Long-term naturalistic follow-up studies among patients with bipolar disorder have shown that on average these people experience symptoms approximately half the time, despite treatment. Reference Kupka, Altshuler, Nolen, Suppes, Luckenbaugh and Leverich1,Reference Judd, Akiskal, Schettler, Endicott, Maser and Solomon2 Even during so-called euthymic periods (i.e. when patients do not fulfil the formal criteria of a mood episode) many have subsyndromal symptoms that increase the risk of relapse and negatively influence their functioning and quality of life. Reference Goossens, Hartong, Knoppert-van der Klein and van Achterberg3–Reference Mazza, Mandelli, Zaninotto, Nicola, Martinotti and Harnic6 Collaborative care was developed and tested in primary care in order to enhance treatment effectiveness in patients with depression. Reference Wagner, Austin and Von Korff7 Some previous studies have tested the effectiveness of collaborative care in bipolar disorder. Reference Bauer, McBride, Williford, Glick, Kinosian and Altshuler8–Reference Kilbourne, Post, Nossek, Drill, Cooley and Bauer11 Although these studies have shown promising results, the majority demonstrated an effect on mania only, not on depressive symptoms. Given the deleterious effect of persistent depressive symptoms, we focused our collaborative care intervention in particular on the management of depressive symptoms by adding problem-solving treatment, and tested in a randomised controlled trial whether collaborative care might be an effective intervention programme. Reference Van der Voort, van Meijel, Goossens, Renes, Beekman and Kupka12
Method
A pragmatic, two-armed, cluster randomised controlled trial (RCT) was conducted in out-patient mental health clinics in The Netherlands. The collaborative care programme was compared with treatment as usual (TAU). Collaborative care was provided by specially trained collaborative care teams, in which mental health nurses functioned as care managers. In The Netherlands there are relatively few barriers to treatment in specialised mental healthcare, and the standard of care is generally high. However, we assumed considerable practice variation between teams. Therefore, we assessed the quality and level of care delivered by the teams that were eligible for participation before the start of the trial, to avoid including teams that were already providing the core elements of collaborative care. Baseline measurements were obtained at inclusion, and follow-up measurements at 6 months and 12 months. The primary outcome measures were time spent with symptoms of mania or depression, and the severity of such symptoms, and the secondary outcome measure was medication adherence. The trial was registered with The Netherlands Trial Registry (NTR2600).
Randomisation
Clustered randomisation was performed at the level of the out-patient teams. Teams that treated at least 20 patients with bipolar disorder were invited to participate. The teams were matched on the number of nurses who were willing to participate in the study, in order to obtain approximately the same number of respondents in both research conditions. The two teams within every matched pair were randomly assigned to either the experimental or the control condition, by use of an internet random generator, performed blind by the second author (B.M.). There was no matching on other characteristics since, despite the practice variation we found, the overall level and quality of care appeared to be comparable among teams. Next, in each team a nurse or psychiatrist compiled a list of patients who met the inclusion criteria. Since executing a new intervention leads to an increased workload for the nurses in the experimental condition, the maximum number of patients to be included was set at ten per nurse. If more than ten patients per participating nurse met the inclusion criteria, these patients were listed in random order and approached by the psychiatrist or nurse for participation, starting at the top of this list. Once the provisional agreement of the patient was obtained, the researcher contacted the patient to give detailed oral information about the study. If patients agreed to participate, additional written information was provided, including an informed consent form. The study protocol was approved by the medical ethical committee of the VU University Medical Centre.
Participants
We included patients aged 18–65 years with a diagnosis of bipolar disorder type 1 or 2 or not otherwise specified (NOS) according to DSM-IV-TR. 13 Diagnoses were derived from the medical records and subsequently confirmed by the treating psychiatrist using the Dutch language version of the Questionnaire for Bipolar Illness (QBP-NL). Reference Leverich, Nolen, Rush, McElroy, Keck and Denicoff14 Since collaborative care is a relatively intensive intervention, it is not appropriate for patients experiencing a severe manic or depressive episode. The intervention programme is also less appropriate for patients who are stable enough to function well with only low-intensity treatment. Based on these considerations we applied the following exclusion criteria:
-
(a) severe or very severe depression or mania, with a score of 6 or 7 on the Clinical Global Impression – Bipolar Disorder scale; Reference Spearing, Post, Leverich, Brandt and Nolen15
-
(b) a stable course of illness in the past year, allowing low intensity of treatment with a maximum of four consultations with the psychiatrist or nurse per year;
-
(c) insufficient command of the Dutch language;
-
(d) inability or unwillingness to give informed consent.
