference Averin and Uzark14,Reference de Souza, Gigoski, Etges and Barbosa18,Reference Dewan, Cephus, Owczarzak and Ocampo19,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25,Reference Pereira, Firpo and Gasparin30,Reference Pourmoghadam, DeCampli and Ruzmetov34,Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley36 Seventeen studies included neonates less than 1 month of age Reference Kogon, Ramaswamy and Todd7,Reference Averin and Uzark14,Reference Coker-Bolt, Jarrard, Woodard and Merrill16,Reference Dewan, Cephus, Owczarzak and Ocampo19,Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Hsieh, Tabbutt and Xu23–Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pierick, Pierick and Reinking32–Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley36 , one study included children less than 12 months of age Reference Yi, Kim and Huh37 , two studies included infants at 2 weeks and 2 months of age Reference Harrison21,Reference Harrison and Ferree22 , one study included infants less than 56 days old Reference Chaves, Baker-Smith and Rosenthal15 , one study included infants less than 2 months of age Reference Pham, Connelly, Wei, Sykes and O’Brien31 , one study included infants 0 to 6 months old Reference de Souza, Gigoski, Etges and Barbosa18 , and one study included infants less than 7 months old. Reference Pereira, Firpo and Gasparin30 One study defined the age of subjects as “infancy,” but other details were not provided Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17 (see Supplementary Table 4).
Variables
A function of the research question and inclusion criteria, the primary variable of interest in all studies is oral feeding status upon discharge from neonatal hospitalisation. Oral feeding as a term was not defined in any of the studies. Instead, terms such as dysphagia were used to describe and define oral feeding. In some instances, how oral feeding was measured provided the best explanation of the variable; however, measurement techniques also varied between studies (see Supplementary Table 5). The term “dysphagia” was used in four studies, but how dysphagia was defined differed among the studies. Six studies measured oral feeding as the time (in days) to achieve full oral feeding prior to discharge from the neonatal hospitalisation. Reference Kogon, Ramaswamy and Todd7,Reference Coker-Bolt, Jarrard, Woodard and Merrill16,Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Indramohan, Pedigo and Rostoker24,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25,Reference Kataria-Hale, Cognata and Hagan27 Fourteen studies measured feeding status by the mechanism of feeding at discharge (i.e., full oral feeding, oral and nasogastric tube, nasogastric tube only, and gastrostomy tube). Reference Averin and Uzark14,Reference Chaves, Baker-Smith and Rosenthal15,Reference Davies, Carver, Schmidt, Keskeny, Hoch and Pizarro17,Reference Dewan, Cephus, Owczarzak and Ocampo19,Reference Hsieh, Tabbutt and Xu23,Reference Lambert, Pike and Medoff-Cooper28,Reference Mckean, Kasparian, Batra, Sholler, Winlaw and Dalby-Payne29,Reference