Background
Worldwide, approximately 15% of children and adolescents suffer from mental health and/or neurodevelopmental disorders, including anxiety, depression, bipolar disorder, obsessive-compulsive disorder (OCD), autism spectrum disorder (ASD), and attention deficit hyperactivity disorder (ADHD), contributing to lifelong morbidity and leading mental health problems among children to be of immense public health concern (Dalsgaard et al., Reference Dalsgaard, Mortensen, Frydenberg and Thomsen2002; Fergusson et al., Reference Fergusson, Boden and Horwood2007). However, the current management of mental health disorders, including pharmacological, psychological, and/or combined approaches, has frequently yielded suboptimal response rates, underscoring the need for new treatment approaches (Holmes et al., Reference Holmes, Ghaderi, Harmer, Ramchandani, Cuijpers, Morrison, Roiser, Bockting, O’Connor, Shafran, Moulds and Craske2018).
A growing body of evidence demonstrates that diet quality may be a modifiable risk factor for mental disorders. The gut microbiome is a leading hypothesized mechanism of the diet–mental health association as its composition is driven by dietary intake (Marx et al., Reference Marx, Moseley, Berk and Jacka2017). Loss of beneficial microbes in the gastrointestinal tract, the emergence of pathobionts (that is bacteria that cause disease only sporadically), and gut microbiome dysbiosis may influence disease progression and/or severity (Petersen and Round, Reference Petersen and Round2014). Systematic reviews of observational studies have reported consistently significant associations between decreased gut microbial diversity and greater psychopathology in adults (Sanada et al., Reference Sanada, Nakajima, Kurokawa, Barcelo-Soler, Ikuse, Hirata, Yoshizawa, Tomizawa, Salas-Valero, Noda, Mimura, Iwanami and Kishimoto2020; Simpson et al., Reference Simpson, Diaz-Arteche, Eliby, Schwartz, Simmons and Cowan2021). Several pathophysiological mechanisms have been hypothesized to underlie these findings and require data to either support or refute these theories. Emerging paediatric research has documented disruptions to the gut microbiome across a range of chronic health conditions, including asthma (Patrick et al., Reference Patrick, Sbihi, Dai, Al Mamun, Rasali, Rose, Marra, Boutin, Petersen, Stiemsma, Winsor, Brinkman, Kozyrskyj, Azad, Becker, Mandhane, Moraes, Sears, Subbarao, Finlay and Turvey2020; Wang et al., Reference Wang, Guo, Sheng and Zhou2021), ASD (Iglesias-Vazquez et al., Reference Iglesias-Vazquez, Van Ginkel Riba, Arija and Canals2020), ADHD (Ligezka et al., Reference Ligezka, Sonmez, Corral-Frias, Golebiowski, Lynch, Croarkin and Romanowicz2021), and cystic fibrosis (Nielsen et al., Reference Nielsen, Needham, Leach, Day, Jaffe, Thomas and Ooi2016).
Both the gut microbiome and the brain undergo significant developmental change over the first years of life, with numerous microbiota colonizing the gastrointestinal tract while simultaneous neurodevelopmental changes take place. Research has demonstrated a link between the gut microbiome and neurodevelopment in young children, suggesting that the alpha diversity of the gut microbiome as well as the level of abundance of specific gut bacteria affect cognitive functioning at an early age (Jena et al., Reference Jena, Montoya, Mullaney, Dilger, Young, McNabb and Roy2020). The microbiota–gut–brain axis, an intricate communication network between the gut microbiota, the gastrointestinal tract, and the brain, is thought to be a conduit of bidirectional communication between the gut microbiome and the brain. The gut microbiota can produce neurotransmitters and neuroactive compounds that directly influence the central nervous system through the neuroendocrine pathway (Niazi et al., Reference Niazi, Hassan, Tufail and Riaz2023). Additionally, the immune pathway allows immune cells and signalling molecules to affect the brain function (Verma et al., Reference Verma, Soni and Gupta2022). The vagus nerve pathway acts as a major route of communication connecting the gut and the brain. This bidirectional communication system plays a crucial role in regulating physiological processes and has been implicated in influencing brain function, behaviour, and overall health. Research on the microbiome–gut–brain axis is rapidly growing and has revealed several associations between the gut microbiome and neurologic and psychiatric disorders across the lifespan (Bäckhed et al., Reference Bäckhed, Roswall, Peng, Feng, Jia, Kovatcheva-Datchary, Li, Xia, Xie, Zhong, Khan, Zhang, Li, Xiao, Al-Aama, Zhang, Lee, Kotowska, Colding, Tremaroli, Yin, Bergman, Xu, Madsen, Kristiansen, Dahlgren and Wang2015; Marx et al., Reference Marx, Moseley, Berk and Jacka2017; Dickerson et al., Reference Dickerson, Dilmore, Godoy-Vitorino, Nguyen, Paulus, Pinto-Tomas, Moya-Roman, Zuniga-Chaves, Severance and Jeste2022). While the adult microbiome is relatively stable, the microbiome of a child continues to evolve through adolescence, potentially serving as a prime opportunity for preventive intervention (Agans et al., Reference Agans, Rigsbee, Kenche, Michail, Khamis and Paliy2011; Ringel-Kulka et al., Reference Ringel-Kulka, Cheng, Ringel, Salojärvi, Carroll, Palva, de Vos and Satokari2013; Flannery et al., Reference Flannery, Stagaman, Burns, Hickey, Roos, Giuliano, Fisher and Sharpton2020). Moreover, the microbial profiles associated with adult mental health disorders may differ from those for children and adolescents.
Understanding current evidence syntheses is a necessary next step in advancing knowledge in this area of research. The rigorous application of umbrella review methodology fulfils this need as it provides a high-quality overview to guide future research. This umbrella review aims to assess current knowledge about the relationship between the gut microbiome and psychiatric disorders and opportunities for future research among children and adolescents.