Masking
Given the nature of the intervention, masking of participants and professionals to the assigned treatment condition was not possible. Because of the cluster randomisation patients were aware of the condition their treatment team was assigned to when their informed consent was asked. It was not possible to ensure masking of the research assistants who interviewed patients. In order to prevent bias, information about the course of illness was obtained by patient self-report through an interview using the strict format of the retrospective National Institute of Mental Health Life Chart. Reference Leverich and Post16
Intervention
The rationale of collaborative care is that people with chronic and intermittent disorders benefit from treatments in which the collaboration between patient and professionals is structured systematically and in which the self-management skills of the patient are enhanced. Reference Woltmann, Grogan-Kaylor, Perron, Georges, Kilbourne and Bauer17 Within the collaborative care framework different treatments can be offered. Our programme consisted of:
-
(a) The formation of a collaborative care team, including at least the patient, the nurse and the psychiatrist, where all decisions concerning treatment and care were made. If the patient consented, a family member or friend was invited to join the team. The team members met at 3 months, 6 months and 12 months. Coordination of care was provided by the mental health nurse in the role of care manager.
-
(b) Contracting, aiming at achieving agreement within the collaborative care team on the most important problems and treatment activities. A treatment plan was made, formulated as a contract, in which goals and treatment activities were recorded.
-
(c) Working with the treatment plan was based on systematic care needs assessment, making use of the Camberwell Assessment of Need. Reference Phelan, Slade, Thornicroft, Dunn, Holloway and Wykes18 The execution and outcomes of the treatment plan were systematically monitored and evaluated by the collaborative care team.
-
(d) Psychoeducation, Reference Honig, Hofman, Rozendaal and Dingemans19,Reference Hofman, Honig and Vossen20 provided to patients and caregivers together in six sessions of 2 h each.
-
(e) Problem-solving treatment (PST). Reference Schreuders, van Oppen, van Marwijk, Smit and Stalman21,Reference Mynors-Wallis, Gath, Lloyd-Thomas and Tomlinson22 This is a brief (six sessions) therapy, based on the principles of cognitive-behavioural therapy, applied according to a strict protocol and aimed at improving practical skills to solve everyday problems. The rationale of PST is that by increasing problem-solving skills, patients’ understanding of the relationship between everyday problems and mood increases, resulting in the experience of regaining control over their own life.
-
(f) Mood charting by means of the prospective Life Chart Method. Reference Leverich and Post16
-
(g) Recognition of early warning signs of relapse, followed by predefined interventions as defined in a relapse prevention plan. Reference Goossens, Kupka, Beentjes and van Achterberg23,Reference Sierra, Livianos, Arques, Castello and Rojo24
-
(h) Pharmacotherapy and somatic care, continued as appropriate. In addition, in the collaborative care team continuous monitoring of effects took place, with specific attention to medication adherence.
Procedures
A manual-based training programme was developed by the investigators with the assistance of an expert panel, consisting of five expert nurses, a psychiatrist, a patient and a family member. Nurses in the experimental condition received this 3-day training programme, with 2 weeks between each training day. The training aimed at enhancing knowledge about the interventions to be delivered, as well as skills training to perform the interventions adequately. Since PST was a new intervention for all nurses, a total of 6 h training in this skill was offered by an experienced, specialised PST trainer. During the entire training in collaborative care the importance of programme fidelity was emphasised, as well as dilemmas that might occur between strict programme fidelity and flexible patient-tailored care. Fifteen nurses were trained in collaborative care. Four psychiatrists participated in part of the training, receiving an overview of the rationale and the various elements of the collaborative care intervention, as well as information about the study procedures. The trained teams in the experimental condition provided collaborative care throughout 1 year. The nurses were primarily responsible for the coordination and continuity of treatment. Supervision of PST was given by the trainer. The primary investigator (N.V.) coached the nurses for the whole duration of the study. These supervisory contacts were offered both individually by telephone and in group sessions in the treatment facility of the teams. A mean number of coaching contacts of 15.4 (range 11–20) was provided. Nurses in the TAU condition received no training, coaching or supervision.
