Significant vegetation change has occurred on native rangelands of the Northern Great Plains of the United States. National analyses have shown invasion of rangeland by nonnative grasses such as Kentucky bluegrass (C3 grass) and smooth brome (Bromus inermis Leyss.; C3 grass) (U.S. Department of Agriculture Natural Resources Conservation Service [USDA–NRCS] 2010). Invasion of native prairie by nonnative grasses may compromise ecosystem function and limit potential ecosystem services (Toledo et al. Reference Toledo, Sanderson, Spaeth, Hendrickson and Printz2014). Dense stands of sod-forming grasses may alter hydrological attributes by reducing water infiltration into the soil and increasing surface water runoff (Spaeth et al. Reference Spaeth, Pierson, Weltz and Awang1996). Invasive C3 grasses have higher nitrogen (N) concentrations and less recalcitrant carbon (C) than native C4 grasses, which contribute to faster decomposition rates and accelerated nutrient cycling (Mahaney et al. Reference Mahaney, Smemo and Gross2008; Wedin and Tilman Reference Wedin and Tilman1996). Greater plant and litter production of some invading C3 grasses compared with native species may also contribute to greater soil microbial activity and soil N mineralization (Piper et al. Reference Piper, Lamb and Siciliano2015).
Box 1 Management Implications
Preventing or managing invasion of native rangeland by exotic grasses has become more difficult because of changing climate conditions and cultural aspects. In some instances, it may be necessary to adaptively manage the resulting invaded state (i.e., a novel ecosystem) rather than attempting to restore the native state. A case study of long-term invasion of native rangeland by an exotic grass at Mandan, ND, illustrates that the resulting invaded state could maintain or increase soil carbon levels. Managing the invaded state, however, involves potential trade-offs in other ecosystem services such as species diversity and ecohydrology.
Bluegrass and smooth brome invasion of native prairie has been associated with lax grazing management or nonuse of grasslands in North Dakota (DeKeyser et al. Reference DeKeyser, Clambey, Krabbenhoft and Ostendorf2009, Reference DeKeyser, Meehan, Clambey and Krabbenhoft2013, Reference DeKeyser, Denhardt and Hendrickson2015) and elsewhere in the Midwest (Ellis-Felege et al. Reference Ellis-Felege, Dixon and Wilson2013). Kentucky bluegrass invasion has also been associated with overgrazing (DeKeyser et al. Reference DeKeyser, Meehan, Sedivec and Lura2010). Recent data from a long-term (100-yr) grazing site at the Northern Great Plains Research Laboratory (NGPRL) near Mandan, ND, have shown aggressive invasion of native prairie by Kentucky bluegrass regardless of grazing intensity (Sanderson et al. Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015). The original vegetation at the long-term site was primarily blue grama [Bouteloua gracilis (Willd. ex Kunth) Lag. ex Griffiths; C4 grass] and needle-and-thread grass [Hesperostipa comata (Trin. & Rupr.) Barkworth; C3 grass] (Sarvis Reference Sarvis1920). Increased abundance of Kentucky bluegrass was noted at the long-term grazing site in the 1990s (Frank et al. Reference Frank, Tanaka, Hofmann and Follett1995), and its abundance has increased steadily (Sanderson et al. Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015).
The differences in stable C isotope ratios between C3 and C4 plants can be used to estimate changes in the composition of soil organic C (Balesdent and Mariotti 1987). Because of differences in photosynthetic pathways, C4 plants discriminate less against the 13C isotope than 12C, resulting in a less negative δ 13C ratio (approx. −9‰ to −15‰) than C3 plants (approx. −25‰ to −30 ‰). Long-term changes in photosynthetic functional groups of plant communities are reflected in the isotopic signatures of soil organic C (Wedin et al. Reference Wedin, Tieszen, Dewey and Pastor1995). This relationship has been exploited to determine the amount of either C3- or C4-derived C in soil organic matter (Derner et al. Reference Derner, Boutton and Briske2006).