Methods
A protocol was created for this umbrella review following the PRISMA extension for Protocols (Rethlefsen et al., Reference Rethlefsen, Kirtley, Waffenschmidt, Ayala, Moher, Page, Koffel, Blunt, Brigham, Chang, Clark, Conway, Couban, de Kock, Farrah, Fehrmann, Foster, Fowler, Glanville, Harris, Hoffecker, Isojarvi, Kaunelis, Ket, Levay, Lyon, McGowan, Murad, Nicholson, Pannabecker, Paynter, Pinotti, Ross-White, Sampson, Shields, Stevens, Sutton, Weinfurter, Wright, Young and Group2021) and was registered on Open Science Framework (Campisi et al., Reference Campisi, Romano, Zhang, Bradley-Ridout, Merriman and Korczak2022). The umbrella review was conducted using the Joanna Briggs Institute Umbrella Review guidelines and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (Aromataris et al., Reference Aromataris, Fernandez, Godfrey, Holly, Khalil and Tungpunkom2015).
Search methodology
The database search strategies were developed by two academic health sciences librarians (K.M. and G.B.R.) and peer-reviewed by an independent librarian following the Peer Review of Electronic Search Strategies for systematic review guidelines (McGowan et al., Reference McGowan, Sampson, Salzwedel, Cogo, Foerster and Lefebvre2016). The search strategy was developed in Ovid Medline and translated into each database using that platform’s command language, including text words, controlled vocabulary, and subject headings when possible. Searches include articles published up until February 2023. The following databases were searched: Child Development and Adolescent Studies (EBSCOhost), Ovid MEDLINE (1946 to present including Epub ahead of print, in-process, and other non-indexed citations), Ovid EMBASE (1947 to present), Ovid APA PsycINFO (1806 to present), EBSCO CINAHL Plus with Full Text (1981 to present), Scopus (Elsevier), and the Cochrane CENTRAL. A modified version of the CADTH SR / MA / HTA / ITC filter was used in the Ovid Medline and Ovid Embase search strategies (CADTH, 2023). Full search strategies can be found in Supplementary Table S1.
Inclusion and exclusion criteria
Review articles were considered eligible if they (1) examined a paediatric population (<18 years of age); (2) included participants with a diagnosis of depression, anxiety, bipolar disorder, ADHD, ASD, or OCD; (3) ensured participants did not have comorbid chronic medical conditions; (4) reported the association between the gut microbiome (i.e. alpha diversity, beta diversity, relative abundances of clusters of DNA or RNA fragment sequences commonly called operational taxonomic units [OTUs]) and mental health disorder or the effect of gut microbiome-targeted treatments (e.g. prebiotic, probiotic, and synbiotic supplementation) on symptoms of mental health disorder with or without the resulting gut microbiome composition changes, in terms of OUT abundance; (5) were peer-reviewed; and (6) were systematic reviews (observational or interventional studies). No limits were imposed on the publication date of records, geographical location, sociodemographic factors, or setting. Primary research, animal studies, narrative reviews, conference abstracts, comments, opinions, letters, and editorials were excluded. Due to the limited capacity within the study for a translator, seven non-English records were excluded.
Study selection and data collection
Deduplication of the search results took place in Covidence online software (Covidence Systematic Review Software, 2023). Screening of all references generated by the database searches occurred independently by four authors (K.R., T.Z., S.C.C., and A.S.) in a two-step process: title and abstract screening were followed by full-text screening. Discrepancies were resolved via discussion with a third author (K.R. or T.Z.). The data extraction template was pilot tested on five included systematic reviews. The following items were extracted: title, author, year, journal, mental health disorder, study review type, date range of studies included in each systematic review, number of studies in the review, sample size, age range, type of intervention and the intervention details (duration of intervention, frequency, timing, and method of intervention delivery, presence of a control group), human gut microbiota data (sequencing platform, relative abundance, richness, alpha and beta diversity), and mental health outcome data (diagnostic tool to assess mental health symptoms, symptom severity/impact). All extracted data were verified by SCC and/or AS.
Methodological quality evaluation
A MeaSurement Tool to Assess Systematic Reviews version 2 (AMSTAR-2) was used to assess the quality of the included systematic reviews and meta-analyses (Shea et al., Reference Shea, Reeves, Wells, Thuku, Hamel, Moran, Moher, Tugwell, Welch, Kristjansson and Henry2017). Quality assessment was performed in duplicate (A.S. and C.Z.) and compared to ensure inter-rater reliability. Further details about individual AMSTAR-2 checklist items can be found in Supplementary Figure S1 (Shea et al., Reference Shea, Reeves, Wells, Thuku, Hamel, Moran, Moher, Tugwell, Welch, Kristjansson and Henry2017). Each item on the AMSTAR-2 checklist was answered with “yes,” “no,” “partial yes,”, or “no meta-analysis conducted”; however, only the “yes” answer counted as a point towards the total score for the assessed review. Based on the total point scores, systematic reviews and meta-analyses were categorized as high-quality, moderate-quality, low-quality, or critically low-quality reviews using previously established definitions (Shea et al., Reference Shea, Reeves, Wells, Thuku, Hamel, Moran, Moher, Tugwell, Welch, Kristjansson and Henry2017).
Taxonomic evidence synthesis
Due to significant heterogeneity among the included systematic reviews, the findings are reported for OTUs that consistently exhibited an association or demonstrated efficacy across multiple reviews concerning mental health disorders. By focusing on the OTUs that demonstrated consistent patterns across the reviewed literature, we aimed to highlight robust signals of these particular associations or efficacies. Results are presented with phylogenetic trees and counts of OTUs reported in systematic reviews. Phylogenetic trees were created using the ‘ape’ package in R (Paradis et al., Reference Paradis, Blomberg, Bolker, Brown, Claude, Cuong and Desper2019).