Pham, Connelly, Wei, Sykes and O’Brien31–Reference Yi, Kim and Huh37 Two studies utilised high-frequency heart rate variability during oral feeding as a surrogate for oral feeding success or failure. Reference de Souza, Gigoski, Etges and Barbosa18,Reference Pereira, Firpo and Gasparin30 Two studies measured oral feeding using two different validated feeding readiness tools. Reference Harrison21,Reference Harrison and Ferree22 One study did not provide details on how oral feeding was measured. Reference Karsch, Irving, Aylward and Mahle26
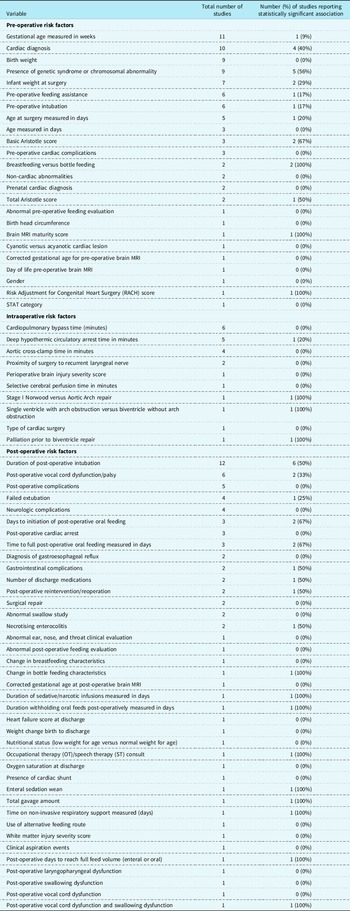
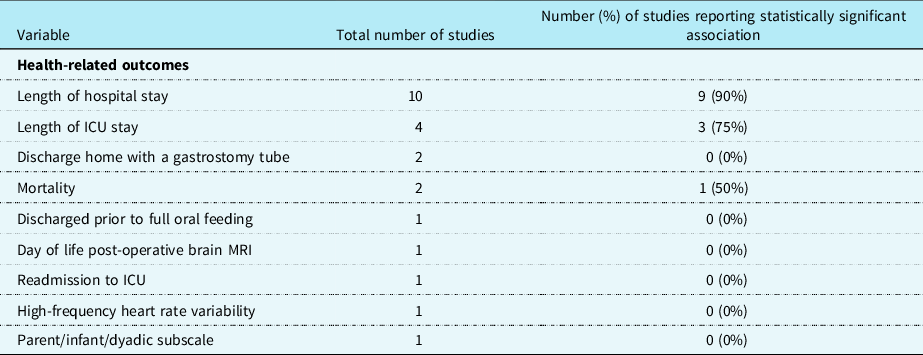
Oral feeding was evaluated as the dependent variable in 18 studies and as an independent variable in 7 studies. Of the 18 studies Reference Kogon, Ramaswamy and Todd7,Reference Chaves, Baker-Smith and Rosenthal15–Reference de Souza, Gigoski, Etges and Barbosa18,Reference Gakenheimer-Smith, Glotzbach and Ou20,Reference Harrison21,Reference Hsieh, Tabbutt and Xu23,Reference Indramohan, Pedigo and Rostoker24,Reference Lambert, Pike and Medoff-Cooper28–Reference Sables-Baus, Kaufman, Cook and Da Cruz35,Reference Yi, Kim and Huh37 where oral feeding was the dependent variable, an association with 75 different independent variables was evaluated (see Table 1). Of the 7 studies Reference Averin and Uzark14,Reference Dewan, Cephus, Owczarzak and Ocampo19,Reference Harrison and Ferree22,Reference Jeffries, Wells, Starnes, Wetzel and Moromisato25–Reference Kataria-Hale, Cognata and Hagan27,Reference Skinner, Halstead, Rubinstein, Atz, Andrews and Bradley36 in which oral feeding was an independent variable, 14 different dependent variables were evaluated as outcome measures (see Table 2).
Table 1. Risk factors

Table 2. Health-related outcomes
Factors associated with oral feeding
For the purposes of this scoping review, risk factors were categorised as follows: pre-operative (n = 22 studies), intraoperative (n = 12 studies), and post-operative (n = 41 studies; See Table 1). Health-related outcomes were categorised for the purposes of this scoping review similarly to risk factors including pre-operative, intraoperative, and post-operative studies; however, only post-operative health-related outcomes were identified (n = 9 studies; See Table 2).
The most commonly evaluated risk factors were gestational age (n = 11 studies), cardiac diagnosis (n = 10 studies), birth weight (n = 9 studies), presence of genetic syndrome or chromosomal abnormality (n = 9 studies), and duration of post-operative intubation (n = 12 studies). The most commonly evaluated health-related outcomes were length of hospital stay (n = 10 studies) and length of ICU stay (n = 4 studies). Of the risk factors described, those with a consistent significant relationship were cardiac diagnosis (4/10 or 40%), presence of genetic syndrome or chromosomal abnormality (5/9 or 56%), and duration of post-operative intubation (6/12 or 50%; See Table 1). Of the health-related outcomes described, length of hospital stay (9/10 or 90%) and length of ICU stay (3/4 or 75%) had the most consistently reported (see Table 2).
