The incidence of hormone-sensitive cancers in Asian countries such as China and Japan is lower than that in Western countries, and this has been attributed in part to differences in dietary factors( Reference Magee and Rowland 1 ). Asian diets are traditionally more plant based, lower in fat and higher in fibre and soya products than Western diets, making them rich in phyto-oestrogens( Reference Tham, Gardner and Haskell 2 ). Phyto-oestrogens are naturally occurring non-steroidal food compounds that have structural and functional similarities to 17β-oestradiol, a potent oestrogenic steroid, allowing them to compete with oestradiol by binding to oestrogen receptors. Their anti-oestrogenic activity has raised the possibility that these compounds may provide some protection against hormone-sensitive cancers( Reference Bingham, Atkinson and Liggins 3 , Reference Setchell 4 ). Phyto-oestrogens have also been shown to have antioxidative activity and to influence malignant cell proliferation, differentiation and angiogenesis in such a way as to make them of interest as potential anti-cancer compounds( Reference Adlercreutz 5 ).
There are three main classes of phyto-oestrogens: isoflavones; lignans; coumestans( Reference Magee and Rowland 1 ). Soya products and chickpeas are the main sources of isoflavones (genistein, daidzein and glycitein), while oil seeds, whole grains, legumes, vegetables and berries are the main food sources of lignans (such as secoisolariciresinol, matairesinol, lariciresinol and pinoresinol)( Reference Lahmann, Hughes and Ibiebele 6 ). Coumestrol is the main coumestan consumed by humans and is present in low concentrations in the diet; however, it is relatively rich in foods such as mung bean sprouts and flaxseeds( Reference Thompson, Boucher and Liu 7 ). Most of the plant lignans in foods are converted by bacteria in the upper part of the colon to the enterolignans enterolactone and enterodiol( Reference Adlercreutz 8 ). Equol, a metabolite of the isoflavone daidzein, is also produced by intestinal bacteria; however, this process occurs in only about one-third of the human population( Reference Adlercreutz 9 ). Humans also consume small amounts of pre-formed enterolignans in animal products such as milk, as a result of their production by intestinal bacterial metabolism in animals( Reference Peterson, Dwyer and Adlercreutz 10 ).
Of all the phyto-oestrogens, isoflavones have been the most widely investigated in relation to gynaecological cancers. Experimental studies have suggested that genistein and daidzein can independently modify cytokine production and reduce ovarian cancer cell proliferation( Reference Chen and Anderson 11 , Reference Gercel-Taylor, Feitelson and Taylor 12 ). Similarly, genistein appears to protect against oestrogen-induced cell proliferation in the uterus( Reference Gaete, Tchernitchin and Bustamante 13 , Reference Sha and Lin 14 ). In observational studies, however, the role of phyto-oestrogens in either ovarian or endometrial cancer has received relatively little attention, and the few studies conducted to date have yielded inconsistent results. To our knowledge, only two cohort studies carried out in the USA( Reference Chang, Lee and Canchola 15 ) and Sweden( Reference Hedelin, Lof and Andersson 16 ) and four case–control studies conducted in the USA( Reference Bandera, King and Chandran 17 , Reference McCann, Freudenheim and Marshall 18 ), Italy( Reference Rossi, Negri and Lagiou 19 ) and China( Reference Zhang, Xie and Lee 20 ) have examined the intake of phyto-oestrogens in relation to ovarian cancer. Overall, the findings have shown inverse( Reference Chang, Lee and Canchola 15 , Reference McCann, Freudenheim and Marshall 18 – Reference Zhang, Xie and Lee 20 ) or null( Reference Hedelin, Lof and Andersson 16 , Reference Bandera, King and Chandran 17 ) associations with the intake of isoflavones and/or lignans. For endometrial cancer, the only cohort study to date has reported inverse associations with the intake of only isoflavones( Reference Ollberding, Lim and Wilkens 21 ). Of three case–control studies, two US studies( Reference Bandera, Williams and Sima 22 , Reference Horn-Ross, John and Canchola 23 ) found no evidence of an association with the intake of total phyto-oestrogens, lignans or isoflavones, while a Chinese study( Reference Xu, Zheng and Xiang 24 ) examining soya foods found an inverse association of borderline significance with higher intakes of isoflavones.