Sensitivity analysis: overlap minimization
A sensitivity analysis was carried out to examine whether the strength of observed signals between mental health disorders and gut OTUs, as well as the effectiveness of prebiotic/probiotic supplementation in treating mental health disorders, were influenced by the inclusion of the same primary studies in multiple systematic reviews. This issue is particularly relevant in umbrella reviews, as the degree of overlap or inclusion of the same primary study in multiple systematic reviews can potentially introduce bias and affect the accuracy of the findings (Pieper et al., Reference Pieper, Antoine, Mathes, Neugebauer and Eikermann2014).
Overlap minimization followed a multi-step procedure to align the primary studies with the current umbrella review’s inclusion criteria. Initially, the primary studies were assessed to determine their alignment with our predefined inclusion criteria, excluding any studies that did not meet the criteria for inclusion. Next, the degree of overlap in primary studies was calculated using the corrected covered area (CCA) formula: (N – r)/(rc – r), where N represents the number of included primary studies accounting for double counting, r is the number of index publications, and c is the number of included systematic reviews (Hennessy and Johnson, Reference Hennessy and Johnson2020). A CCA within the range of 0%–5% indicates a slight overlap, 6%–10% suggests a moderate overlap, 11%–15% indicates a high overlap, and > 15% shows a very high overlap (Hennessy and Johnson, Reference Hennessy and Johnson2020). We generated a CCA and an overlap matrix for observational and interventional systematic reviews separately with the use of the Graphical Representation of Overlap for OVErviews tool (GROOVE) (Pérez-Bracchiglione et al., Reference Pérez-Bracchiglione, Meza, Bangdiwala, de Guzmán, Urrútia, Bonfill and Madrid2022; Gosling et al., Reference Gosling, Solanes, Fusar-Poli and Radua2023). Whenever possible, higher quality reviews were given priority for retention. To minimize overlap and improve the CCA, systematic reviews with a primary study overlap exceeding 25% were identified, and the review with fewer primary studies was eliminated, prioritizing the retention of higher quality reviews whenever feasible (Lipsey and Wilson 2001, Lunny et al., Reference Lunny, Brennan, McDonald and McKenzie2018). The systematic reviews that remained after the overlap optimization were included in the taxonomic evidence sensitivity analysis.
Results
The database searches yielded 2,099 potential records for this umbrella review following the removal of duplicates (Figure 1). Among the 39 included review studies, 23 (59%) were observational and 16 (41%) were interventional. Of the included reviews, 36 (92%) examined ASD, 6 (15%) examined ADHD, 1 (3%) examined depression, 1 (3%) examined anxiety, and no reviews examined OCD or bipolar disorder [Note: totals add to more than 100% as some reviews examined more than one disorder]. The publication date for the included review studies ranged from 2013 to 2023, with 31 (80%) published between 2020 and 2023. There was significant heterogeneity among the included primary studies regarding demographics (participant characteristics, age range, sex), methodological reporting (stool collection, storage and sequencing approach that is 16S rRNA or shotgun metagenomics), statistical reporting (library preparation that is collection of DNA fragments for sequencing, choice of sequencing platform), and intervention parameters (prebiotic or probiotic combination trialled, intervention duration). Review study characteristics can be found in Supplementary Tables S2, S3 and Table 1.

Figure 1. PRISMA flowchart.
Table 1. Summary of prebiotic, probiotic, and synbiotic interventions along with reported gut microbiome and mental health outcomes among the interventional reviews (n = 16).

Methodological quality assessment
Methodological quality assessment was performed for observational and interventional systematic reviews. Among observational systematic reviews (n = 23), two scored in the high-quality range, eight scored in the moderate-quality range, three scored in the low-quality range, and 10 reviews scored in the critically low-quality range. Interventional systematic reviews and meta-analyses (n = 16) included seven reviews of moderate quality, two of low quality, and seven of critically low quality, using AMSTAR-2 evaluation guidelines. Several criteria of the AMSTAR-2 checklist were under-reported in the included reviews, leading to the heterogeneous outcome of the quality assessment. In detail, none of the reviews reported on the sources of funding for the included studies. Only one review (3%) provided a list of excluded primary studies, and similarly, only one review (3%) used a comprehensive literature search strategy. Furthermore, only 7 (18%) reviews had described their review methods in advance via study registration. Adequate detail regarding the included studies was described in only 9 (23%) reviews. The assessment of the risk of bias was adequately performed in 21 (54%) reviews, and 20 (51%) reviews took into consideration the risk of bias in primary outcomes when discussing the results. No systematic reviews were eliminated from further synthesis based on methodological quality since this was not one of the a priori exclusion criteria. Further detail regarding the quality assessment of individual reviews is presented in Supplementary Table S4.
Gut microbiome taxonomic evidence synthesis
Very few reviews examined the richness and diversity between the gut microbiome and mental health disorders in children. Therefore, it was not feasible to include these findings in the evidence synthesis. As a result, the focus of this umbrella review is on reported OTUs.
Observational systematic reviews – ASD
Among the 23 observational systematic reviews, there were consistent findings regarding the increased relative abundance of certain OTUs in youth with ASD. The most commonly reported OTUs across the reviews were Clostridium clusters, Candida, Dorea, Roseburia, Bacteroides, Oscillospira, Ruminococcus, Barnesiella, Desulfovibrio, and Lactobacillus.
On the other hand, a decreased abundance of specific OTUs was repeatedly observed in youth with ASD. These OTUs included Bifidobacterium, Prevotella, Dialister, Veillonella, Escherichia, Fusobacterium, Streptococcus, and Coprococcus.
It is worth noting that the remaining OTUs were only reported in one review, suggesting limited evidence regarding their association with ASD. Figures 2 and 3 offer a comprehensive overview of the OTUs reported in the observational systematic reviews for ASD and ADHD, including the specific studies that reported them and the corresponding patterns of increased or decreased abundance.
