Disordered lipid metabolism, beyond circulating lipid levels, is the underlying cause of insulin resistance and increased cardiovascular risk (Reaven, Reference Reaven2003). This condition usually precedes the diagnosis of type 2 diabetes mellitus by decades while the clinical expression of the disease could be prevented by dietary and lifestyle modification (Tuomilehto et al. Reference Tuomilehto, Lindstrom, Eriksson, Valle, Hamalainen, Ilanne-Parikka, Keinanen-Kiukaanniemi, Laakso, Louheranta and Rastas2001). Many aspects of diet composition have been considered to be important in the modulation of insulin resistance, but in the last few years more attention has been given to the ability of the quality of dietary fat, independent of total amount. Epidemiological evidence and intervention studies in terms of this particular aspect indicate that the quality of dietary fat influences insulin resistance in man; in particular saturated fat worsens it, while monounsaturated and polyunsaturated fats improve it (Perez-Jimenez et al. Reference Perez-Jimenez, Lopez-Miranda and Pinillos2001; Rivellese et al. Reference Rivellese, De Natale and Lilli2002). Therefore, we need to highlight that most gene markers for CVD are not determinant of disease, but predisposing factors in combination with unhealthy environmental exposures; thus, the relevance of understanding the cross-talk between genes and environment and more specifically with dietary factors (Ordovas & Corella, Reference Ordovas and Corella2004a ). In this respect, nutrigenetics is emerging as a multidisciplinary field focusing on studying the interactions between nutritional and genetics factors, and health outcomes (Ordovas & Corella, Reference Ordovas and Corella2004b ).
Several apo B polymorphic sites have been studied for their potential use as markers for CHD in the population (Boekholdt et al. Reference Boekholdt, Peters, Fountoulaki, Kastelein and Sijbrands2003). Moreover, some of them have been investigated for potential gene–diet interactions (Rantala et al. Reference Rantala, Rantala, Savolainen, Friedlander and Kesaniemi2000; Masson et al. Reference Masson, McNeill and Avenell2003). Previously, a common functional polymorphism of the APOB gene promoter was described by van't Hooft et al. (Reference van't Hooft, Jormsjo, Lundahl, Tornvall, Eriksson and Hamsten1999) involving a C to T change located at 516 bp upstream from the transcription start site. This polymorphism was found to induce a significant increase in the transcription rate of the APOB gene and, consequently, in circulating levels of LDL-cholesterol (van't Hooft et al. Reference van't Hooft, Jormsjo, Lundahl, Tornvall, Eriksson and Hamsten1999). Recently, Sposito et al. (Reference Sposito, Gonbert, Turpin, Chapman and Thillet2004) have demonstrated that the -516T APOB allele is an independent predictor of carotid atherosclerosis disease in subjects presenting a broad range of plasma cholesterol levels. These findings are consistent with the fact that the APOB gene promoter plays a major role in cholesterol homeostasis. Previous studies suggest that the effect of the apo B polymorphisms on lipid and glucose parameters could be mediated through linkage to genes with known effect on glucose metabolism or through NEFA exerting their effect on glucose metabolism (Bentzen et al. Reference Bentzen, Poulsen, Vaag and Fenger2003; Gerhard et al. Reference Gerhard, Ahmann, Meeuws, McMurry, Duell and Connor2004). Therefore, genes regulating primarily dyslipidaemia can also modify the insulin sensitivity response to change in dietary fat. Based on this previous evidence, our goal was to study whether the presence of the -516C/T polymorphism in the APOB gene promoter is related to significantly different insulin sensitivity in response to changes in the quality of dietary fat in young healthy subjects.
Experimental methods
Fifty-nine healthy subjects (thirty men and twenty-nine women) were recruited from among 250 students at the University of Cordoba and had an age of 23·1 (sd 1·8) years. Informed consent was obtained from all participants. All subjects showed no evidence of any chronic disease (hepatic, renal, thyroid, or cardiac dysfunction), obesity, or unusually high levels of physical activity (for example, sports training). None of the subjects had a family history of premature coronary artery disease or had taken medications or vitamin supplements in the 6 months before the study. Physical activity and diet, including alcohol consumption, were recorded in a personal log for 1 week and the data were used to calculate individual energy requirements. Mean BMI was under 25 kg/m2 at the onset of the study and remained constant throughout the experimental period. Subjects were encouraged to maintain their regular physical activity and lifestyle and were asked to record in a diary any event that could affect the outcome of the study, such as stress, change in smoking habits and alcohol consumption or intake of foods not included in the experiment design. All studies were carried out in the Research Unit at the Reina Sofia University Hospital (Córdoba, Spain), and the experimental procedure was approved by the hospital's Human Investigation Review Committee.