In the present study, we used data from two large population-based case–control studies to evaluate the associations between dietary intake of phyto-oestrogens, including the major subclasses and their components, and the risk of ovarian and endometrial cancers overall and by histological subtype. To our knowledge, this is the first observational study of gynaecological cancers to investigate the dietary intake of enterolignans and the first to examine associations by histological subtype. We hypothesised that increasing intakes of total phyto-oestrogens and the major phyto-oestrogen subclasses would be inversely associated with the risk of both ovarian and endometrial cancers.
Subjects and methods
Study participants
Study participants were women aged 18–79 years from two population-based case–control studies conducted in Australia: the Australian Ovarian Cancer Study (AOCS, diagnosis between January 2002 and June 2005) and the Australian National Endometrial Cancer Study (ANECS, diagnosis between May 2005 and December 2007). Full details of study recruitment and data collection methods of the AOCS( Reference Merritt, Green and Nagle 25 ) and ANECS( Reference Rowlands, Nagle and Spurdle 26 ) have been reported previously. In brief, for the AOCS, 3550 women with suspected ovarian cancer were identified through major treatment centres and state-based cancer registries throughout Australia. Of these, 307 died before contact could be made, and a further 498 women were excluded because of language difficulties or mental incapacity or because they were too sick, could not be contacted or the physicians refused permission to contact them. The remaining 2745 with suspected ovarian cancer were invited to participate in the study, and of these, 2319 (84 %) agreed to participate. After histopathology review, 590 women were excluded because they were found not to have primary epithelial ovarian cancer, nineteen because their cancer was first diagnosed before the study period, and one because she was not an Australian resident at the time of diagnosis. Of the final 1709 eligible participants, 1612 (94 %) returned the study questionnaire, and the median time between diagnosis and questionnaire completion was 90 d (3 months). Similarly for the ANECS, 2707 women diagnosed with incident endometrial cancer were identified, and of these, 476 women were excluded because of language difficulties or mental incapacity, because they were too sick or could not be contacted, or because physicians refused permission to contact them. The remaining 2231 women were invited to participate in the study, and of these, 1497 (67 %) agreed to participate. We excluded thirty-nine women who did not have primary endometrial cancer or who were diagnosed before the study period, leaving a final sample of 1458 eligible women. Of these, 1399 (96 %) completed an interview where the median time between diagnosis and interview was 167 d (5·5 months).
Population controls for both the AOCS and ANECS were women aged 18–79 years randomly selected from the National Electoral Roll (enrolment to vote is compulsory in Australia) and frequency-matched to cancer cases by age (in 5-year groups) and state of residence. Of the 3442 women contacted for the AOCS, 1615 (47 %) consented to participate; however, women with a history of ovarian cancer or a bilateral oophorectomy were excluded from the AOCS analyses, leaving 1509 women in the control group. Population controls for the ANECS were sampled using methods identical to those used in the AOCS, with 740 women (participation rate 53 %) being recruited between 2005 and 2007. Informed written consent was obtained from all the participants, and both studies were approved by the Human Research Ethics Committees at the Queensland Institute of Medical Research and all participating institutions.
To increase the power of endometrial cancer analyses, we also included a random sample of 799 AOCS control women (after exclusion of women who reported a prior hysterectomy or endometrial cancer), selected to match the state of residence and age distribution of the endometrial cancer case group, giving a total of 1539 control women. The two subsets of controls were very similar with regard to menopausal status, parity, oral contraceptive use, hormone replacement therapy (HRT) use, smoking, BMI and diabetes (all P>0·1). There were also no significant differences between the two groups of controls with respect to the intake of phyto-oestrogens overall or any subclass except lignans, where the median intake for the endometrial cancer study controls was approximately 13 % higher (P< 0·001) than that for the subset of ovarian cancer study controls. Therefore, we conducted sensitivity analyses restricted to the original ANECS participants to assess whether the inclusion of additional controls had affected the final results.