Changes in the natural abundance of δ15N in ecosystems may reveal patterns in N processes (Robinson Reference Robinson2001). Soil N with a δ15N of near zero or less could indicate that N inputs came primarily from atmospheric N2 fixation (atmospheric δ 15N ~ 0). Soil N with a highly positive δ15N may indicate significant inputs of inorganic N or fertilizer. Changes in δ15N have been used to investigate N cycling in semiarid grasslands (Clark Reference Clark1977) and mechanisms associated with cool-season grass invasion of native grasslands (Sperry et al. Reference Sperry, Belknap and Evans2006). Greater plant diversity in temperate grasslands has been linked to decreased soil δ15N, which may indicate increased N use by vegetation (Kleinebecker et al. Reference Kleinebecker, Holzel, Prati, Schmitt, Fischer and Klaus2014). Grazed grasslands may have higher soil δ15N than similar ungrazed areas because of the discrimination against 15N in N-cycling processes associated with dung and urine decomposition (Frank et al. Reference Frank, Evans and Tracy2004).
Soils from sampling campaigns conducted in 1959 and 1991 at the long-term grazing site have been archived at Mandan. The long-term pastures at Mandan were dominated by blue grama for about 70 to 80 yr (Sanderson et al. Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015). We hypothesized that Kentucky bluegrass invasion altered the 13C and 15N levels in the soil. We expected earlier soil samples to be less negative in δ13C (associated with greater blue grama abundance) than samples in later years, a consequence of the increased abundance of the invasive C3 grass aboveground. We expected later soil samples to have higher δ15N than earlier samples because of losses of the lighter N isotope during recycling of N from dung and urine of grazing cattle. We analyzed archived soils from 1959 and 1991 along with soil samples collected in 2014 for 13C and 15N and related results to changes in vegetation to test the hypotheses.
Materials and Methods
The research site was at the USDA–ARS NGPRL near Mandan, ND. The climate is semiarid, continental with long-term mean annual temperature of 4C and average precipitation of 416 mm year−1. The two long-term pastures used in the study were established in 1915 on native rangeland that had not been tilled or farmed previously (Sanderson et al. Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015). The pastures are adjacent to each other (46°46′11.43″N, 100°54′55.16″W and 46°45′51.03″N, 100°55′14.46″W) and uniform in slope (<3%) and have soils with a blend of Temvik and Wilton silt loams (Fine-silty, mixed, superactive, frigid Typic and Pachic Haplustolls; USDA–NRCS 2016).
Blue grama, needle-and-thread grass, and prairie junegrass [Koeleria macrantha (Ledeb.) J.A. Schultes] along with threadleaf sedge (Carex filifolia Nutt.) and needleleaf sedge (Carex duriuscula C.A. Mey) dominated the vegetation in 1915 (Sarvis Reference Sarvis1920). Forbs and shrubs present were Louisian wormwood (Artemisia ludoviciana Nutt.), fringed sagebrush (Artemisia frigida Willd.), and silverleaf Indian breadroot (Pediomelum argophyllum Rydb.) The pastures have been stocked at either a low rate (1.0 animal unit month [AUM] ha−1) or a high rate (2.4 AUM ha−1) since 1916. During the past century, both pastures have been maintained without tillage, fertilizer, herbicide, or fire. Sanderson et al. (Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015) describe the management details and scientific history of these pastures since 1915.
Soils collected and archived from both pastures in 1959 (NGPRL, unpublished annual report) and 1991 (Frank et al. Reference Frank, Tanaka, Hofmann and Follett1995) were used for C and N analyses. The soil samples from 1959 were collected by H. J. Haas (NGPRL, unpublished annual report) from nine locations in each pasture at a 0- to15.2-cm depth, processed, and stored in sealed glass jars. In autumn 1991, six sites were selected in each pasture, and four soil cores were collected at each site to a 106.7-cm depth in several increments (0 to 7.6, 7.6 to 15.2, 15.2 to 22.8, 22.8 to 30.4, 30.4 to 45.6, 45.6 to 76.2, and 76.2 to 106.7 cm) (Frank et al. Reference Frank, Tanaka, Hofmann and Follett1995). Soil from the four cores was composited for each depth increment at each site. Soil samples from the 0- to 7.6-cm and 7.6- to 15.2-cm depths were used for C and N analyses in this study.