Diets
The study design included an initial 28 d period during which all subjects consumed an SFA-enriched diet, with 15 % protein, 47 % carbohydrate (CHO) and 38 % fat (20 % SFA, 12 % MUFA and 6 % PUFA) (Fig. 1). We used the SFA diet period as a baseline regimen, as this Western-style diet is frequently consumed in Western countries, including most areas of Spain. The fixed order of administration of the diets was based on findings (KANWU study, etc; Vessby et al. Reference Vessby, Unsitupa, Hermansen, Riccardi, Rivellese, Tapsell, Nalsen, Berglund, Louheranta and Rasmussen2001) that saturated fat is associated with worsening of insulin sensitivity. Our design purpose was to analyse the substitution of this type of diet with two others (high-CHO or high-monounsaturated fat diets). Therefore, after the SFA period, thirty subjects received a MUFA-enriched diet for 28 d in a randomised, cross-over design. The diet contained 15 % protein, 47 % CHO and 38 % fat ( < 10 % SFA; 6 % PUFA; 22 % MUFA). The MUFA-enriched diet was followed by a high-CHO diet for 28 d containing 15 % protein, 55 % CHO and < 30 % fat ( < 10 % SFA; 6 % PUFA; 12 % MUFA). The other twenty-nine subjects received the CHO diet before the MUFA diet. Volunteers were randomly assigned to this sequence of diets. Cholesterol content remained constant (under 300 mg/d) during the three periods. Virgin olive oil, which was used for cooking, salad dressing and as a spread, provided 80 % of the MUFA diet. CHO intake of the CHO diet was based on the consumption of biscuits, jam and bread. Butter and palm oil were used during the SFA dietary period.
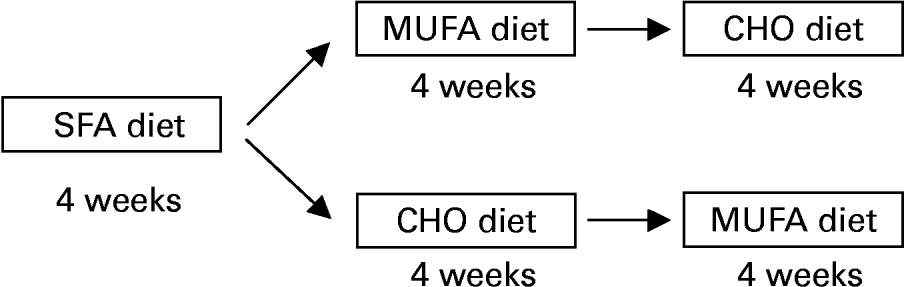
Fig. 1 Study design. Subjects consumed three diets during 4 weeks each following a randomised cross-over design.
The composition of the experimental diets was calculated using the US Department of Agriculture (Human Nutrition Information Service, 1987) food tables and Spanish food composition tables for local foodstuffs (Varela, Reference Varela1980). All meals were prepared in the hospital kitchen and were supervised by a dietitian. Lunch and dinner were eaten in the hospital dining room, whereas breakfast and an afternoon snack were eaten in the medical school cafeteria. Fourteen menus were prepared with regular solid foods and rotated during the experimental period (for example, stew (chickpeas, potatoes, green beans and broth); hake with salad (tomatoes, carrot and lettuce); apples and bread).
Duplicate samples from each menu were collected, homogenised, and stored at − 70°C. The protein, fat, and CHO contents of the diet were analysed by standard methods (Association of Official Analytical Chemists, 1990). Dietary compliance was verified by analysing the fatty acids in LDL-cholesteryl esters at the end of each dietary period (Ruiz-Gutierrez et al. Reference Ruiz-Gutierrez, Prada and Perez-Jimenez1993). The study took place during January and March to minimise seasonal effects and academic stress.