Dietary intake assessment
For both the studies, dietary intake information was obtained using a 135-item semi-quantitative FFQ based on the instrument developed by Willett et al. ( Reference Willett, Reynolds and Cottrell-Hoehner 27 ), but modified and validated for use in Australia( Reference Marks, Hughes and van der Pols 28 – Reference McNaughton, Marks and Gaffney 30 ). The questionnaire was designed to reflect the typical dietary intakes of Australians and has been found to be reproducible among Australian adult populations( Reference Ibiebele, Parekh and Mallitt 31 ). For each food, a commonly used portion size was specified, and women were asked as to how often they had consumed each food item in the previous year (controls) or in the year before their diagnosis (cases). There were nine response options ranging from ‘never’ to ‘four or more times per day’. Of particular interest for the estimation of isoflavone intake, the soya food group included the following food items: soya milk; low-fat soya milk; soyabeans; soya-based meat substitutes; soyabean and linseed bread. Women who reported that they had changed their diet in the last year or two were asked to report their usual diet before the change. The participants also provided detailed information on the use of dietary supplements. Daily intake of energy (kJ/d) was estimated using the 2007 electronic release version of the Australian food composition table NUTTAB 2006( 32 ).
Because the Australian food composition table lacked information on the phyto-oestrogen content of foods, we used a database developed in the UK by the European Investigation into Cancer and Nutrition-Norfolk( Reference Ward and Kuhnle 33 , Reference Kuhnle, Dell'aquila and Low 34 ) to estimate the dietary intake of individual isoflavones (daidzein, genistein, glycitein, biochanin A and formononetin), enterolignans (enterolactone, enterodiol and equol), and coumestrol and the lignans matairesinol and secoisolariciresinol from food sources. The database used is based on direct analyses of foodstuffs using validated methods( Reference Kuhnle, Dell'aquila and Low 34 ). Development of the database included an investigation of the variability of foods from different sources( Reference Kuhnle, Dell'Aquila and Runswick 35 ). We compared published data on the isoflavone content of bread, soyabeans and soya products in Australia with those in the UK database and found values to be comparable( Reference Lahmann, Hughes and Ibiebele 6 ). Furthermore, as in the UK( Reference Kuhnle, Dell'aquila and Aspinall 36 ), in Australia, soya is used in the production of bread to help improve bread quality. Dietary intake of the lignans lariciresinol and pinoresinol( Reference Thompson, Boucher and Liu 7 ) and intake of phyto-oestrogens from supplements were estimated using a published Canadian database( Reference Thompson, Boucher and Cotterchio 37 ). Although equol is a metabolite of daidzein and not a lignan, we included equol in the estimation of the intake of the subclass of enterolignans because it is produced by intestinal microflora, as are enterodiol and enterolactone( Reference Lahmann, Hughes and Ibiebele 6 , Reference Ward and Kuhnle 33 ). As the use of supplements containing phyto-oestrogens was very low in the populations of the present study (8–11 %), the results were almost identical whether the intake from only food sources or combined intake from foods and supplements was examined. Therefore, we considered the intake of phyto-oestrogens from only food sources in all the subsequent analyses.
Statistical analyses
Of the 1612 cases and 1509 controls who completed the main questionnaire in the ovarian cancer study, we further excluded 155 cases and forty-eight controls who did not return the FFQ, twenty-eight cases and four controls with more than 10 % of FFQ items missing, and sixty-three cases and forty-three controls whose estimated daily energy intake was deemed implausible ( < 2940 kJ or >16 800 kJ), leaving 1366 cases and 1414 controls for the final analysis. Of the 1399 cases and 1539 controls who completed an interview in the endometrial cancer study, we further excluded thirty-three cases and forty-two controls who did not return the FFQ, fifteen cases and sixteen controls with more than 10 % of FFQ items missing, and sixty-three cases and forty-six controls with extreme energy intake, leaving 1288 cases and 1435 controls for the final analysis.
Dietary phyto-oestrogen intake was adjusted for total energy intake using the residual method as described by Willett et al. ( Reference Willett, Sampson and Stampfer 38 ). Because the distributions of dietary intakes of phyto-oestrogens were skewed towards higher values, four categories of phyto-oestrogen intakes were created, which allowed us to better contrast the highest dietary intake (top 15 %) with the lowest (bottom 20 %, used as the reference category). OR and 95 % CI were estimated using unconditional multivariable logistic regression. To test for linear trend across the intake levels of phyto-oestrogens, we modelled the median value in each category as a continuous variable.