Measures
Measurements were performed at baseline (T 0) and after 6 months (T 6) and 12 months (T 12). At baseline, demographic data, illness history, diagnosis, illness characteristics and current treatment were recorded by both patient and treating psychiatrist using the patient and clinician versions of the QBP-NL. Reference Leverich, Nolen, Rush, McElroy, Keck and Denicoff14 Course of illness and recurrence of mood episodes were assessed with the retrospective Life Chart Method (LCM) during a telephone interview by a research assistant. Reference Leverich and Post16 Patients were asked to rate retrospectively their average mood, in each consecutive month, over the past 6 months; scores were based on the severity of mood symptoms and the associated degree of functional impairment. At T 0 the 6 months preceding study entry were assessed. The LCM consists of a scale for manic symptoms (+1 to +3) and a scale for depressive symptoms (–1 to –3); a score of 0 indicates a euthymic state. Scores of ±2 and ±3 refer to syndromal episodes, whereas scores of ±1 refer to subthreshold symptoms with only mild functional impairment. Severity of depressive symptoms during the past week was measured with the 16-item self-report version of the Quick Inventory for Depressive Symptomatology (QIDS). Reference Rush, Trivedi, Ibrahim, Carmody, Arnow and Klein25 Symptoms of mania during the past week were assessed with the Altman Self-Rating Mania (ASRM) scale. Reference Altman, Hedeker, Peterson and Davis26 Medication adherence was assessed with the ten-item Drugs Attitude Inventory (DAI-10); Reference Hogan, Awad and Eastwood27 all ten items have a dichotomous outcome (adherent yes/no).
Nurses in the experimental group completed a fidelity checklist during the study, in order to register the collaborative care elements actually delivered. To avoid contamination bias, nurses in the control condition were not asked to fill in this checklist. Care consumption was measured in both groups with the Trimbos and iMTA Questionnaire for Costs Associated with Psychiatric Illness, to register elements of treatment actually delivered in each group. Reference Hakkaart-van Roijen28
Statistical analysis
Our primary outcomes were the duration of symptoms (measured longitudinally with the retrospective LCM) and the severity of symptoms at follow-up (measured at three time points with the QIDS and the ASRM scale). Independent t-tests for continuous variables and χ2 statistics for categorical variables were carried out to compare the two groups on baseline characteristics. These analyses were also performed to compare participants withdrawing from and completing the trial on baseline characteristics, in the total sample. First, means and standard deviations were calculated for the primary outcome variables (months spent with depression or mania, and severity of symptoms) on the three measurements. Next, data were analysed according to the intention to treat (ITT) principle. Differences in outcome between collaborative care and TAU were evaluated by means of linear mixed-model analysis for fixed and random effects. This method is statistically rigorous, allows for longitudinal testing of continuous data and is able to handle missing observations due to patients leaving the study. Our analyses were performed with a random intercept, and with condition and time as fixed effects.
A group × time interaction term was entered into the model to test for differences in treatment effects over time. Next, effect sizes were calculated, based on the estimated differences between T 0 and T 6 and between T 0 and T 12, between groups, based on pooled pretest standard deviations. Reference Morris29 The analyses were extended using multilevel analyses that take the nesting of measurements into account. We also took into account the extent to which patients were exposed to the intervention, by conducting a per protocol analysis. Finally, we repeated all analyses described above for the secondary outcome, medication adherence.
Power calculation
The a priori power calculation concerned the comparison of outcomes from the experimental and control condition at T 12 compared with T 0. By the time we planned this study we were unable to detect studies sufficiently comparable to ours to estimate the expected effect size. Therefore, we used an effect size of Cohen’s d = 0.5, because this is considered to be a clinically relevant effect. With α = 0.05 (two-tailed) and a power (1 – α) of 0.80, the required sample size was 63 patients per arm of the trial. In cluster randomisation the rule of thumb is to add 25% to this amount, bringing the total to 279. Taking into account an expected drop-out rate of 30%, a sample of 103 patients in each group was needed.
Results
A total of 138 participants were included (Fig. 1). Initially, informed consent was obtained from 71 participants in the intervention group and from 82 participants in the control group. However, due to organisational circumstances unrelated to the study, two teams with in total 15 patients withdrew from the experimental arm of the study, leaving 56 patients in the collaborative care arm and 82 in the control arm. After the baseline measurement 13 patients in the collaborative care condition stopped the allocated treatment, of whom 2 continued to participate in the study, leaving 11 patients not assessed at T 12. Four patients in the control condition stopped allocated treatment, of whom two continued to participate in the study. In total 21 participants were lost to follow-up (controls n = 10, intervention n = 11). Of the 45 patients in the collaborative care group at the 12-month assessment 43 received the allocated intervention, and of the 72 patients who completed the study in the TAU group 70 received the allocated intervention. When the baseline characteristics of patients who left the study were compared with those who continued, these groups differed significantly only with respect to family history of bipolar disorder. Logistic regression was conducted to determine whether illness characteristics in patients randomised to collaborative care predicted withdrawal from the care programme. Only longer duration of mania symptoms in the 6 months preceding baseline predicted stopping collaborative care.