In May 2014, 20 sites were randomly selected and geolocated in both pastures for soil sampling and vegetation analysis. An additional six sites within a cattle exclosure (no grazing treatment; maintained since 1916) in the pasture stocked at 1.0 AUM were also selected and geolocated. Immediately after site selection, soil samples for C and N analyses were collected from each site with a truck-mounted probe. Four cores were collected to a depth of 106.7 cm and separated by depth increments outlined previously (Frank et al. Reference Frank, Tanaka, Hofmann and Follett1995). The four cores at each depth were composited, dried at 35C for 3 to 4 d, and then ground by hand to pass through a 2-mm sieve. Identifiable plant material (>2 mm) was removed during sieving. The 2-mm ground samples were then ground in a roller mill to pass through a 0.106-mm sieve. Soil-processing protocols followed in 2014 matched those used for samples collected 1959 and 1991.
A 1-m2 (2 by 0.5 m) quadrat was evaluated for plant species composition and percent canopy cover at each of the 20 randomly located sites in the pastures and the six sites in the exclosure in May 2014. All species within the quadrat were identified, and canopy cover was visually estimated. The live and dead vegetation was clipped to ground level in two 0.09-m2 (0.3 by 0.3 m) frames at each site and dried at 50°C for 48 h to determine total biomass. The dried biomass from one frame was ground to pass through a 1-mm screen in a shear mill, and a subsample of this material was ground to pass through a 0.5-mm screen in an impact mill. The vegetation from the second frame was separated into live and dead fractions. In addition to bulk plant biomass, four samples of two C3 plant species (Kentucky bluegrass and smooth brome) and two C4 plant species (blue grama and purple threeawn [Aristida purpurea Nutt.]) were collected for 13C and 15N analysis (Table 1). In autumn 2014, one hundred 10-point frames were evaluated for plant species composition (Warren-Wilson Reference Warren-Wilson1963) in each pasture, including one frame on each of the 20 randomly selected clipping sites. Previous vegetation analyses in 1964, 1984, and 1998 (Frank et al. Reference Frank, Tanaka, Hofmann and Follett1995; Sanderson et al. Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015) were based on similar point-frame analyses and are included in Table 2.
Table 1 Carbon and nitrogen isotope analysis of C3 and C4 grasses in the pastures (n=4).
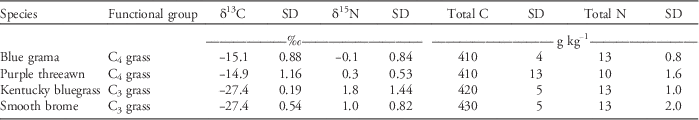
Table 2 Changes in the relative foliar cover of dominant grasses in two long-term pastures [stocking rates of 1.0 and 2.4 animal unit months (AUM) ha−1] between 1964 and 2014 and canopy cover at the time of soil sampling in May 2014 at Mandan, ND.
a Data for 1964, 1984, and 1994 are from Frank et al. (Reference Frank, Tanaka, Hofmann and Follett1995). Relative foliar cover data for 1998, 2004, and 2014 are based on point-frame analysis of vegetation: one hundred 10-pin frames per sampling date in late summer or autumn.
b Canopy cover data from 2014 are averages of visual observations in 20 1-m2 quadrats in the pastures and six quadrats in the exclosure.
Vegetation and soil samples were analyzed for total C and N along with 13C and 15N isotopes with a continuous-flow stable isotope ratio mass spectrometer (Europa Scientific Integra) by the Stable Isotope Facility at the University of California–Davis. Carbon isotope ratios (13C:12C) were expressed relative to the Pee Dee Belemnite standard as a delta-value (δ) with units of parts per thousand (‰). In 2014, we did not acidify soil samples to eliminate carbonates. Reanalysis of soil samples for the 0- to 7.6-cm and 7.6- to 15.2-cm depths from Frank et al. (Reference Frank, Tanaka, Hofmann and Follett1995; acidified) revealed no significant difference in δ13C between nonacidified (−20.41) and acidified (−20.81) soils. Soil pH from these depths was 6.7 or lower. Accordingly, no carbonates would be expected in soil at these depths (Liebig et al. Reference Liebig, Gross, Kronberg, Hanson, Frank and Phillips2006). Nitrogen isotope ratios (15N:14N) were expressed relative to the atmospheric standard and expressed in parts per thousand (‰).