For both the studies, multivariable analyses were adjusted for factors strongly associated with phyto-oestrogen intake and/or cancer risk including age (in years), parity (0, 1–2 or ≥ 3), oral contraceptive use (never, < 60 months or ≥ 60 months), HRT use (never, < 3 months or ≥ 3 months), BMI ( < 25, 25–29·9 or ≥ 30 kg/m2), alcohol consumption (number of standard drinks per week), smoking status (current, ex-smoker or non-smoker) and energy intake (log transformed). For the ovarian cancer study, we also adjusted for education (high school, technical college or university), hysterectomy (yes/no) and menopausal status (pre/peri-menopausal or postmenopausal). Additional covariates for the endometrial cancer study included age at menarche ( ≤ 12, 12–14 or >14 years) and type 2 diabetes (yes/no).
Multiplicative interaction terms were entered into the logistic regression model to test for effect modification by menopausal status and HRT use (pre/peri-menopausal or postmenopausal and no HRT use or postmenopausal with HRT use) for both cancer studies. For endometrial cancer, we also tested for the possibility of effect modification by obesity (BMI < 30 v. ≥ 30 kg/m2). These variables were selected a priori based on our knowledge of the relationship between these factors and oestrogen levels and the importance of BMI as a strong risk factor for endometrial cancer.
Analyses were carried out for all the tumour subtypes combined and then separately by histological subtype. For ovarian cancer, we examined the endometrioid (cases n 113), mucinous (cases n 158), clear-cell (cases n 69), invasive serous (cases n 693) and borderline serous (cases n 120) subtypes separately, and for endometrial cancer, we defined type I as low-grade endometrioid tumours (cases n 993) and type II (cases n 295) as all other epithelial subtypes (including serous and clear-cell, high-grade endometrioid and carcinosarcoma subtypes). All the statistical analyses were carried out using SAS version 9.2 (SAS Institute, Inc.).
Results
The characteristics of the study participants are given in Table 1. Women with ovarian cancer were older and more likely to be postmenopausal, to have had a hysterectomy and to be current smokers compared with the control women. Endometrial cancer cases were more likely to have never smoked, to be obese and to have type 2 diabetes compared with control women. For both cancers, cases were more likely than controls to be nulliparous, to have never used oral contraceptives or HRT, and to abstain from alcohol consumption. Almost all the participants (96 %) reported Caucasian ethnicity.
Table 1 Characteristics of ovarian and endometrial cancer cases and controls (Mean values and standard deviations; number of cases and controls and percentages)
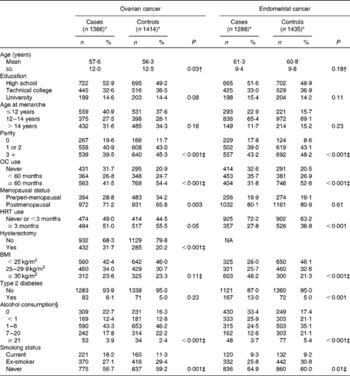
OC, oral contraceptive; HRT, hormone replacement therapy; NA, not applicable.
* Numbers may not sum to total due to missing data.
† t test.
‡ χ2 test for trend.
§ Standard drinks/week (1 standard drink = 10 g alcohol).
Among control women, phyto-oestrogen intake was equal to 1·27 mg/d in the ovarian cancer study and 1·31 mg/d in the endometrial cancer study, of which 0·59/0·63 mg were derived from isoflavones, 0·63/0·66 mg from lignans, 0·02/0·02 mg from enterolignans and 0·01/0·01 mg from coumestrol. In the ovarian cancer study, median total intake of phyto-oestrogens and lignans from food sources was significantly higher in controls than in cases (Table 2). Endometrial cancer cases and controls did not differ significantly according to median total intake of phyto-oestrogens or any phyto-oestrogen subclass except for the intake of coumestrol, which, although low, was significantly higher among controls (Table 2).
Table 2 Energy and energy-adjusted* phyto-oestrogen intakes from foods in ovarian and endometrial cancer cases and controls (Median values and interquartile ranges (IQR))
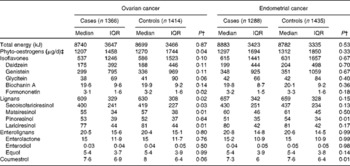
* Energy adjusted using the residual method.
† P value from the Wilcoxon two-sample test.
‡ Total phyto-oestrogen intake = sum of intakes of isoflavones, lignans, enterolignans and coumestrol.