In the included studies, when factors associated with oral feeding are considered, there is a lack of consistency with risk factors, but some reliability with health-related outcomes. For example, greater consistency was reported for length of hospital stay (9 of 10, or 90% of studies) and length of ICU stay (3 of 4, or 75% of studies) compared to 56% of studies for the presence of genetic syndrome or chromosomal abnormality (5 of 9 studies), duration of post-operative intubation (6 of 12, or 50% of studies), or 56% of studies), and cardiac diagnosis (4 of 10, or 40% of studies) (see Supplementary Table 6). The health-related outcome of length of hospital stay and length of ICU stay were consistently associated with oral feeding in full-term neonates and infants with CHD.
Discussion
This scoping review is the first to identify and examine the existing literature regarding post-operative feeding ability and challenges for neonates and infants with CHD. Although feeding difficulties is a clearly identified problem in clinical practice, the variability in the literature in this scoping review suggests this problem is not sufficiently addressed in currently published studies. To date, 25 studies have evaluated factors that are associated with oral feeding in this population.
In applying a broad definition of oral feeding to capture studies that mentioned, studied, or otherwise addressed the problem, this scoping review identified that oral feeding was not clearly defined in the studies reviewed. However, all the included studies measured oral feeding at discharge from the neonatal hospitalisation. In the four studies that used the term “dysphagia,” definition of the term was different between the studies. Although the oral feeding measurement methods had similarities across some studies, none of the 25 studies measured oral feeding in the same way. This suggests a noteworthy inconsistency in the literature on oral feeding in neonates and infants with CHD and makes comparisons among studies difficult.
Among the included studies, there was inconsistency in the risk factors associated with oral feeding and some consistency among the health-related outcomes. Risk factors more commonly investigated in the reviewed studies include gestational age (n = 11 studies) and cardiac diagnosis (n = 10 studies). However, these risk factors were not evaluated in all or even the majority of the 25 included studies. Of particular interest is that only one study analysed brain MRI data to determine the presence of any correlation between structural brain characteristics and/or acquired brain injury, with oral feeding ability at time of discharge in subjects with single-ventricle physiology. Further, to this and acknowledging the complexity of understanding predictors of oral feeding in this heterogeneous population, even with the same cardiac diagnosis, neonates and infants often have different pre-operative and post-operative clinical courses that potentially impact their oral feeding abilities. Conversely, length of ICU stay and hospital length of stay were the health-related outcomes most commonly evaluated and were also the factors most consistent with significant associations of oral feeding ability.
There are also a multitude of risk factors and health-related outcomes important to understanding oral feeding in neonates and infants with CHD that were not represented within the included studies. For example, pre-operative oral and enteral feeding is believed to be confounded by the use of prostaglandins, which may increase the risk of necrotising enterocolitis, delaying the initiation of oral or enteral feeding pre-operatively. Reference Kataria-Hale, Cognata and Hagan27 Variation in feeding practices between institutions was also not acknowledged as a potential variable, as some cardiac programmes adhere to standardised post-operative feeding protocols which dictate when oral feeding is initiated post-operatively. This was not evaluated as a factor in the studies included in this review.
The majority of the studies were cohort studies that utilised retrospective chart review for data collection. Sample size was inconsistent between studies, with the majority of studies representative of single institutions. In studies with small sample sizes, this precluded identification of statistically significant correlations by the authors and, when correlations were identified, they limited the generalisability of the findings. The gestational age of the subjects was inconsistent between studies, with either all full-term subjects included or a mixed sample of full-term and pre-term subjects. Some studies performed sub-analyses for gestational age to determine the difference in oral feeding ability between the full-term and premature cohorts, whereas other studies did not clearly state or report findings of additional sub-analyses conducted. Excluding premature neonates in future studies or performing sub-analyses if premature neonates are included is recommended because premature neonates have a constellation of additional risk factors for poor oral feeding, which can confound the conclusions when not taken into consideration during statistical analysis.