Lipids analysis and biochemical determinations
Venous blood samples for glucose, lipid and lipoprotein analysis were collected into EDTA-containing (1 g/l) tubes from all subjects after a 12 h overnight fast at the beginning of the study and at the end of each dietary period. Plasma was obtained by low-speed centrifugation for 15 min at 4°C within 1 h of venepuncture. Plasma cholesterol and TAG levels were determined by enzymic techniques (Bucolo & David, Reference Bucolo and David1973). HDL-cholesterol was determined after precipitation with fosfowolframic acid (Assmann et al. Reference Assmann, Schierwer, Schmitz and Hägele1983). Apo A-I and B were determined by immunoturbidimetry (Riepponem et al. Reference Riepponem, Marniemi and Rautaoja1987). LDL-cholesterol concentration was calculated using the Friedewald formula (Friedewald et al. Reference Friedewald, Levy and Fredrickson1972). NEFA concentrations were analysed by an enzymic colorimetric assay (Boehringer Mannheim, Mannheim, Germany) (Shimizu et al. Reference Shimizu, Inoue, Tani and Yamada1979). To reduce interassay variation, plasma was stored at − 80°C and analysed at the end of the study.
Insulin suppression test
A modified insulin suppression test was carried out on all the subjects at the end of the dietary period (Harano et al. Reference Harano, Ohgaku, Hidaka, Haneda, Kikkawa, Shigeta and Abe1977; Laws et al. Reference Laws, Jeppesen, Maheux, Schaaf, Chen and Reaven1994). The study began at 08.00 hours, after 12 h of fasting. A continuous infusion of somatostatin (214 nmol/h), insulin (180 pmol/m2 per min) and glucose (13·2 mmol/m2 per min) were infused in the same vein. Somatostatin was used to inhibit endogenous insulin secretion. Blood was sampled every 30 min for the first 2.5 h, by which time steady-state plasma glucose (SSPG) and steady-state plasma insulin (SSPI) concentrations were achieved. Blood was then sampled at 10 min intervals for the last 30 min (at 150, 160, 170 and 180 min) for measurement of plasma glucose and insulin concentrations. These four values determined the SSPG and SSPI concentrations. Since SSPI concentrations were similar in all subjects, SSPG concentrations provided a measure of the ability of insulin to promote the disposal of infused glucose. Subjects with high SSPG are relatively more insulin resistant than others with lower SSPG.
Genetic analysis
DNA extraction was performed by standard procedures. Genotyping for the -516C/T polymorphism was carried out using the restriction enzyme Ear I (New England Biolabs, Inc., Ipswich, MA, USA) as described previously (van't Hooft et al. Reference van't Hooft, Jormsjo, Lundahl, Tornvall, Eriksson and Hamsten1999).
Statistical methods
The Statistical Package for the Social Sciences (version 9.0; SPSS Inc., Chicago, IL, USA) was used for the statistical comparisons. ANOVA for repeated measures was used to analyse the differences between SSPG and SSPI concentrations among several study groups. When statistically significant effects were demonstrated, Tukey's post hoc test was used to identify differences between groups. The Kolmogorov–Smirnov one-sample test was used to test the normality of the distribution. A probability value less than 0·05 were considered significant. All data presented in the text and tables are expressed as means and standard deviations.
Results
Thirty-six subjects were homozygotes for the -516C allele (C/C) (nineteen males and seventeen females) and twenty-three heterozygotes for the -516T allele (C/T) (eleven males and twelve females). There were no TT homozygotes, so the frequency was lower than that reported in an older population from a different geographical area (Sposito et al. Reference Sposito, Gonbert, Turpin, Chapman and Thillet2004). The distribution of genotypes was as expected from the Hardy–Weinberg equilibrium. No differences were recorded between C/C and C/T subjects with respect to age, BMI, serum lipids and glucose concentration at baseline, which are shown in Table 1. No significant differences for any of the variables examined were observed between genotype groups or sex. Daily nutrient intakes of the participants are shown in Table 2. Plasma lipids and apolipoproteins were determined at the end of each dietary period (Table 3).