Fig. 1 Flow of participants through the trial.
Since the outcomes of multilevel analyses and analyses that ignore nesting were not significantly different, we present the analyses ignoring nesting. Moreover, as no significant difference was found between the results of ITT v. per protocol analyses, we report ITT only. In the final analyses, sample sizes may differ per questionnaire, owing to the fact that not all measurements were entirely completed by the remaining participants. At T 12, measurements of 117 patients (85%) were included in the analyses (intervention 80%, control 88%; P = 0.3).
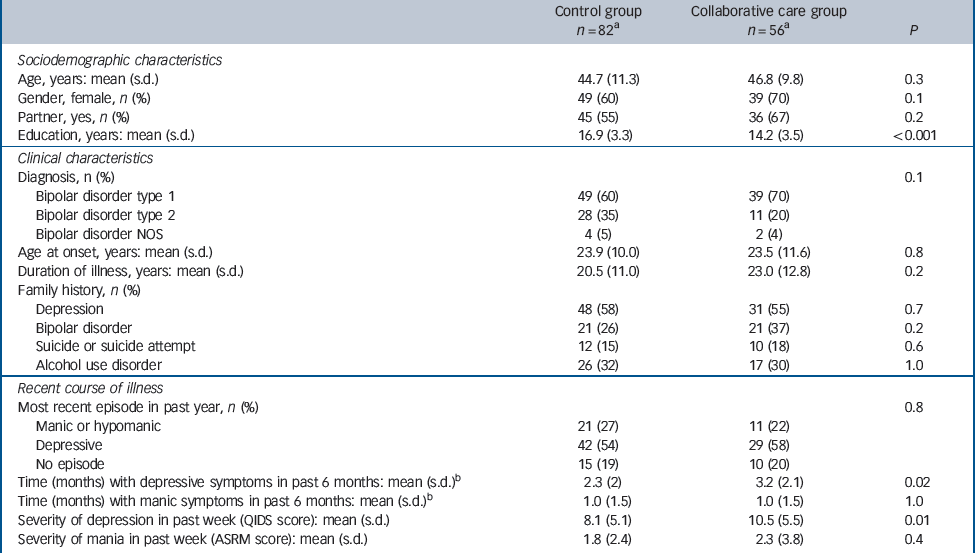
Sample characteristics are summarised in Table 1. The mean duration of illness of the patients included was 21 years. At baseline, a few significant differences existed between the experimental and control conditions. Participants randomised to collaborative care reported more months with depressive symptoms during the 6 months prior to baseline than patients in the control group (mean 3.2 months, s.d. = 2.1, v. 2.3 months, s.d. = 2.2; P = 0.02). Patients in the experimental condition had a greater severity of depressive symptoms than the control group in the week preceding baseline (mean QIDS score 10.5, s.d. = 5.5, v. 8.1, s.d. = 5.1; P = 0.01). Furthermore, patients randomised to collaborative care on average had a lower educational level than patients in the control condition (mean years of education 14.2, s.d. = 3.5, v. 16.9, s.d. = 3.3; P<0.01).