Possible reasons for the lack of consistency in findings pertaining to factors associated with oral feeding in the current literature include the variation in how oral feeding was defined across studies, along with the differences in study design and data collection methods. In addition, the use of univariate analysis as the only statistical analysis technique in 12 of the studies did not allow for the identification of independent risk factors. Small sample sizes and single-institution studies also prevent adequate assessment of significant findings related to oral feeding in this population. Even when significant findings are revealed, the small sample sizes and single-institution studies limit their generalisability to the wider population of neonates with CHD and the practical significance of the findings. Some studies exclude neonates with an underlying genetic syndrome to avoid confounding the results. However, as has been identified, up to 35% of children with CHD have an underlying genetic syndrome that can certainly influence oral feeding abilities both pre-operatively and post-operatively.Reference Simmons and Brueckner 39 It is therefore crucial to include neonates with underlying genetic syndromes to determine if presence of a known genetic disorder does, in fact, have predictive value for poor post-operative oral feeding in this population.
Neonates and infants with CHD who require cardiopulmonary bypass in the neonatal period are extremely complex. While predictors of poor post-operative oral feeding are likely multifactorial, further rigorous investigation is needed to better understand this phenomenon within this high-risk paediatric population. Based on this scoping review, several recommendations for research are identified. Future quantitative studies with large sample sizes of neonates and infants inclusive of all CHD lesions requiring cardiopulmonary bypass are needed, as these are reflective of the true population and therefore will improve the external validity of the findings. Including neonates and infants with known genetic syndromes and/or chromosomal abnormalities is crucial across studies, as specific types of CHD can be associated with known, suspected, or unknown genetic abnormalities.Reference Pierick, Pierick and Reinking32, Reference Simmons and Brueckner39 Therefore, excluding neonates and infants with genetic syndromes removes a subset of the population that may be clinically relevant.
Limitations of this scoping review should be acknowledged. The language of the included references was limited to English due to the language fluency of the research team. In addition, although the search strategy was developed by a project team member with expertise in library science, it is possible that potential studies may have been missed for inclusion. Lastly, the framework of Arksey and O’Malley used to guide this scoping review does not include the determination of the quality of individual studies; therefore, while some indications of quality (e.g., study design and sample size) were highlighted, this synthesis does not provide a comprehensive understanding of the quality of the included studies nor how quality may have influenced the results (i.e., significant associations). Reference Arksey and O’Malley13 Of note, an abbreviated electronic database search for scoping reviews and systematic reviews on oral feeding outcomes in similar neonatal surgical populations as a comparison for this scoping review was conducted. However, the search yielded no applicable reviews. Another potential limitation was the exclusion of premature neonates from this scoping review given the potential confounding factors known to be associated with poor oral feeding outcomes related to prematurity. Future syntheses should consider including this population with planned analysis to evaluate differences in factors and outcomes between the premature and full-term infant populations. Lastly, the authors intentionally allowed for a broad definition of oral feeding for this review; however, the lack of a more specific and refined definition may have contributed to the variability of the included studies.
Conclusion
It is widely established that poor oral feeding is a major, long-standing challenge for neonates and infants with CHD. Understanding what influences oral feeding is important to potentially reduce long-term morbidities in a vulnerable population that is now surviving into adulthood. The findings of this scoping review indicate that factors associated with oral feeding among neonates and infants with CHD are being evaluated in the literature. Although the application of Arksey and O’Malley’s (2005) framework does not incorporate a formal assessment of the risk of bias or quality of the included studies, there is some indication that the studies may be sub-optimal in their methodology for various reasons. In addition to the use of a broad range of study designs, there is inconsistency in the variables evaluated and results reflecting the association of these variables with oral feeding across studies. This indicates the need for more rigorous study designs that allow for larger sample sizes and more consistent evaluation of the variables that have been understudied. Despite this variation among studies, this scoping review has identified a sufficient number of studies evaluating similar variables that warrant future conduct of a more focused systematic review. A systematic review would formally appraise and synthesise available evidence to determine if consistently evaluated variables can be considered true factors influencing post-operative oral feeding among neonates and infants with CHD with an identification of certainty in the findings based on an evaluation of methodological rigour. Reference Guyatt, Oxman and Vist38 Findings from such a review could assist in developing and implementing interventions to address significant factors to promote improvement of short- and long-term neurodevelopmental outcomes in neonates and infants with CHD.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122001299
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Not applicable.