Table 1 Baseline characteristics according to the -516C/T polymorphism in the APOB gene promoter (Mean values and standard deviations)
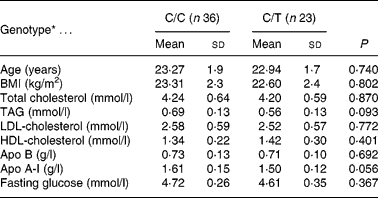
* There were no significant differences between genotypes (ANOVA).
Table 2 Mean daily intake during each experimental diet period

Table 3 Plasma lipids and apolipoproteins at the end of each dietary period (Mean values and standard deviations)

* Mean value was significantly different from that at the end of the SFA-rich dietary period (P < 0·05).
† Mean value was significantly different from that at the end of the low-fat, high-carbohydrate dietary period (P < 0·05).
‡ ANOVA for repeated measures.
Insulin values during the three SSPI periods (MUFA, CHO and SFA) at 150, 160, 170 and 180 min of the insulin suppression test were similar in subjects who were homozygous for the C allele (C/C) (9·54 (sd 1·98), 10·03 (sd 1·74) and 8·87 (sd 1·90) mmol/l) and in subjects carrying the T allele (C/T) (10·23 (sd 2·20), 8·88 (sd 1·32) and 10·49 (sd 2·53) mmol/l; P = 0·685) at the -516C/T apo B polymorphism. On the other hand, SSPG values were higher in C/T than in C/C subjects after the MUFA, CHO and SFA diets (Fig. 2; 6·51 (sd 2·79), 6·77 (sd 2·75) and 8·43 (sd 4·04) mmol/l respectively in C/T v. 5·29 (sd 2·45), 5·51 (sd 2·41) and 5·83 (sd 2·36) mmol/l in C/C subjects; P = 0·010 by ANOVA). In addition, a significant effect on SSPG values was observed for the effect of this mutation in relation to sex (Fig. 3). Thus, male carriers of the -516T showed a significantly greater decrease in SSPG concentrations in changing from the SFA-rich diet (9·18 (sd 1·35) mmol/l) to the MUFA (6·55 (sd 0·74) mmol/l) and CHO (6·31 (sd 0·93) mmol/l) diets than did those who were homozygous for C/C (P = 0·040). This effect was not observed in females (P = 0·391) (Fig. 4).
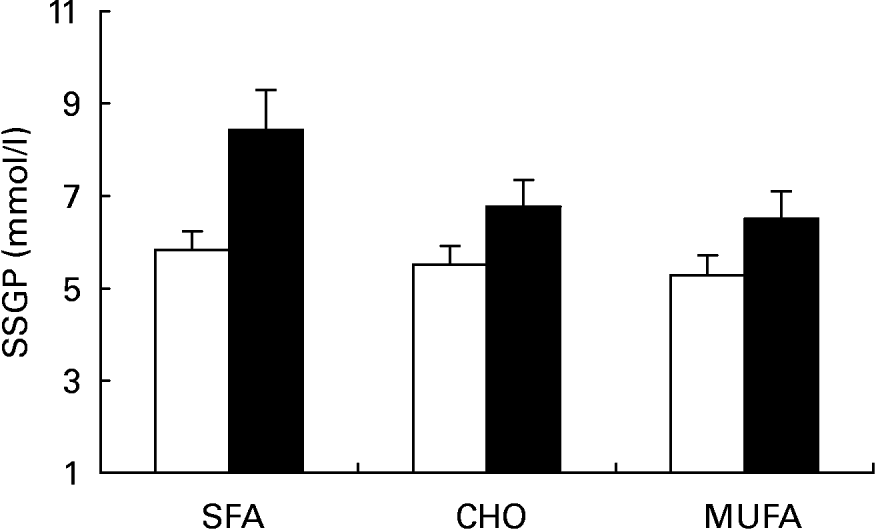
Fig. 2 Steady-state plasma glucose (SSPG) during the insulin suppression test according to the -516C/T polymorphism in the APOB gene promoter after SFA, carbohydrate (CHO)-rich and MUFA diets in the total population. (□), C/C subjects; (■), C/T subjects. Values are means, with standard deviations represented by vertical bars. The effect of diet was significant (P < 0·004); the effect of genotype was significant (P < 0·001); the diet–genotype interaction was not significant (P < 0·126) (ANOVA).