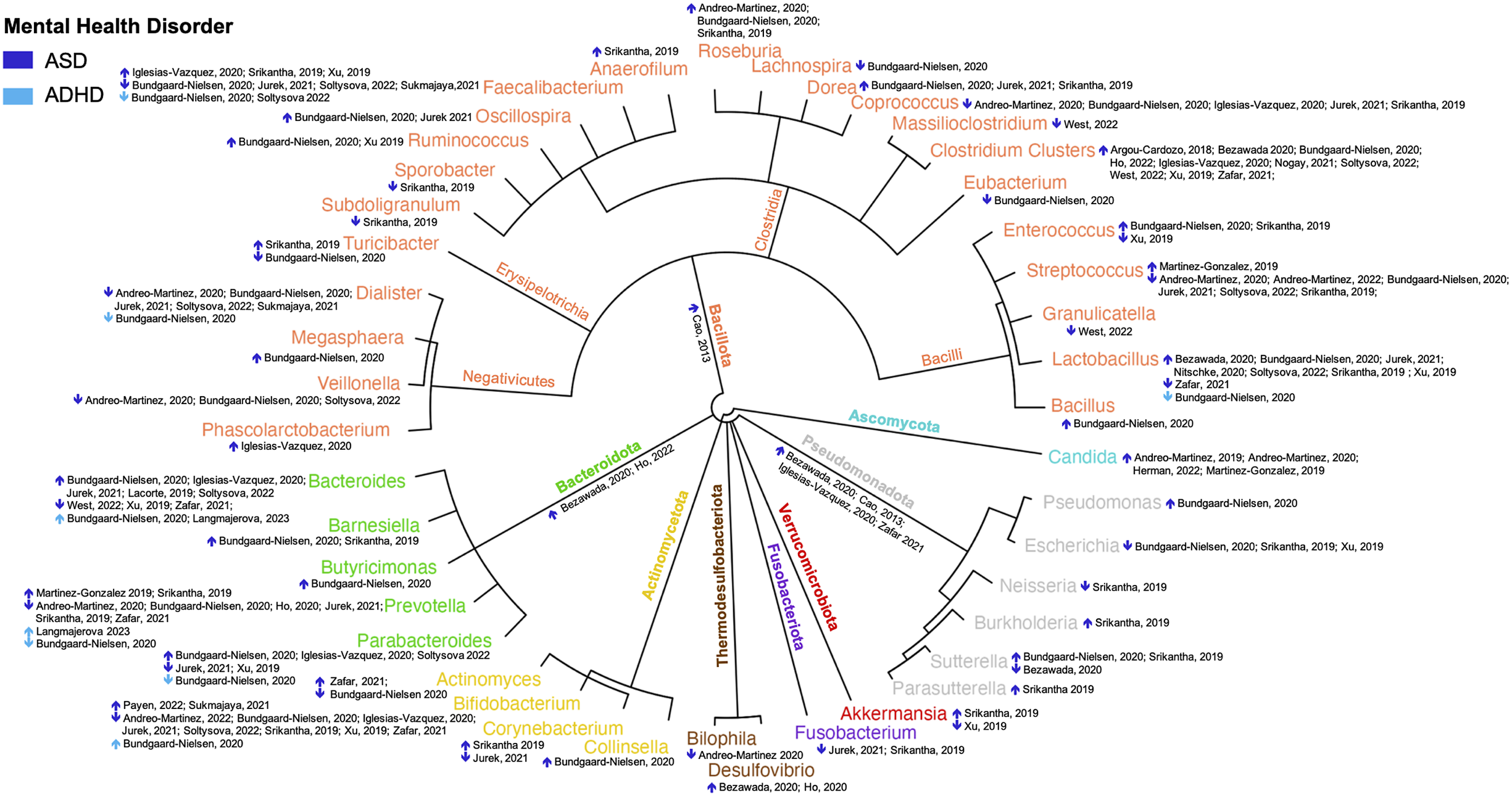
Figure 2. Reported abundance of gut operational taxonomic units in youth with ASD and ADHD (n = 23). The outer circle represents the genus level, with outer colours distinguishing the phylum. Dark blue arrows indicate reviews of ASD, and light blue arrows indicate reviews of ADHD. ↑ indicates higher abundance in youth with the disorder, while ↓ indicates lower abundance in the disorder.

Figure 3. Reported changes in gut microbiota operational taxonomic units following prebiotic, probiotic, and synbiotic supplementation among youth with ASD (n = 16). The outer circle represents the genus level, with outer colours distinguishing phylum. Dark blue arrows indicate reviews of ASD. ↑ indicates higher abundance in youth with the disorder compared to baseline, while ↓ indicates lower abundance in the disorder compared to baseline.
Observational systematic reviews – ADHD
Only two reviews reported an abundance of OTUs in youth with ADHD. Observational reviews consistently reported an increase in the relative abundance of OTUs belonging to the Bacteroides genus in youth with ADHD. On the other hand, a consistent decrease in the relative abundance of certain OTUs belonging to the Faecalibacterium genus was reported in observational reviews in youth with ADHD. The remaining OTUs were only reported in one review, suggesting limited evidence regarding their association with ADHD (Figure 2).
Interventional systematic reviews
Among the 16 interventional reviews, 15 examined the efficacy of probiotics, eight examined the efficacy of prebiotics, and four examined the efficacy of synbiotics on ASD symptoms. One review examined the efficacy of probiotics and prebiotics on anxiety. All supplements were administered orally, as a capsule or packet, one, two, or three times daily, with dosages ranging from 0.5 to 90 × 1010 colony-forming units of bacteria. Treatment duration ranged from 21 days to 15 months.