Table 1 Sample characteristics at baseline
| Control group n = 82Footnote a |
Collaborative care group n = 56Footnote a |
P | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age, years: mean (s.d.) | 44.7 (11.3) | 46.8 (9.8) | 0.3 |
| Gender, female, n (%) | 49 (60) | 39 (70) | 0.1 |
| Partner, yes, n (%) | 45 (55) | 36 (67) | 0.2 |
| Education, years: mean (s.d.) | 16.9 (3.3) | 14.2 (3.5) | <0.001 |
| Clinical characteristics | |||
| Diagnosis, n (%) | 0.1 | ||
| Bipolar disorder type 1 | 49 (60) | 39 (70) | |
| Bipolar disorder type 2 | 28 (35) | 11 (20) | |
| Bipolar disorder NOS | 4 (5) | 2 (4) | |
| Age at onset, years: mean (s.d.) | 23.9 (10.0) | 23.5 (11.6) | 0.8 |
| Duration of illness, years: mean (s.d.) | 20.5 (11.0) | 23.0 (12.8) | 0.2 |
| Family history, n (%) | |||
| Depression | 48 (58) | 31 (55) | 0.7 |
| Bipolar disorder | 21 (26) | 21 (37) | 0.2 |
| Suicide or suicide attempt | 12 (15) | 10 (18) | 0.6 |
| Alcohol use disorder | 26 (32) | 17 (30) | 1.0 |
| Recent course of illness | |||
| Most recent episode in past year, n (%) | 0.8 | ||
| Manic or hypomanic | 21 (27) | 11 (22) | |
| Depressive | 42 (54) | 29 (58) | |
| No episode | 15 (19) | 10 (20) | |
| Time (months) with depressive symptoms in past 6 months: mean (s.d.)Footnote b | 2.3 (2) | 3.2 (2.1) | 0.02 |
| Time (months) with manic symptoms in past 6 months: mean (s.d.)Footnote b | 1.0 (1.5) | 1.0 (1.5) | 1.0 |
| Severity of depression in past week (QIDS score): mean (s.d.) | 8.1 (5.1) | 10.5 (5.5) | 0.01 |
| Severity of mania in past week (ASRM score): mean (s.d.) | 1.8 (2.4) | 2.3 (3.8) | 0.4 |
ASRM, Altman Self-Rating Mania scale; NOS, not otherwise specified; QIDS, Quick Inventory for Depressive Symptomatology.
a. Number of respondents differs slightly among measurements.
b. Assessed with the Life Chart Method.
Concerning treatment characteristics at baseline, we found that several elements of collaborative care were already provided to a considerable number of patients in both conditions. Teams in both conditions worked with a Life Chart in almost half of the cases (intervention group 43%, control group 43%; χ2 = 0.005, d.f. = 1, P = 0.2); relapse prevention plans were present in more than half of the cases (intervention 52%, control 63%; χ2 = 1.6, d.f. = 1, P = 0.2). In the 5 years prior to the trial significantly more patients in the control condition than patients in the experimental condition had participated in a psychoeducation course (intervention group 37%, control group 64%; χ2 = 9.6, d.f. = 1, P = 0.003). In two-thirds of cases one or more relatives were involved in treatment (intervention group 67%, control group 69%; χ2 = 1.06, d.f. = 1, P = 0.9). None of the teams had provided PST to their patients. The mean number of consultations with a nurse or psychiatrist in the 3 months preceding baseline did not differ between the two groups (intervention 5.8, s.d. = 5.5, v. controls 5.4, s.d. = 6.3; P = 0.8).
Primary outcomes
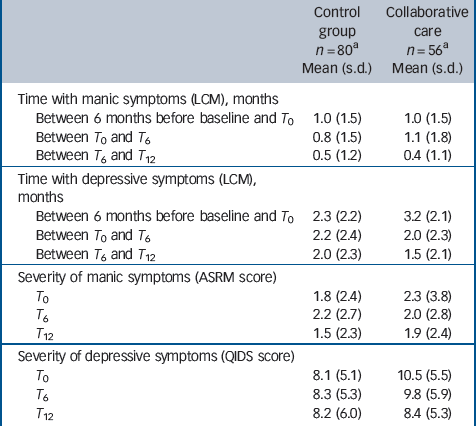
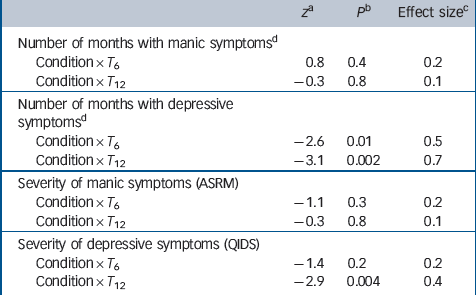
Table 2 shows observed means and standard deviations of number of months spent with manic or depressive symptoms, as well as severity of symptoms. Table 3 shows the results of mixed-models analyses. After 6 months patients in collaborative care demonstrated a larger reduction in the number of months with depressive symptoms than patients in the TAU group, with a medium effect size (z = –2.6, P= 0.01, d= 0.5). After 12 months this reduction was even larger (z = –3.1, P= 0.002, d= 0.7). Severity of depressive symptoms improved more after 12 months in patients who received collaborative care, compared with patients treated as usual (z = –2.9, P= 0.004, d= 0.4). There was no significant difference between the two conditions in time with mania symptoms or in change in severity of mania symptoms over 12 months.