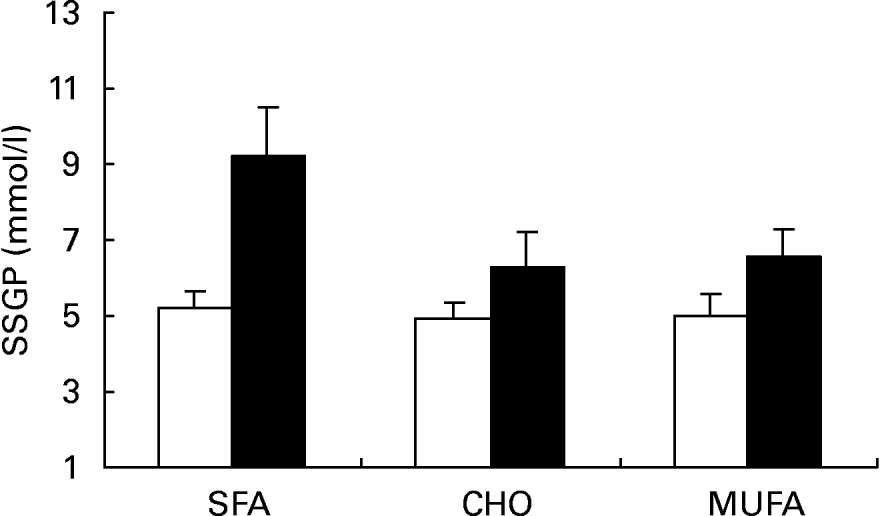
Fig. 3 Steady-state plasma glucose (SSPG) during the insulin suppression test according to the -516C/T polymorphism in the APOB gene promoter after SFA, carbohydrate (CHO)-rich and MUFA diets in the group of males. (□), C/C subjects; (■), C/T subjects. Values are means, with standard deviations represented by vertical bars. The effect of diet was significant (P < 0·012); the effect of genotype was significant (P < 0·011); the diet–genotype interaction was significant (P < 0·04) (ANOVA).

Fig. 4 Steady-state plasma glucose (SSPG) during the insulin suppression test according to the -516C/T polymorphism in the APOB gene promoter after SFA, carbohydrate (CHO)-rich and MUFA diets in the group of females. (□), C/C subjects; (■), C/T subjects. Values are means, with standard deviations represented by vertical bars. The effect of diet was significant (P < 0·039); the effect of genotype was not significant (P < 0·272); the diet–genotype interaction was not significant (P < 0·908) (ANOVA).
Furthermore, C/T subjects presented higher plasma NEFA values than C/C subjects after the consumption of the SFA diet compared with the MUFA and CHO diets (P = 0·001).
Discussion
Our data show that male carriers of the -516T allele, C/T, have a significant increase in insulin resistance after the consumption of all diets, but the difference is more exaggerated after an SFA diet compared with MUFA- and CHO-rich diets. These findings were not observed among female participants.
Epidemiological evidence and intervention studies strongly support the hypothesis that the quality of dietary fat influences insulin sensitivity in man. In contrast, the results are generally difficult to interpret in terms of the presence of some confounders, such as the small number of subjects involved in those studies, the influence of total amount of fat, and dietary fatty acids present in the phospholipids and cholesteryl esters of serum or cell membranes. Even after accounting for all the mentioned caveats, the majority of the epidemiological studies show that a higher intake of saturated fat is associated with the worsening of insulin sensitivity, while the contrary is true for unsaturated fat (Hu et al. Reference Hu, van Dam and Liu2001; Vessby et al. Reference Vessby, Unsitupa, Hermansen, Riccardi, Rivellese, Tapsell, Nalsen, Berglund, Louheranta and Rasmussen2001). On the other hand, other studies have shown that a high-CHO diet is an adequate alternative for improving glucose metabolism in healthy subjects. However, these findings could be related to the individual genetic background.