Pastures and the exclosure were not replicated, so strong inferences are not drawn, but P-values are used as an indication of evidence of differences. We used an ANOVA approach to compare changes in soil and plant biomass characteristics with time (2 yr) and grazing intensity (three levels). Soil and biomass data were analyzed on grazing intensity (exclosure=no grazing; 1.0 and 2.4 AUM ha−1) and years (1991 and 2014 only) in a two-way factorial separately for each sampling depth. Comparisons were only made across AUM grazing levels or within year (1991 vs. 2014) using a Tukey-Kramer multiple comparison adjustment for conservative comparison of evidence of differences; as no other interactions were of meaningful interest (Schabenberger and Pierce Reference Schabenberger and Pierce2002; and PROC MIXED in the Statistical Analysis System [SAS Institute 2015] software).
Results and Discussion
Blue grama has predominated in the pastures for several decades (Table 2). Blue grama began to decline in the pasture stocked at 1.0 AUM sometime between 1964 and 1984 (Table 2). During the 1990s, there was a major change from blue grama to dominance by Kentucky bluegrass in both the exclosure and the pasture stocked at 1.0 AUM (Table 2). In the pasture stocked at 2.4 AUM, dominance by Kentucky bluegrass did not occur until the early 2000s. By 2014, the pastures were dominated by Kentucky bluegrass. Smooth brome was prevalent in the exclosure in 2014.
The soil δ13C at the 0- to 7.6-cm depth in the pastures and exclosure became more negative between 1991 and 2014 (Table 3). There was a small change in δ13C at the 7.6- to 15.2-cm depth in the pasture stocked at 1.0 AUM ha−1 between 1991 and 2014, but no changes in the exclosure or the pasture stocked at 2.4 AUM ha−1. Soil C concentrations in the surface 7.6 cm increased an average of 35% (12 g C kg−1; P-values<0.006) from 1991 to 2014; however, soil C concentrations at the 7.6- to 15.2-cm depth did not change (Table 3). Soil C concentrations at either depth did not differ among grazing treatments within 1991 or 2014 (P-values>0.8). In 2014, the estimated proportion of C from C4 plants in the surface 7.6 cm of soil was 18% for the pasture stocked at 1.0 AUM and 30% for the pasture stocked at 2.4 AUM. Corresponding values for the 7.6- to 15.2-cm depth were 13% and 21%.
Table 3 Changes in soil δ13C and soil C from 1991 to 2014 in two long-term pastures [stocking rates of 1.0 and 2.4 animal unit months (AUM) ha−1] and an exclosure (no grazing) at Mandan, ND.Footnote a
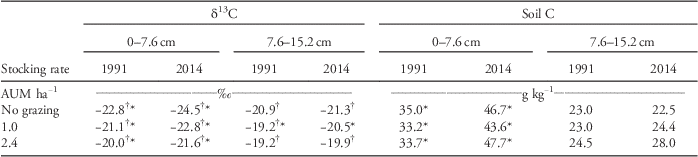
a Comparisons are made within year across AUM levels and between years for each AUM level. Statistical comparisons for differences are indicated with † for within-year comparison and * for between-year grazing-treatment comparison. If values in columns or rows have the same symbol, it indicates a statistical difference (Tukey-Kramer adjusted for multiple comparisons) at P≤0.05. If no symbols are present within a column, then there were no differences between values.
Soil δ13C became less negative with increased grazing intensity at both soil depths in 1991 and 2014 (Table 3). The pasture stocked at 2.4 AUM ha−1 had a greater abundance of blue grama than the lightly stocked pasture and exclosure, contributing C with a less negative δ13C value.
The amount of standing live biomass was greatest in the pasture stocked at 2.4 AUM ha−1, whereas the amount of dead biomass was greatest in the exclosure compared with other grazing treatments in May 2014 (Table 4). There were differences in δ13C, δ15N, C, and N values for live biomass among the exclosure and pastures; however, these differences were very small and of little practical significance. The δ13C, δ15N, C, and N values for dead biomass were similar among the exclosure and pastures.
Table 4 Amount of live and dead biomass in two long-term pastures [stocking rates of 1.0 and 2.4 animal unit months (AUM) ha−1] and an exclosure (no grazing) at Mandan, ND in May 2014 along with carbon (C) and nitrogen (N) concentrations and isotope composition.Footnote a
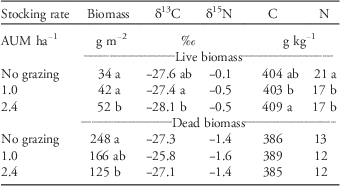
a Mean values with different letters are significantly different from each other at P≤0.05 (Tukey-Kramer adjusted for multiple comparisons). If no letters are present within a column, then there were no differences between values.