Table 2 Duration and severity of mania or depressive symptoms in the two groups
| Control group n = 80Footnote a Mean (s.d.) |
Collaborative care n = 56Footnote a Mean (s.d.) |
|
|---|---|---|
| Time with manic symptoms (LCM), months | ||
| Between 6 months before baseline and T 0 | 1.0 (1.5) | 1.0 (1.5) |
| Between T 0 and T 6 | 0.8 (1.5) | 1.1 (1.8) |
| Between T 6 and T 12 | 0.5 (1.2) | 0.4 (1.1) |
| Time with depressive symptoms (LCM), months |
||
| Between 6 months before baseline and T 0 | 2.3 (2.2) | 3.2 (2.1) |
| Between T 0 and T 6 | 2.2 (2.4) | 2.0 (2.3) |
| Between T 6 and T 12 | 2.0 (2.3) | 1.5 (2.1) |
| Severity of manic symptoms (ASRM score) | ||
| T 0 | 1.8 (2.4) | 2.3 (3.8) |
| T 6 | 2.2 (2.7) | 2.0 (2.8) |
| T 12 | 1.5 (2.3) | 1.9 (2.4) |
| Severity of depressive symptoms (QIDS score) | ||
| T 0 | 8.1 (5.1) | 10.5 (5.5) |
| T 6 | 8.3 (5.3) | 9.8 (5.9) |
| T 12 | 8.2 (6.0) | 8.4 (5.3) |
ASRM, Altman Self-Rating Mania scale; LCM, Life Chart Method; QIDS, Quick Inventory for Depressive Symptomatology; T 0, T 6, T 12, assessments at baseline, 6 months and 12 months.
a. Number of respondents varies slightly among measurements.
Table 3 Test statistics and effect sizes of the condition × time interaction terms for duration and severity of symptoms (mixed model regression analysis)
| z Footnote a | P Footnote b | Effect sizeFootnote c | |
|---|---|---|---|
| Number of months with manic symptomsFootnote d | |||
| Condition × T 6 | 0.8 | 0.4 | 0.2 |
| Condition × T 12 | –0.3 | 0.8 | 0.1 |
| Number of months with depressive symptomsFootnote d |
|||
| Condition × T 6 | –2.6 | 0.01 | 0.5 |
| Condition × T 12 | –3.1 | 0.002 | 0.7 |
| Severity of manic symptoms (ASRM) | |||
| Condition × T 6 | –1.1 | 0.3 | 0.2 |
| Condition × T 12 | –0.3 | 0.8 | 0.1 |
| Severity of depressive symptoms (QIDS) | |||
| Condition × T 6 | –1.4 | 0.2 | 0.2 |
| Condition × T 12 | –2.9 | 0.004 | 0.4 |
ASRM, Altman Self-Rating Mania scale; QIDS, Quick Inventory for Depressive Symptomatology; T0, T6, T12, assessments at baseline, 6 months and 12 months.
a. Z test statistic concerns the fixed effects regression parameters of the interaction condition × time in a mixed effects regression model.
b. P value concerns the z statistic.
c. Effect size between groups, using pooled pretest standard deviation.
d. Number of months with symptoms measured with retrospective Life Chart Method.
We conducted sensitivity analyses, adjusting for all outcomes that differed at baseline, to investigate the impact of these baseline differences on the dependent variables. Reference Twisk30,Reference Fitzmaurice, Laird and Ware31 Both the effect of collaborative care at T 12 on duration of depressive symptoms (z = –2.1, P = 0.04, d = 0.4) and severity of depressive symptoms remained significant (z = 2.2, P = 0.03, d = 0.3). However, the effect on duration of symptoms at T 6 lost significance (z = –1.5, P = 0.1, d = 0.3).
Medication adherence
No difference was found between treatment conditions in change in the secondary outcome of medication adherence between baseline and T 6 (z = 0.3, d.f. = 238.3; P = 0.8), or between baseline and T 12 (z = 0.2, d.f. = 237.8; P = 0.8).
Implementation of collaborative care
After 12 months almost 80% of patients randomised to collaborative care reported using a relapse prevention plan, 84% had been following a psychoeducation course, 55% used a Life Chart, 86% had relatives involved in treatment and 72% had received one or more sessions of PST. The total number of contacts with mental healthcare providers did not differ between patients in the control group compared with patients who received collaborative care.