Improvements in ASD symptoms were reported in all reviews following prebiotic, probiotic, and synbiotic supplementation. One review (Basso et al., Reference Basso, Johnstone, Knytl, Nauta, Groeneveld and Kadosh2022) reported improvement in anxiety symptoms following probiotic or prebiotic supplementation. Details regarding supplement types, duration, dosage, and species as well as gut microbiome and mental health outcomes can be found in Table 1.
Eight reviews reported changes in OTUs. Consistent findings were for the effect of prebiotics, probiotics, and synbiotics on specific bacterial groups. The use of prebiotics consistently increased the abundance of Lachnospira, while the use of probiotics increased the abundance of Lactobacillus and Enterococcus. Furthermore, probiotic intervention decreased the abundance of Desulfovibrio, and probiotics as well as synbiotic treatment consistently decreased the abundance of Clostridium clusters.
The impact of prebiotic, probiotic, and synbiotic supplementation on Bifidobacterium in the gut was inconsistent. Some studies (Yang et al., Reference Yang, Fu, Liao and Li2020; Mitchell and Davies, Reference Mitchell and Davies2021; Prosperi et al., Reference Prosperi, Santocchi, Guiducci, Frinzi, Morales, Tancredi, Muratori and Calderoni2022) reported an increase in Bifidobacterium levels, while others reported decreased levels (Lasheras et al., Reference Lasheras, Gracia-Garcia and Santabarbara2021; Amadi et al., Reference Amadi, Orish, Frazzoli and Orisakwe2022).
The remaining OTUs were only reported in one review, indicating limited findings. Thus, the effects of prebiotics, probiotics, and synbiotics on these specific OTUs remain uncertain and require further investigation (Figure 3).
Sensitivity analysis
Reduction of overlap bias was conducted separately for observational and interventional systematic reviews.
The 23 observational systematic reviews included 92 primary studies, of which 26 were excluded for the following reasons: animal studies (n = 6), other mental health disorders (n = 15), and non-gut microbiome studies (n = 5). Forty-two primary studies were unique and 50 primary studies were reported in ≥2 systematic reviews, yielding a CCA of 12.5% and indicating a high degree of overlap (Supplementary Figure S2). Reduction methods eliminated 13 observational systematic reviews that had >25% overlap, leaving 80 primary studies (40 were unique, and 40 were reported in ≥2 systematic reviews) reported in 10 observational systematic reviews. The CCA was 8.47%, indicating a moderate degree of overlap (Supplementary Figure S3).
The overlap minimization reduced the number of reported OTUs among observational reviews of ASD youth from 43 to 32. The 11 OTUs lost were Bacillus, Eubacterium, Lachnospira, Megasphaera, Oscillospira, Ruminococcus, Butyricimonas, Collinsella, Bilophila, Desulfovibrio, and Pseudomonas. Exaggeration, the replication of primary study’s findings in multiple reviews, was evident for increased and decreased abundance of OTUs. Exaggerated increased abundance was reported for Clostridium clusters, Dorea, Enterococcus, Roseburia, Barnesiella, and Candida. Exaggerated decreased abundance was reported for Coprococcus, Dialister, Veillonella, Fusobacterium, and Escherichia. Reporting inconsistencies remained for Faecalibacterium, Lactobacillus, Prevotella, and Bacteroides, while clearer signals emerged for Streptococcus, Turicibacter, Parabacteroides, Bifidobacterium, Corynebacterium, Akkermansia, and Sutterella (see Figure 4A,B).

Figure 4. Reported increase/decrease in abundance of gut operational taxonomic units in youth with autism spectrum disorder (ASD) (dark blue) and ADHD (light blue) across (A) included systematic reviews (n = 23) and (B) after overlap bias was minimised (n = 10).
The overlap minimization reduced the number of reported OTUs among observational reviews of ADHD youth from seven to three. The four OTUs lost were Dialister, Lactobacillus, Parabacteroides, and Bifidobacterium. Exaggeration in reporting was evident for increased and decreased abundance of OTUs. Exaggerated increased abundance was reported for Bacteroides and decreased abundance for Faecalibacterium. A clearer signal emerged for Prevotella (see Figure 4A,B).
The 16 interventional systematic reviews included 36 primary studies, of which 26 were excluded for the following reasons: not biotic supplementation (n = 11) and not a paediatric population (n = 15). Thirteen primary studies were unique and 23 primary studies were reported in ≥2 systematic reviews yielding a CCA of 18.9% indicating a high degree of overlap (Supplementary Figure S4). Reduction methods eliminated 10 interventional reviews with >25% overlap leaving 28 primary studies (17 were unique and 11 were reported in ≥2 systematic reviews) reported in six interventional systematic reviews. The CCA was 9.3%, indicating a moderate degree of overlap (Supplementary Figure S5).
The overlap minimization reduced the number of reported OTUs among ASD youth for the interventional reviews from 16 to 7. The eight OTUs removed were Acidaminococcus, Blautia, Eubacterium, Faecalibacterium, Ruminococcus, Streptococcus, Veillonella, Bacteroides, and Odoribacter. Exaggerated increased abundance was reported for Enterococcus and Lachnospira and decreased abundance for Clostridium clusters and Desulfovibrio. Inconsistent reporting remained for Bifidobacterium, and a clearer signal emerged for Lactobacillus (see Figure 5A,B).

Figure 5. Reported increase/decrease in abundance of gut operational taxonomic units in youth with ASD following prebiotic (black), probiotic (red), or synbiotic (green) supplementation (A) across included interventional systematic reviews (n = 16) and (B) after overlap bias was minimized (n = 6).
Discussion
This umbrella review is the first to synthesize the current literature, to our knowledge, examining the gut microbiome and psychiatric disorders among children and adolescents. Child and adolescent psychiatric disorders were found to be unequally represented in the included systematic reviews. The majority of reviews focused on ASD, accounting for 92% of the total reviews. A smaller proportion of reviews examined ADHD, with only 15% of the reviews dedicated to this disorder. Furthermore, there was one review each on depression and anxiety, while no reviews specifically addressed OCD or bipolar disorder. The nascency of this research area was further evident as 80% of reviews were published since 2020.