Changes in soil N in the exclosure and pastures were limited. The soil δ15N at the 0- to 7.6-cm depth was higher in the pastures than in the exclosure in 1991 and 2014 (Table 5). Soil δ15N decreased from 1991 to 2014 at the 0- to 7.6-cm depth in the exclosure and pastures and at the 7.6- to 15.2-cm depth only for the pasture stocked at 1.0 AUM ha−1. Soil N concentrations did not differ among the pastures or exclosure, except for a slightly lower soil N at the 0- to 7.6-cm depth in the pasture stocked at 1.0 AUM in 2014. Soil N in the surface 7.6 cm increased slightly (average of 0.9 g N kg−1) from 1991 to 2014, and levels at the 7.6- to 15.2-cm depth did not change.
Table 5 Changes in soil δ15N and total N from 1991 to 2014 in two long-term pastures [stocking rates of 1.0 and 2.4 animal unit months (AUM) ha−1] and an exclosure (no grazing) at Mandan, ND.Footnote a
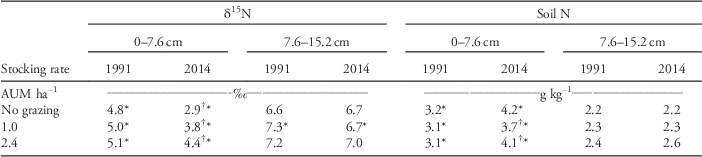
a Comparisons are made within year across AUM levels and between years for each AUM level. Statistical comparisons for differences are indicated with † for within-year comparison and * for between-year grazing-treatment comparison. If values in columns or rows have the same symbol, it indicates a statistical difference (Tukey-Kramer adjusted for multiple comparisons) at P≤0.05. If no symbols are present within a column, then there were no differences between values.
We had soil samples only to a 15.2-cm depth from 1959 from the two pastures (none from the exclosure); thus, we were able to make only qualitative comparisons of depth-averaged values between 1959 and 2014 (unpublished data). The δ13C of soil to a 15.2-cm depth in 1959 was −20.8‰ and −19.4‰ for the pastures stocked at 1.0 AUM and 2.4 AUM, respectively. In 2014, the depth-averaged values for the same pastures were −21.7‰ and −20.8‰. Soil C concentrations were 30 and 33 g C kg−1 soil for the two pastures in 1959, compared with average values of 34 and 38 g C kg−1 in 2014. The δ15N of soil to a 15.2-cm depth in 1959 was 5.5‰ and 5.8‰ for the pastures stocked at 1.0 AUM and 2.4 AUM, respectively. In 2014, the depth-averaged values of δ15N for the same pastures were 5.2‰ and 5.7‰. Soil N concentrations were 2.7 and 3.0 g N kg−1 soil for the two pastures in 1959, compared with average values of 3.0 and 3.4 g N kg−1 in 2014.
The vegetation in the pastures and exclosure had been mainly native C3 and C4 grasses for more than 70 yr (Sanderson et al. Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015). For example, in 1916, blue grama accounted for 40% to 60% of the pasture vegetation and needle-and-thread made up about 15% to 20% (by dry weight based on hand-clipped samples; Sarvis Reference Sarvis1920). Lorenz and Rogler (Reference Lorenz and Rogler1967) reported that the pasture stocked at 2.4 AUM ha−1 had become dominated by blue grama and upland sedges in the 1950s, whereas the pasture stocked at 1.0 AUM ha−1 had remained relatively unchanged in vegetative composition since 1916. By 2014, the pastures were dominated by Kentucky bluegrass (Table 2). Smooth brome was prevalent in the exclosure in 2014. Smooth brome is less tolerant of grazing than Kentucky bluegrass (Hendrickson and Lund Reference Hendrickson and Lund2010); thus, protection from grazing may have allowed it to flourish. Sanderson et al. (Reference Sanderson, Liebig, Hendrickson, Kronberg, Toledo, Derner and Reeves2015) speculated that Kentucky bluegrass invasion on these pastures may have been aided by higher than normal precipitation since the 1990s along with a longer frost-free period as a result of climate change. Propagules of Kentucky bluegrass are very abundant because of its ubiquitous use in turf and other amenity grasslands (DeKeyser et al. Reference DeKeyser, Denhardt and Hendrickson2015). Similar long-term increases of Kentucky bluegrass occurred in prairie pastures of southwestern Manitoba, Canada. After 41 yr of grazing exclusion (1969 to 2010), bluegrass was the most abundant species in prairie pastures that had a history of moderate (defined as 26% to 50% defoliation) and heavy (>51% defoliation) grazing (Sinkins and Otfinowski Reference Sinkins and Otfinowski2012).