The original study by van't Hooft et al. (Reference van't Hooft, Jormsjo, Lundahl, Tornvall, Eriksson and Hamsten1999) reported a significant association between the -516C/T polymorphism and plasma concentrations of LDL-cholesterol in healthy males. In that population, subject carriers of the -516T allele had increased LDL-cholesterol concentrations as compared with subjects who were homozygous for the -516C allele. In addition, Bentzen et al. (Reference Bentzen, Poulsen, Vaag and Fenger2003) analysed the effect of five other genetic polymorphisms in the APOB gene on parameters of lipid and glucose metabolism in a Danish population (Gerhard et al. Reference Gerhard, Ahmann, Meeuws, McMurry, Duell and Connor2004). They observed a significant interaction between genotype in T71I, A591V, R3611Q, and E4154K and glucose tolerance on lipid-related parameters and between genotype in L2712P and E4154K and sex on lipid and glucose-related parameters. Thus, they suggest that the effect of these polymorphisms on lipid and glucose parameters could be mediated through NEFA exerting their effect on glucose metabolism.
The present study to our knowledge is the first to examine the association between the -516C/T polymorphism in the APOB gene promoter and insulin sensitivity in response to dietary intervention. Our data indicate that only male carriers of the -516T allele showed a significantly greater decrease in SSPG concentrations in changing from an SFA-rich diet to MUFA or CHO diets than did those who were homozygous for C/C. Such a trend was also observed for the entire group, although it is not statistically significant for young females. Although the sex difference in diet–gene interaction was not hypothesised a priori, the sex interaction parallels findings for blood lipids in these same data; for example, Perez-Martinez et al. (Reference Perez-Martinez, Perez-Jimenez, Ordovas, Bellido, Moreno, Gomez, Marin, Fernandez de la Puebla, Paniagua and Lopez-Mirandain press) have demonstrated a genotype–sex interaction at the apo B-516C>T polymorphism and the lipid profile in the group of the male participants. The sample size could be considered small, but the feeding study design is highly controlled and small sample sizes are often sufficient in this design. Further, the fact that this effect was not observed in females has a reasonable a posteriori explanation. The finding suggests a role of sex hormones in the biological expression of the effects associated with the presence of this polymorphism; for example, there may be a region in the APOB gene promoter that is regulated by oestrogens. At the cellular level, oestrogens regulate mRNA production for particular proteins, among which there are proteins involved in lipid metabolism. Thus, in young females it is likely that the -516C/T polymorphism in the APOB gene promoter would not have an effect on insulin resistance. One interesting finding is that subjects carrying the -516T allele exhibit increasing plasma concentrations of NEFA after the consumption of an SFA-rich diet compared with C/C subjects. This situation may inhibit glucose utilisation by peripheral cells and reduce the effect of peripheral insulin. Another hypothesis to explain our findings should be if the presence of the -516C/T polymorphism in the APOB gene could modulate postprandial lipaemia, in the same way as it has already been observed in other genotypes (Perez-Martinez et al. Reference Perez-Martinez, Lopez-Miranda, Ordovas, Bellido, Marin, Gomez, Paniagua, Moreno, Fuentes and Perez-Jimenez2004). This must be corroborated in future studies.
On the other hand, functional analyses have not been performed and it is difficult to predict whether this polymorphism could have an impact on the expression and/or on the activity of the apo B. Currently, the mechanisms underlying the observations of the present study are not well understood.
In conclusion, allele variability at the apo B-516C/T polymorphism could partly explain the interindividual differences in insulin sensitivity response after the MUFA olive oil-rich and CHO-rich diets in healthy males. Although highly speculative at the moment, the present study proposes an attractive linkage of apolipoproteins of central importance in lipid metabolism to glucose metabolism.
Acknowledgements
The present study was supported by research grants from the Plan Nacional de Investigación (Ministerio de Educación y Ciencia) (SAF 01/2466-C05 04 to F. P.-J., SAF 01/0366 to J. L.-M.), the Spanish Ministry of Health (FIS 01/0449), Consejería de Salud, Servicio Andaluz de Salud (00/212, 00/39, 01/239, 01/243, 02/64, 02/65, 02/78), Consejería de Educación, Plan Andaluz de Investigación, Universidad de Córdoba and by NIH/NHLBI grant no. HL54776 and contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research Service. None of the authors had any conflict of interest.