Findings from observational reviews reveal consistent signals of association between specific OTUs and symptoms of ASD in youth. These findings remained following overlap minimization. The most consistent evidence points to an increased abundance of Clostridium clusters and a decreased abundance of Bifidobacterium. Clostridium clusters are spore-forming bacteria, known to release pro-inflammatory toxins that have the potential to reach the brain through the bloodstream (Argou-Cardozo and Zeidan-Chulia, Reference Argou-Cardozo and Zeidan-Chulia2018). Lower ratios of Clostridium clusters have been observed in vegetarians and vegans versus omnivores (Rinninella et al., Reference Rinninella, Cintoni, Raoul, Lopetuso, Scaldaferri, Pulcini, Miggiano, Gasbarrini and Mele2019). Additionally, certain metabolites derived from the activity of Clostridium, such as short-chain fatty acids (SCFAs), are produced through the fermentation of dietary fibre. SCFAs play a critical role in maintaining the proper functioning of the gut immune system by modulating gene expression. SCFAs promote gut health through increasing epithelial cell turnover and their use as sources of energy for intestinal cells (Xing et al., Reference Xing, Pettersson and Kundu2020). Imbalances in SCFA concentrations can disrupt gut homeostasis and potentially trigger peripheral inflammation. Additionally, SCFAs can reach the brain through the bloodstream, where they influence its development by modulating the production of neurotransmitters, for example, serotonin and dopamine (Srikantha and Mohajeri, Reference Srikantha and Mohajeri2019). Bifidobacterium are considered to be beneficial bacteria whose presence is consistent with a greater adherence to Mediterranean diets and lower adherence to high fat intakes similar to those seen in Western diets (Rinninella et al., Reference Rinninella, Cintoni, Raoul, Lopetuso, Scaldaferri, Pulcini, Miggiano, Gasbarrini and Mele2019). Evidence from animal studies suggests that certain species of Bifidobacterium have a positive effect on the hypothalamic–pituitary–adrenal axis by lowering dopamine and adrenaline and may also have an anti-inflammatory effect on the gastrointestinal tract (Moya-Pérez et al., Reference Moya-Pérez, Perez-Villalba, Benítez-Páez, Campillo and Sanz2017). Bifidobacterium also has the capability to produce gamma-aminobutyric acid, an important inhibitory neurotransmitter that is closely associated with the metabolism of glutamate, the primary excitatory neurotransmitter in the brain (Perna et al., Reference Perna, Alalwan, Alaali, Alnashaba, Gasparri, Infantino, Hammad, Riva, Petrangolini, Allegrini and Rondanelli2019).
While the full analysis may exaggerate the importance of certain species, the sensitivity analysis may potentially underestimate the importance of certain species. OTU signals remaining after the sensitivity analysis indicate a lower abundance of beneficial bacteria and higher levels of harmful bacteria in ASD youth contributing to gut dysbiosis, characterized by an imbalance or altered composition of OTUs. For example, weak but consistent increased abundance of OTUs remained for harmful bacteria in ASD youth such as Candida, which releases ammonia and toxins (Reichelt and Knivsberg, Reference Reichelt and Knivsberg2009), Dorea which promotes inflammation (Schirmer et al., Reference Schirmer, Smeekens, Vlamakis, Jaeger, Oosting, Franzosa, Ter Horst, Jansen, Jacobs and Bonder2016), Bacteroides, which produces propionic acid-neurotoxic SFCA (Abdelli et al., Reference Abdelli, Samsam and Naser2019), and SCFA producers such as Roseburia. Moreover, weak but consistently decreased abundance of OTUs remained for beneficial bacteria in ASD youth such as Coprococcus, which are necessary for carbohydrate digestion and fermentation (Kovatcheva-Datchary et al., Reference Kovatcheva-Datchary, Nilsson, Akrami, Lee, Vadder, Arora, Hallen, Martens, Björck and Bäckhed2015), and Dialister, which are known to assist with the maintenance of the normal gastrointestinal tract function (Taylor et al., Reference Taylor, Serrano‐Contreras, McDonald, Epstein, Fell, Seoane, Li, Marchesi and Hart2020).
The inconsistent findings regarding specific OTUs and their association with ASD symptoms may be attributed to several factors. Firstly, the high degree of natural variability observed in the composition of the gut microbiome across different populations can contribute to divergent results. Each individual’s gut microbiome is influenced by numerous factors, including genetics, environment, and lifestyle, leading to significant variation (Vandeputte et al., Reference Vandeputte, Commer, Tito, Kathagen, Sabino, Vermeire, Faust and Raes2021). Secondly, the heterogeneity of the ASD phenotype, characterized by variations in symptom severity and presentation, may also influence the composition of the gut microbiome and obscure clear associations with specific OTUs. One potential explanation for ASD-related gut dysbiosis is that children and youth with ASD have been noted to have lower vegetable intake compared with neurotypical youth. This discrepancy in vegetable consumption has been attributed to selective eating patterns and sensory disturbances commonly experienced by children with ASD (Emond et al., Reference Emond, Emmett, Steer and Golding2010; Siddiqi et al., Reference Siddiqi, Urooj and D’Souza2019). The complex nature of ASD and the diverse symptoms experienced by individuals with ASD make it challenging to establish consistent relationships between specific OTUs and ASD symptoms (Jacob et al., Reference Jacob, Wolff, Steinbach, Doyle, Kumar and Elison2019). Lastly, the variability in the age of subjects included in studies examining the gut microbiome in relation to ASD could contribute to the inconsistent findings. The gut microbiome undergoes developmental changes during childhood, and differences in age among study participants could influence the composition and functioning of the microbiome (Ronan et al., Reference Ronan, Yeasin and Claud2021). These factors collectively emphasize the complexity of studying the gut microbiome in relation to ASD and highlight the need for further research that takes these variables into account to provide more conclusive insights.