As we hypothesized, the change in vegetation from C4 to C3 grass dominance was accompanied by a change in the isotopic composition of soil C. The soil δ13C at the 0- to 7.6-cm soil depth became more negative during the past 23 yr, indicating greater input of C3 carbon. Changes in atmospheric δ13C may have partially contributed to this change, as there has been about a −0.6‰ change in atmospheric δ13C since 1990 and about a −1.5‰ change since 1959 (Carbon Dioxide Information Analysis Center 2016). The increase in soil δ13C with grazing intensity at both soil depths in 1991 and 2014 (Table 3) probably reflects the relative abundance of blue grama in the pastures (Table 2; Frank et al. Reference Frank, Tanaka, Hofmann and Follett1995). Blue grama was more abundant in the heavily stocked pasture than in the lightly stocked pasture or the exclosure for many years before 2014 (Table 2). The relatively low δ13C in soil from 1959 (−19.4‰ to −20.8‰) indicates that C3 plants have also been a significant component of pasture vegetation. The most abundant C3 graminoids in the Mandan pastures in earlier years included needle-and-thread grass, prairie junegrass, threadleaf sedge, and needleleaf sedge (Sarvis Reference Sarvis1920). The recent changes in δ13C in soil, however, probably reflect C input from invasive C3 grasses. Soils under long-term C3 grasslands have a δ13C of approximately −23‰ to −27‰ (e.g., Balesdent and Mariotti Reference Balesdent and Mariotti1987; −26.2‰ for a temperate meadow). In C3-dominated grasslands of the Cedar Creek Minnesota site, soil to a 16-cm depth had a δ13C of −23.9‰ compared with −26.4‰ for C3 plant biomass (Wedin et al. Reference Wedin, Tieszen, Dewey and Pastor1995). Corresponding soils under long-term C4 grassland typically have a δ13C of approximately −12‰ to −18‰ (e.g., Derner et al. [Reference Derner, Boutton and Briske2006] for short-, mid-, and tallgrass plant communities).
Roots constitute the primary input of C to soil organic matter (Rasse et al. Reference Rasse, Rumpel and Dignac2005). We did not measure root biomass; however, Liebig et al. (Reference Liebig, Kronberg, Hendrickson, Dong and Gross2013) reported root biomass amounts of 3.67 and 2.95 Mg ha−1 for the pastures stocked at 1.0 and 2.4 AUM ha−1, respectively. They did not measure root biomass in the exclosure. Lorenz and Rogler (Reference Lorenz and Rogler1967) measured root biomass in the two pastures in 1961 and reported no difference in root mass to a 122-cm depth; however, the proportion of roots in the 0- to 30-cm layer was 78% and 74% for the pastures stocked at 1.0 and 2.4 AUM ha−1, respectively.
The δ13C in soil from the exclosure and pastures may also reflect the amount of dead biomass (pool of decomposing organic matter) present (Table 4). Dead biomass had an average δ13C of −26‰ (Table 4) and was most abundant in the exclosure. The relatively large amount of dead plant material accumulating in the exclosure and pastures as a result of Kentucky bluegrass invasion not only affects nutrient cycling but can also alter hydrological attributes such as surface-water runoff and infiltration of water into the soil (Printz and Hendrickson Reference Printz and Hendrickson2015) and evaporation from the soil surface (Facelli and Pickett Reference Facelli and Pickett1991). Excess litter can suppress germination and emergence of other plants (Facelli and Pickett Reference Facelli and Pickett1991), which may reduce the diversity of native species in grasslands (Letts et al. Reference Letts, Lamb, Mischkolz and Romo2015).