Risk estimates for total intake of phyto-oestrogens, major subclasses and individual compounds are given in Table 3. In crude analyses, there was an indication of inverse associations with higher intakes of phyto-oestrogens, isoflavones and lignans; however, after adjustment, the only statistically significant results were obtained for the lignan compounds matairesinol and lariciresinol (Table 3). The adjusted OR for the highest v. the lowest intake category was 0·72 (95 % CI 0·54, 0·96; P for trend = 0·02) for matairesinol and 0·72 (95 % CI 0·55, 0·96; P for trend = 0·03) for lariciresinol.
Table 3 Adjusted OR for the risk of ovarian and endometrial cancers associated with phyto-oestrogen intake from foods (Adjusted odds ratios and 95 % confidence intervals)
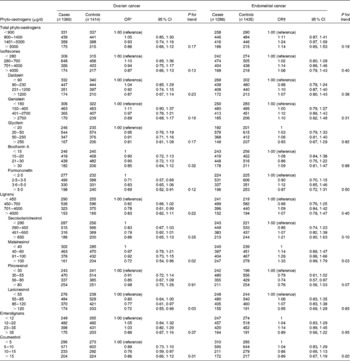
* OR and 95 % CI adjusted for age, energy intake (log transformed), hysterectomy, menopausal status, education, parity, oral contraceptive use, hormone replacement therapy use, BMI, alcohol consumption and smoking status.
† OR and 95 % CI adjusted for age, energy intake (log transformed), age at menarche, parity, oral contraceptive use, hormone replacement therapy use, BMI, type 2 diabetes, alcohol consumption and smoking status.
In contrast, there was no clear pattern of endometrial cancer risk with increasing intakes of total phyto-oestrogens or any of the subclasses. However, a borderline significant positive association was observed for matairesinol, where the adjusted OR for the highest v. the lowest intake category was 1·33 (95 % CI 0·99, 1·79; P for trend = 0·03). As we had previously observed a significant difference between the AOCS and ANECS controls included in the endometrial cancer analyses with respect to the intake of lignans, we conducted sensitivity analyses including only the ANECS controls (n 626) recruited at the same time as the endometrial cancer cases. Overall, the results were similar to those observed from the larger study sample, confirming the null results for phyto-oestrogens, lignans, isoflavones and coumestrol. Of note, there was no longer any association between matairesinol intake and endometrial cancer risk (OR 0·91, 95 % CI 0·63, 1·33; P for trend = 0·71).
In the ovarian cancer study, we observed significant interactions between the combined menopausal status–HRT use (never or < 3 months v. ≥ 3 months) variable and total intake of phyto-oestrogens (P for interaction = 0·03) and enterolignans (P for interaction = 0·03). The interaction test for isoflavones approached significance (P for interaction = 0·07). There was a significant inverse trend for phyto-oestrogens (OR for the highest v. the lowest intake category: 0·52 (95 % CI 0·31, 0·88); P for trend = 0·002) and for isoflavones (OR 0·50, 95 % CI 0·30, 0·84; P for trend = 0·003) only among postmenopausal women who had never used HRT, whereas the intake of enterolignans was inversely associated with ovarian cancer risk among postmenopausal women who had used HRT (OR 0·47, 95 % CI 0·29, 0·76; P for trend = 0·01). In the endometrial cancer study, no significant effect modification was observed between the combined menopausal status–HRT use variable or obesity and total intake of phyto-oestrogens or any subclass (data not shown).
We also investigated the phyto-oestrogen–cancer associations with the different histological subtypes of ovarian and endometrial cancers (data not shown). For ovarian cancer, we found an inverse association between total intake of phyto-oestrogens and the risk of mucinous ovarian tumours (OR for the highest v. the lowest intake category: 0·47 (95 % CI 0·24, 0·93); P for trend = 0·04) with borderline significant associations for isoflavones (OR 0·55, 95 % CI 0·28, 1·07; P for trend = 0·06) and lignans (OR 0·56, 95 % CI 0·29, 1·07; P for trend = 0·07). However, there were no significant associations with non-mucinous tumours, separately or combined. For endometrial cancer, the results did not differ by subtype (type I or type II).
Discussion
The findings of the present study suggest a possible inverse association between consumption of phyto-oestrogens and the risk of ovarian cancer. However, most results did not reach statistical significance at intake levels reported in the Australian study population considered in the present study, for whom the median intake of phyto-oestrogens was 1·27 mg/d. Furthermore, when we examined associations by histological subtype, the inverse results appeared to be largely driven by mucinous ovarian tumours, and there was little or no association for other subtypes. We found no evidence of an association between dietary intake of phyto-oestrogens or any of the main classes and the risk of endometrial cancer overall or by histological subtype.