Discussion
Although treatment for bipolar disorder is widely available in The Netherlands, many patients have persistent symptoms that have a considerable impact on their daily functioning and quality of life. Depressive symptoms are especially debilitating and naturalistic studies have shown that in general depression is the more difficult to treat condition of bipolar disorder. Reference Altshuler, Post, Black, Keck, Nolen and Frye4,Reference Judd, Akiskal, Schettler, Endicott, Leon and Solomon32 The majority of previous collaborative care studies have shown positive effects on mania symptoms but no effect on depression symptoms. Reference Bauer, McBride, Williford, Glick, Kinosian and Altshuler8–Reference Kilbourne, Post, Nossek, Drill, Cooley and Bauer11,Reference Kilbourne, Goodrich, Lai, Post, Schumacher and Nord33,Reference Kilbourne, Goodrich, Lai, Clogston, Waxmonsky and Bauer34 For this reason we designed an intervention aimed not only at symptoms of mania, but also specifically at depressive symptoms by adding PST to the programme, which in previous studies has proved to be an effective treatment for (non-bipolar) depression.
Patients randomised to collaborative care showed more improvement, both in terms of the proportion of time they reported depressive symptoms and in terms of depression severity at the 12-month follow-up. Collaborative care had no effect on symptoms of mania and no effect on medication adherence.
Collaborative care
Collaborative care has been tested in several treatment settings and in a diversity of patient populations. Reference Woltmann, Grogan-Kaylor, Perron, Georges, Kilbourne and Bauer17 Most studies found collaborative care to be effective, albeit with small effect sizes. Reference Beekman, van der Feltz-Cornelis and van Marwijk35,Reference Miller, Grogan-Kaylor, Perron, Kilbourne, Woltmann and Bauer36 Most studies that investigated collaborative care in patients with bipolar disorder found improvements in mania symptoms but not in depressive symptoms. In one study of the effects of collaborative care in patients with bipolar disorder and cardiovascular risk, post hoc analysis showed a decrease in depressive
symptoms in a subgroup of patients with elevated cardiovascular risk. Reference Kilbourne, Goodrich, Lai, Clogston, Waxmonsky and Bauer34 In contrast to most studies, we found a clear effect on depressive symptoms, with moderate effect sizes. Although it is not possible to assess which specific elements of collaborative care account for this effect, we presume PST to be important. No other collaborative care programme for bipolar disorder has included this treatment. High levels of heterogeneity exist between studies concerning the effect of PST on depressive symptoms; however, a recent meta-analysis suggested that PST is as effective as pharmacological therapy and other psychosocial therapies in decreasing depressive symptoms. Reference Cuijpers, Andersson, Donker and van Straten37 One might think that our finding of the effect of collaborative care on bipolar depression might be mainly due to PST and that perhaps PST could also be effective in bipolar depression when offered as a stand-alone intervention. Testing this would require a separate study, which might be worthwhile. Our hypothesis is, however, that the effect of collaborative care is due to the combination of interventions in the programme.
It is striking that no effect was found on symptoms of mania. This could be explained by the limited sample size. Research shows that these symptoms are less prevalent than depression, occurring on average 10% of the time; Reference Judd, Akiskal, Schettler, Endicott, Maser and Solomon2 as a consequence the chance of finding these symptoms in a relatively small sample is limited. In our sample only a few patients experienced mania symptoms. During the 12 months of the trial the mean number of months with symptoms of mania in the total sample was 1.4 (s.d. = 2.3), so patients spent on average approximately 10% of the time with mania. Only 15 patients reached the cut-off point indicating a high probability of manic or hypomanic condition on the ASRM scale (>6). Given these small numbers we presume that our study did not have enough power to find differences between groups in time spent with manic symptoms or the severity of such symptoms.