Weak signals also indicate the presence of gut dysbiosis in ADHD youth. The sensitivity analysis conducted for ADHD revealed an elevated level of replication of primary study findings in multiple reviews, which further highlights the limited primary research available in this area. Therefore, caution should be exercised when interpreting these findings. Only one review reported an increased abundance of OTUs belonging to the Bacteroides and Prevotella genus in youth with ADHD. Bacteroides metabolize polysaccharides and oligosaccharides, providing nutrition and vitamins to the host and other intestinal microbial residents. However, when dietary habits include the consumption of high animal protein foods, which promote the growth of Bacteroides, it may lead to a relative decrease in other bacterial taxa, creating an imbalance in the microbial community (Desai et al., Reference Desai, Seekatz, Koropatkin, Kamada, Hickey, Wolter, Pudlo, Kitamoto, Terrapon and Muller2016; Rinninella et al., Reference Rinninella, Cintoni, Raoul, Lopetuso, Scaldaferri, Pulcini, Miggiano, Gasbarrini and Mele2019). One review also reported decreased relative abundance of beneficial OTUs belonging to the Faecalibacterium genus which produce butyric acid, bioactive peptides, and other anti-inflammatory substances with immunomodulatory effects, consistent with a diet low in dietary fibre, fruit, and vegetables (Quévrain et al., Reference Quévrain, Maubert, Michon, Chain, Marquant, Tailhades, Miquel, Carlier, Bermúdez-Humarán and Pigneur2016, Zou et al., Reference Zou, Lin, Xue, Tuo, Chen, Chen, Sun, Li, Liu, Dai, Kristiansen and Xiao2021). While these weak signals suggest gut dysbiosis in ADHD youth, it is important to note that the current understanding is based on a limited number of reviews and their findings should be interpreted with caution. Additionally, the complexity of ADHD, which involves various genetic, environmental, and neurological factors, further complicates the interpretation of the results.
Interventions for ASD consistently demonstrated improvements in ASD symptoms across all reviews that evaluated biotic supplements. This trend persisted even after conducting the sensitivity analysis to account for potential overlap. These findings provide support for the hypothesis that the gut–brain axis, which involves bidirectional communication between the gut and the brain, plays a role in the manifestation of ASD. Among the reviews of interventions for ASD, the sensitivity analysis unveiled a high degree of overlap among primary studies, indicative of a reduced volume of primary research available. Therefore, when interpreting the findings, it is essential to consider the implications of minimizing the results due to overlap. Specifically, probiotic supplementation promoted a healthier state of the gut microbiota, known as gut eubiosis, as consistently reported in the reviewed studies. Enhanced gut microbiome eubiosis was characterized by an increase in the abundance of beneficial bacteria, such as Lactobacillus and Enterococcus, and a decrease in the abundance of detrimental bacteria, including clusters of Clostridium. Among this early evidence, a consistent link between enhanced gut microbiome eubiosis and positive outcomes in ASD supports the notion that targeting the gut microbiota through biotic supplements may hold therapeutic potential for individuals with ASD.
The effect of prebiotic, probiotic, and synbiotic supplementation on Bifidobacterium demonstrated inconsistent findings in interventional reviews before and after overlap minimization (Figure 5A,B). This highlights the need for additional research to provide a more comprehensive understanding of the impact of biotic supplementation on Bifidobacterium and to address the lack of evidence.
This umbrella review identified a significant issue of primary study overlap in the systematic evidence gathered so far. A significant amount of overlap was found in the primary studies included in observational systematic reviews and interventional reviews. Approximately 54% of primary studies in the observational reviews and 64% of primary studies in the interventional reviews were reported in two or more reviews. This overlapping inclusion of studies can introduce a high to very high level of bias when reviewing the systematic evidence. This bias was demonstrated in the sensitivity analysis, which revealed exaggerated reporting of OTUs in the included studies. Moreover, the review found that a considerable proportion (56%) of the observational and interventional reviews had low or critically low methodological quality. This was due to several factors, including missing details about excluded primary studies, failure to provide a registered a priori protocol, and inadequate use of comprehensive search strategies. These methodological shortcomings may limit the reliability and generalizability of the findings and underscore the importance of following rigorous systematic review protocols.
This umbrella review also highlights a significant issue of reporting inconsistency within the literature regarding the gut microbiota of children and adolescents with mental health disorders. Although all studies reported relative taxon abundances, very few report diversity measures such as richness, which is crucial in understanding the gut microbiome’s complexity. Diversity, richness, and evenness are all vital concepts that provide a more comprehensive understanding of the gut microbiome. A greater diversity of bacteria in the gut is generally considered to be more beneficial than a less diverse microbiome (Young and Schmidt, Reference Young and Schmidt2008). Therefore, the lack of diversity reporting in the literature is a significant gap that needs to be addressed to better understand the relationship between the gut microbiome and mental health disorders in children and adolescents. Additionally, the compositional nature of sequence datasets may also contribute to the conflicting findings, as an increase in the relative abundance of a taxon can occur even if its absolute abundance decreases (Gloor et al., Reference Gloor, Macklaim, Pawlowsky-Glahn and Egozcue2017). This could lead to confounding findings from many of the included reviews, possibly providing an explanation for the inconsistent results observed in different studies.
This umbrella review has several strengths. Firstly, the search and review process was rigorous, and the broad search strategy resulted in a large number of records being captured without limiting the search by date or country of publication. Secondly, this review addressed the issue of primary study overlap, which is a known source of bias in umbrella reviews. By minimizing overlap, the review was able to provide a more comprehensive and unbiased synthesis of the available evidence. Additionally, the inclusion of both observational and interventional reviews allowed for a comprehensive assessment of the literature on the gut microbiome and mental health disorders among youth.