Ovarian cancer
Previous studies in Western populations have found null( Reference Hedelin, Lof and Andersson 16 , Reference Bandera, King and Chandran 17 ) or inverse( Reference Chang, Lee and Canchola 15 , Reference Rossi, Negri and Lagiou 19 – Reference Ollberding, Lim and Wilkens 21 ) associations between increasing intakes of isoflavones and the risk of ovarian cancer. Of two other case–control studies, one carried out in Italy has reported significant inverse associations with the highest quintile of intake of isoflavones( Reference Rossi, Negri and Lagiou 19 ), while a US population-based study( Reference Bandera, King and Chandran 17 ) has suggested that isoflavones and their components may decrease ovarian cancer risk, although similar to those of the present study, the results did not reach statistical significance for isoflavones, daidzein, genistein or glycitein. Among other Western populations, a recent Swedish cohort( Reference Hedelin, Lof and Andersson 16 ) study has found no association between phyto-oestrogen intake and overall ovarian cancer risk. However, in that study, the mean daily intake of total isoflavones (including genistein, daidzein, formononetin, biochanin A and equol) was only 0·07 mg/d.
In contrast, a case–control study conducted in China( Reference Zhang, Xie and Lee 20 ) has reported that the highest intakes of total isoflavones and the compounds daidzein, genistein and glycitein are each associated with about half the risk of ovarian cancer compared with the lowest intakes. However, soya is widely consumed in China, unlike the Western diet in which lignans are the predominant phyto-oestrogens. The mean daily intake of isoflavones in the Chinese study controls was 24·7 mg/d, much higher than the mean 2·7 mg/d consumed by the population controls in the present study, in which the majority of participants were classified as being of Caucasian ethnicity. Since then, a cohort study conducted in the USA has reported that compared with the relative risk associated with the consumption of less than 1 mg isoflavones/d, that associated with the consumption of more than 3 mg/d is 0·56 in women (95 % CI 0·33, 0·96; P for trend = 0·04)( Reference Chang, Lee and Canchola 15 ).
With respect to lignans, we found that increasing intakes of the lignans lariciresinol and matairesinol were associated with a reduced risk of ovarian cancer, despite their relatively small contribution to overall lignan intake. Similarly, a US population-based case–control study has found a reduced risk for women with the highest quintile of intake of total lignans (combined secoisolariciresinol and matairesinol) compared with those with the lowest intake( Reference McCann, Freudenheim and Marshall 18 ). In contrast, both the Swedish cohort study( Reference Hedelin, Lof and Andersson 16 ) and another US population-based case–control study( Reference Bandera, King and Chandran 17 ) have found no association between intake of total lignans and the risk of ovarian cancer.
It is not clear why associations might be stronger for lariciresinol and matairesinol than for other lignans; however, Adlercreutz( Reference Adlercreutz 8 ) has suggested that the oestradiol-binding nuclear type II binding sites may mediate the anti-cancer action of lignans and that, of the lignans tested, matairesinol is the most effective at binding to these sites( Reference Adlercreutz, Mousavi and Clark 39 ). Others have reported decreased risks of breast cancer associated with higher intakes of matairesinol, but not with those of secoisolariciresinol or total lignans( Reference Linseisen, Piller and Hermann 40 ).
In the ovarian cancer study, we observed significant interactions between the combined menopausal status–HRT use variable and total intake of phyto-oestrogens and enterolignans. There was a significant inverse trend for total intake of phyto-oestrogens and for the intake of only isoflavones among postmenopausal women who had never used HRT, whereas the intake of enterolignans was inversely associated with ovarian cancer risk only among postmenopausal women who had used HRT. Due to the anti-oestrogenic activity attributable to phyto-oestrogens, we hypothesised that inverse associations might be stronger among women who had the highest levels of oestrogen, i.e. pre-menopausal women and postmenopausal women taking HRT. However, this was not the case; the inconsistent results that we observed may have been due to our inability to differentiate between different types of HRT or they may be chance findings due to the multiple comparisons made.