Sample size
Treatment as usual in The Netherlands is assumed to be of relatively high quality, which probably decreased the chance of finding significant effects with our sample size. Adding PST, however, probably increased the strength of our collaborative care programme, thus increasing the chance of finding significant effects. With our sample size, based on an effect size of Cohen’s d = 0.5, we were able to detect significant differences between conditions, concerning the decrease of depressive symptoms after a year, with effect sizes ranging from 0.4 to 0.7 (see Table 3). Therefore, retrospectively, we still assume our a priori power analysis to be adequate for this study. The effect sizes we found are relatively high when compared with a recent review by Miller et al, who found small effect sizes (0.33) for collaborative care programmes across mental health conditions. Reference Miller, Grogan-Kaylor, Perron, Kilbourne, Woltmann and Bauer36
Treatment as usual
Given the level of usual care in The Netherlands, we expected that some elements of collaborative care would be provided in TAU in non-systematic ways. At baseline we assessed the presence of collaborative care elements in both treatment conditions. The level of care was relatively high in both groups. Given this high level of care at baseline, the room for improvement due to collaborative care was limited. The fact that significant differences were nonetheless found is encouraging for further improvement of quality of care when interventions are planned and applied in a structured format. Our structured collaborative care programme with accompanying training may have contributed to a higher quality of (nursing) care, compared with probably less systematically performed TAU. Reference Goossens, Beentjes, de Leeuw, Knoppert-van der Klein and van Achterberg38
Strengths and limitations
The quality of this study is enhanced in several ways. First, we included the expertise of patients, informal caregivers, psychiatrists and nurses during the process of developing the collaborative care intervention. Second, its implementation was optimised by structured implementation of collaborative care in the experimental group, with 3 days of training, individual coaching for the nurses and programme fidelity assessments. Implementation succeeded to a satisfactory degree; however, it should be noted that the number of patients working with the Life Chart was low. Possible explanations for the latter finding are that patients experienced this long-lasting, daily home assignment as a burden, and also that nurses occasionally failed to stress the importance of the Life Chart and support the patient in completing it. This is in line with the report of Goossens et al, Reference Goossens, Beentjes, de Leeuw, Knoppert-van der Klein and van Achterberg38 who studied the activities nurses actually perform, and concluded that although nurses state the Life Chart to be important, their care for patients with bipolar disorder lacks a systematic approach. Third, the total number of contacts with the nurse and psychiatrist did not differ between the two treatment conditions, which makes the assumption plausible that the extra costs of collaborative care would be limited. Finally, attrition of respondents was limited, since 85% of respondents completed the assessments.
The first limitation of our study is that baseline differences concerning illness characteristics were present between treatment conditions. This might be explained by our method of including participants. After having obtained consent to approach eligible patients, the investigator provided them with more details about the study. When informed about the collaborative care programme, some patients declined participation, expecting that the programme would to be too intensive for them given their care needs. These were probably patients with less severe symptoms, which would explain why the collaborative care group reported more depressive symptoms at baseline. We showed, however, that after adjusting for these differences, the results remained. A second limitation was the fact that masking was not possible in the Life Chart interviews. However, the retrospective Life Chart is highly structured, and was administered on the basis of patient’s self-report not the clinical judgement of the interviewer, which limits the possibility of bias. The third limitation was the withdrawal of two teams in the collaborative care condition, due to organisational circumstances unrelated to this study. Although there is no reason to assume that this biased the results, it did reduce the statistical power of analyses.
We aimed to study the potential benefits of collaborative care for patients with bipolar disorder in actual clinical practice, which enables us to generalise our findings to real life, but also implied that full implementation of collaborative care could not be achieved in every patient. Incomplete implementation may have led to underestimation of the effects of collaborative care. Moreover, collaborative care was tailored to the specific needs of patients and their caregivers, resulting in not all elements of the programme being delivered to all patients. Still, the overall implementation of the programme was successful to a satisfactory degree. In the collaborative care group at T 12 there was a clear increase in the use of a relapse prevention plan, the use of a Life Chart, having followed psychoeducation and the involvement of relatives in treatment, compared with baseline. Problem-solving treatment showed the best degree of implementation, which supports the presumption that it was primarily accountable for the effect we found on depression.
Implications of the study
This pragmatic trial is the first to evaluate the effectiveness of collaborative care for patients with bipolar disorder, including specific interventions aimed at improving depressive symptoms. During the study, patients randomised to collaborative care spent less time with depressive symptoms compared with patients in the control condition. Furthermore, a decline in severity of depressive symptoms was found in patients who received collaborative care. No difference was found in mania symptoms between groups, nor in medication adherence. Although it is not possible to determine which components of collaborative care were responsible for the results, we assume that PST significantly contributed to these effects. Moreover, prompting mental healthcare professionals to deliver care in a more systematic way may have contributed to the effectiveness of this intervention.







eLetters
No eLetters have been published for this article.