Maintaining an appropriate amount of litter is necessary to sustain productivity of native cool-season grasses. Bork and Irving (Reference Bork and Irving2015) reported that litter accumulation benefited cool-season grass (mainly needle-and-thread grass and western wheatgrass [Pascopyrum smithii (Rydb.) A. Love]) production in mixed-grass prairie in southeastern Alberta, Canada. Litter amounts in that study were 1,684 kg ha−1 on sand dune ecological sites and 2,109 kg ha−1 on loam sites. Litter presumably lowered soil temperatures and conserved soil moisture, which may have contributed to greater cool-season grass production. We observed, however, that greater forage biomass at spring clipping was associated with less dead biomass (litter plus standing dead material; Table 4). Deutsch et al. (Reference Deutsch, Bork and Willms2010) manipulated litter (defined as detached litter, standing dead, and partially decomposed plant material not incorporated into the mineral soil) amounts on two Park grassland sites in southeastern Alberta. The sites had not been grazed for 10 yr and had accumulated 5,500 to 7,000 kg ha−1 of litter. Maintaining litter amounts or doubling litter levels reduced soil temperature from May to August compared with plots with litter removed. Soil moisture in that study was greater on the plots with large amounts of litter compared with litter-removed plots; however, this could have been confounded with differences in soil moisture use by differences in vegetation cover and amounts among the plots.
There have been no outside inputs of N (except from natural sources) to the pastures over the years. Thus, the changes in soil δ15N probably resulted from N cycling via urine and dung of grazing animals and decomposition of litter over the years. For example, soil from grazed grasslands in the Lamar Valley of Yellowstone National Park had higher δ15N than soils from areas excluded from grazing by wild ungulates (Frank and Evans Reference Frank and Evans1997). The higher δ15N in grazed soils was attributed to ammonia volatilization from urine and dung, which results in a preferential loss of the lighter N isotope (Frank et al. Reference Frank, Evans and Tracy2004). Losses of N via dung and urine of grazing cattle probably accounted for some of the increase in δ15N with grazing in our study. The increase in δ15N with grazing intensity in 2014 agreed with our stated expectation; however, the overall decrease in δ15N between years did not. We expected that soil δ15N would have increased with more years of cattle grazing and associated inputs and recycling of dung and urine. Dead biomass (standing dead, litter, and thatch) had a negative δ15N (−1.5‰, which nearly matches the −1.2 ‰ change from 1991 to 2014; Table 5). The decomposition of this organic matter may have also contributed to the decrease in δ15N from 1991 to 2014. There was less dead biomass in the grazed pastures compared with the exclosure, which may have also accounted for some of the differences in δ15N in the surface 7.6 cm of soil among the pastures and exclosure.
Wedin and Tilman (Reference Wedin and Tilman1996) indicated that N loading of grasslands caused a reduction in plant species diversity and a shift in composition from native C4 grass (little bluestem [Schizachyrium scoparium (Michx.) Nash]) to invasive C3 grass (quackgrass [Elymus repens (L.) Gould]). The shift in species composition reduced the C:N ratio of above- and belowground vegetation. In that study, both bluegrass and quackgrass were less nitrogen-use efficient. With the changes in C:N ratio, N retention in their grassland system decreased and soil C storage decreased.
The dramatic changes from C4 to C3 grass dominance in the long-term pastures at Mandan have altered the physical structure of vegetation and affected C cycling with little effect on soil N. The change in δ 13C (more negative) in soil organic C from 1991 to 2014 indicates significant input of C from C3 grasses. There appears to have been an effect of invasion on N cycling, which may have been influenced by both grazing animals and large amounts of dead vegetation. There was a gain in C in both pastures from 1991 to 2014, indicating that C3 grass invasion altered C balance and cycling.
Invasion of these long-term prairie pastures by Kentucky bluegrass does not appear to have reduced soil C storage. The more deleterious effect of invasion, however, has been a buildup of standing dead vegetation and litter, which may reduce germination and emergence of native plants and potentially reduce species diversity.
Acknowledgment
Mention of commercial products and organizations in this manuscript is solely to provide specific information. It does not constitute endorsement by USDA–ARS over other products and organizations not mentioned.