In the present study, we also examined associations by histological subtype of ovarian cancer and found an indication of an inverse association between total intake of phyto-oestrogens and the risk of mucinous ovarian tumours, but no significant associations with non-mucinous subtypes, suggesting different aetiologies. Previous epidemiological studies have reported differences in associations with both reproductive and non-reproductive exposures for mucinous v. non-mucinous cancers( Reference Gram, Lukanova and Brill 41 – Reference Lahmann, Cust and Friedenreich 46 ). Future epidemiological studies should consider the possibility that the effects of phyto-oestrogen intake may also differ by ovarian cancer subtypes.
Endometrial cancer
Contrary to our a priori hypothesis, we found no evidence of an association between total dietary phyto-oestrogens or any phyto-oestrogen subclass and endometrial cancer risk. Similarly, findings from two US population-based case–control studies( Reference Bandera, Williams and Sima 22 , Reference Horn-Ross, John and Canchola 23 ) did not support a protective effect of dietary phyto-oestrogens on the risk of endometrial cancer. Although Bandera et al. ( Reference Bandera, Williams and Sima 22 ) reported a suggestion of an inverse association with the intake of total isoflavones in women with BMI < 25 kg/m2, but no effect modification by HRT use, we found no evidence of effect modification by BMI or HRT use.
To our knowledge, the Multiethnic Cohort Study( Reference Ollberding, Lim and Wilkens 21 ) is the only study to have reported a significantly reduced risk of endometrial cancer with higher intakes of total isoflavones. Notably, the association was found only at consumption levels approximately eleven times higher than the estimated US average of 1·0 mg/d( Reference Chun, Chung and Song 47 ). In contrast, an Asian case–control study has reported limited evidence of an inverse association with isoflavones, even at intake levels >63 mg/d (highest quartile: OR 0·77, 95 % CI 0·56, 1·05; P for trend = 0·05)( Reference Xu, Zheng and Xiang 24 ). The mean intake of isoflavones in our Australian population controls was only 2·6 mg/d (median 0·6 mg/d). It is possible that the strong effects of unopposed oestrogen on the risk of endometrial cancer may be overriding any effect produced by the relatively low intake of dietary phyto-oestrogens in our Western population. Furthermore, our null results for dietary lignans and enterolignans are corroborated by the lack of an association found in the only study to date that has investigated circulating enterolactone levels as a biomarker for plant lignan intake( Reference Zeleniuch-Jacquotte, Lundin and Micheli 48 ).
Conclusions
Several mechanisms have been postulated to explain the potential protective effects of phyto-oestrogens on hormone-related cancers. Phyto-oestrogens have been shown to exert effects on sex hormone metabolism, as well as possess several non-hormonal cancer-protective properties that influence antioxidative activity, intracellular enzyme activity, protein synthesis, and malignant cell proliferation, differentiation and angiogenesis( Reference Adlercreutz 5 ). Specifically, isoflavones (found in soya-based foods) can bind to oestrogen receptors, although weakly compared with endogenous oestrogen, and have a number of other mechanisms of action including antioxidant activity, inhibition of tyrosine kinases (which influence unregulated cell growth) and inhibition of metastasis( Reference Barnes 49 ). The lack of an association between intake of isoflavones and the risk of endometrial cancer, which is strongly associated with oestrogen exposure, may suggest that any association with ovarian cancer may be mediated more by the non-oestrogenic effects of isoflavones. Both lignans and enterolignans increase plasma sex hormone-binding globulin levels, thus reducing the levels of free biologically active sex hormones( Reference Adlercreutz 8 ). In addition, plant-based diets contain more fibre, which reduces intestinal reabsorption of oestrogen( Reference Adlercreutz 8 ). Overall, there is limited but suggestive evidence that the intake of non-starchy vegetables may decrease the risk of both ovarian and endometrial cancers, although the exact mechanisms of protection remain mostly unknown and any protective effect may result from a combination of influences on several pathways involved in carcinogenesis( 50 ).