Despite these strengths, some limitations should be considered when interpreting the results. Due to a high degree of heterogeneity, we were unable to conduct a meta-analysis. Caution should be exercised when interpreting findings related to OTUs, as the significance of certain OTUs may be overstated due to primary study overlap, while the importance of others may remain unclear or ambiguous. Additionally, due to the limitations inherent in a high-level overview, we were unable to delve into the specifics of individual treatments and their direct effects on the gut microbiome and mental health. However, our study contributes by synthesizing and presenting a broader perspective on the overall trends and implications of biotic treatments on mental health. Also, we did not report OTUs that exhibited no associations or lack of efficacy with the disorders under investigation to maintain clarity and avoid presenting conflicting or inconclusive information. It is important to note that the research field on this topic is still in its early stages, and as more studies emerge, this will improve. Additionally, the current review may under-represent research conducted in a language other than English, as our inclusion criteria led to the exclusion of seven non-English reviews. Finally, although this review focused on alterations of gut microbiota, other research has shown that the gut–brain axis links numerous peripheral intestinal functions to brain centres through a wide range of processes and pathways, potentially in a bidirectional manner (Arneth, Reference Arneth2018).
To further advance our understanding of the relationship between the gut microbiome and mental health disorders among children and adolescents, future studies should consider several important methodological improvements. Researchers should consider using amplicon sequence variants (ASVs) in study analyses. ASVs allow for better comparisons across different studies and enable the identification of rare taxa that would otherwise be lost in OTU-based analyses (Amir et al., Reference Amir, McDonald, Navas-Molina, Kopylova, Morton, Xu, Kightley, Thompson, Hyde and Gonzalez2017; Chiarello et al., Reference Chiarello, McCauley, Villéger and Jackson2022). This is because OTUs can vary according to the dataset, as they rely on (and are sensitive to) clustering of sequences, while ASVs will be consistent and are not impacted by the dataset. Future research accounting for lifestyle factors and other drivers of microbiome composition such as antibiotic use is needed to determine whether putative variations in the microbiome are mechanistically important or simply non-specific correlates of psychiatric disorders. Research examining potential mechanisms of action is also needed, whereby the pathways by which the gut microbiome may influence mental health can be more clearly elucidated. Although studies using 16S analysis can provide data on species, the resolution is too limited for this purpose, given dramatic differences in gene complements even across strains of the same species. Thus, future studies should also incorporate more mechanistic approaches such as metagenomics, metatranscriptomics, and metabolomics. These technologies can provide a deeper understanding of the functional potential of the microbiome and identify specific pathways and metabolites that may be involved in mediating the relationship between the gut microbiome and mental health. Accordingly, future research should consider metabolomic changes over microbiome changes, whereby researchers can identify specific metabolites that are influenced by alterations in the gut microbiota composition or activity. These changes can be associated with various health conditions, including metabolic disorders, inflammatory bowel diseases, mental health disorders, and more. Understanding these metabolomic alterations could potentially provide valuable information about the mechanistic links between gut microbiota and host health, facilitating the development of targeted interventions or therapeutic approaches. Finally, standardization of intervention approach and reporting is needed to compare results more accurately across clinical trials.
Conclusion
The research investigating the connection between the gut microbiome and mental health disorders in children and adolescents is expanding, with the majority of studies focusing primarily on ASD. A smaller proportion of studies examined the relationship between the gut microbiome and ADHD. However, there is still a significant gap in our understanding of the gut microbiome’s role in other common and debilitating mental health disorders such as depression, anxiety disorders, OCD, and bipolar disorder. Further research in these areas is crucial to gain a better understanding of the potential involvement of the gut microbiome in the development and treatment of these disorders.
In relation to ASD, this umbrella review reveals a consistent pattern across multiple reviews, indicating associations between an increased abundance of detrimental bacteria and a decreased abundance of beneficial bacteria. Although the body of review literature on interventions targeting the gut microbiome in ASD is relatively smaller, emerging evidence suggests that the use of biotic supplements can promote gut microbiome eubiosis, leading to positive impacts on ASD symptoms. These findings support the need for further investigation into the role of biotic supplementation in the management of ASD. Furthermore, considering the diverse array of clinical and microbial phenotypes found in individuals with ASD and the specificity of emerging therapies in targeting specific traits and microbial populations, it is increasingly apparent that a personalized treatment approach should be adopted to effectively address the unique requirements of each patient.
To inform the development of more targeted and effective prebiotic interventions, future research should also consider potential mediators and moderators of the gut microbiome–ASD relationship, including the mechanisms through which the gut microbiome may contribute to ASD development and the factors that may modulate this relationship. Finally, this review highlights the need for the development of consensus reporting guidelines for a core set of gut microbiome outcome measures, which will be essential for comparing results across studies and informing future research efforts in this field.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/gmb.2023.16.
Author contribution
Conceptualization: D.J.K., K.R., T.Z., S.C.C.; Funding acquisition: D.J.K., S.C.C.; Methodology: D.J.K., S.C.C.; Supervision: D.J.K., S.C.C.; Writing – review & editing: D.J.K., K.R., T.Z., A.S., C.Z., J.P., K.M., P.S., S.C.C.; Data curation: K.R., T.Z., A.N.S., C.Z., K.M., S.C.C.; Formal analysis: K.R., A.S., A.N.S., C.Z., J.P., P.S.; Writing – original draft: K.R., A.S., A.N.S., S.C.C.; Visualization: A.S., S.C.C.; Project administration: S.C.C.
Disclosure statement
The authors declare none.
Funding
This work was supported by the SickKids Hospital Precision Child & Youth Mental Health Research Initiative.
Research transparency and reproducibility
Reasonable requests for data can be made to Daphne J. Korczak, 1145 Burton Wing, Department of Psychiatry, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada M5G 1X8; Tel 416 813–6936; Fax 416–813-5236; email [email protected].