The strengths of the present study include samples of large sizes obtained from two national population-based case–control studies, the use of a comprehensive and reproducible FFQ( Reference Ibiebele, Parekh and Mallitt 31 ) and the use of comprehensive food composition databases to estimate phyto-oestrogen intake( Reference Thompson, Boucher and Liu 7 , Reference Ward, Kuhnle and Mulligan 51 ). Overall, the intake of phyto-oestrogens and subclasses in the present study was comparable to other reports based on Western populations( Reference Bandera, King and Chandran 17 , Reference McCann, Freudenheim and Marshall 18 , Reference Bandera, Williams and Sima 22 , Reference Chun, Chung and Song 47 , Reference Ward, Kuhnle and Mulligan 51 ). However, selection bias is a particular problem in case–control studies, and we acknowledge that the response rate among controls in the two studies investigated was low at around 50 %. Previous comparisons of the AOCS controls with the 2004 Australian National Health Survey participants( 52 ) have shown that distributions of key variables (education level, parity, BMI and smoking status) are comparable with national data( Reference Pandeya, Williams and Green 53 ). Furthermore, the two studies were conducted in comparable populations of Australian women at similar time periods (2002–5 and 2005–7) and using identical methods, yet we found a suggestive overall pattern of reduction in the risk of ovarian cancer with increasing intakes of phyto-oestrogens, but no association for endometrial cancer. It seems reasonable to assume that any selection bias would be similar in the two studies and so could not produce differing results.
As is common to all case–control studies, there may have been recall bias associated with reporting of dietary intake data and other factors are difficult to quantify. We acknowledge that there is a high variability in the isoflavone content of different soya products( Reference Setchell and Cole 54 ). Although the FFQ that was used was designed to include the most commonly consumed soyabean food items in the Australian diet, it was not designed to specifically investigate the intake of phyto-oestrogens or their respective subclasses. The absorption of plant lignans and subsequent bioconversion to enterolignans vary greatly between individuals( Reference Peterson, Dwyer and Adlercreutz 10 ). In addition to dietary intakes, many other factors such as smoking and obesity affect the levels of circulating lignans( Reference Adlercreutz 8 ), although we were able to adjust for smoking and obesity in the present study. Comparable to that observed in other studies in Western populations, we observed much lower intakes of phyto-oestrogens than those typically observed in Asian populations, and these may have been insufficient to observe an association, if in fact one exists. Similarly to other observational studies( Reference Chang, Lee and Canchola 15 – Reference Xu, Zheng and Xiang 24 ), we measured dietary intake using a validated FFQ and restricted information to recent dietary history (previous 1–2 years). This may have attenuated our findings if the participants had changed their diets, as it has been suggested that a lifetime exposure to phyto-oestrogens may be needed to gain full health benefits( Reference Adlercreutz 8 , Reference Setchell, Zimmer-Nechemias and Cai 55 ).
In summary, the results of these large population-based case–control studies suggest that phyto-oestrogens have no major protective role in either ovarian or endometrial cancer at the levels typically consumed by Western populations. However, the results suggest a possible inverse association between higher intakes of dietary phyto-oestrogens and the risk of ovarian cancer. Studies examining the circulating levels of phyto-oestrogens and the risk of ovarian cancer overall and by histological subtype may be helpful in exploring this association further.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513003899
Acknowledgements
The authors thank the other ANECS (Amanda Spurdle, Andreas Obermair and Joanne Young) and AOCS (David Bowtell, Adele Green, Georgia Chenevix-Trench and Anna deFazio) investigators and the AOCS and ANECS nurses and research assistants for their contributions. They also thank Angela A. Mulligan (University of Cambridge) and Gunter G. C. Kuhnle (University of Reading) for their assistance with the European Investigation into Cancer and Nutrition-Norfolk (UK) phyto-oestrogen database.
The AOCS Study (http://www.aocstudy.org/) was supported by the US Army Medical Research and Material Command (grant no. DAMD17-01-1-0729); the National Health and Medical Research Council (NHMRC) of Australia (grant no. 199600); Cancer Council Tasmania and Cancer Foundation of Western Australia. The ANECS Study (www.anecs.org.au/) was supported by the NHMRC (grant no. 339435) and Cancer Council Tasmania (grant no. 403031 and 457636). P. M. W. and C. M. N. were funded by fellowships from the NHMRC. Annette Neill was supported by a Cancer Council Queensland scholarship. The US Army Medical Research and Material Command, the NHMRC of Australia, Cancer Council Tasmania, the Cancer Foundation of Western Australia and Cancer Council Queensland had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: A. S. N. had the primary responsibility for preparing the manuscript; A. S. N. and T. I. I. conducted data analyses for both the studies; P. H. L., M. C. H., C. M. N. and P. M. W. were involved in the design of the study and interpretation of the results and critically reviewed the manuscript. All authors approved the final manuscript.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.





