Introduction
Carnivores play an important role in the functioning of natural ecosystems as apex predators (Miller et al., Reference Miller, Dugelby, Foreman, del Rio, Noss and Phillips2001). However, anthropogenic activities such as agricultural and urban expansion resulting in habitat loss and degradation, introduction of invasive species, and hunting, threaten carnivores and put them at risk of extinction (Purvis et al., Reference Purvis, Gittleman, Cowlishaw and Mace2000; Crooks, Reference Crooks2002; Farris et al., Reference Farris, Golden, Karpanty, Murphy, Stauffer and Ratelolahy2015). Decreases in carnivore populations can lead to changes in abundance of other species through trophic cascade effects (Duffy, Reference Duffy2002). For example, through the consumption of rodents, carnivores indirectly control the transmission of parasites between animals and humans and limit the spread of zoonotic diseases (Ostfeld & Holt, Reference Ostfeld and Holt2004). Carnivores also act as umbrella species in conservation because they require large areas of suitable habitat to maintain viable populations. Protecting areas large enough to support carnivore populations is likely to benefit many other species and natural communities with smaller habitat requirements (Noss et al., Reference Noss, Quigley, Hornocker, Merrill and Paquet1996; Thorne et al., Reference Thorne, Cameron and Quinn2006). The expansion of human-modified landscapes and resulting habitat loss have led to more frequent interactions between humans and wild carnivores (Randa & Yunger, Reference Randa and Yunger2006; Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017), with negative consequences such as increasing competition for food resources and transmission of diseases from domestic dogs to wild predators (Silva-Rodríguez et al., Reference Silva-Rodríguez, Ortega-Solís and Jiménez2010; Acosta-Jamett et al., Reference Acosta-Jamett, Chalmers, Cunningham, Cleaveland, Handel and Bronsvoort2011; Moreira-Arce et al., Reference Moreira-Arce, Vergara and Boutin2015). Carnivores are also at risk from road traffic and retaliatory killings in response to poultry and livestock depredation (Sanderson et al., Reference Sanderson, Sunquist and Iriarte2002; Inskip & Zimmermann, Reference Inskip and Zimmermann2009; Farris et al., Reference Farris, Golden, Karpanty, Murphy, Stauffer and Ratelolahy2015).
Population-level responses to habitat loss can vary amongst carnivore species depending on whether they are habitat generalists or specialists (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004; Zúñiga et al., Reference Zúñiga, Muñoz-Pedreros and Fierro2009). Forest specialists such as the guiña Leopardus guigna, for example, are more affected by habitat fragmentation than habitat generalists (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004; Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013, Reference Gálvez, Guillera-Arroita, St. John, Schüttler, Macdonald and Davies2018; Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017). The responses of different carnivore populations also vary depending on the spatial and temporal scale of anthropogenic disturbances (Lyra-Jorge et al., Reference Lyra-Jorge, Ribeiro, Ciocheti, Tambosi and Pivello2010; Moreira-Arce et al., Reference Moreira-Arce, Vergara and Boutin2015, Reference Moreira-Arce, Vergara, Boutin, Carrasco, Briones, Soto and Jiménez2016).
Covering 18% of the Chilean continental territory, the Mediterranean region of central Chile is considered a biodiversity hotspot because of its high proportion (c. 50%) of endemic flora and fauna (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000). The area is dominated by the sclerophyllous forest-shrubland ecosystem (Dinerstein et al., Reference Dinerstein, Olson, Graham, Webster, Primm, Bookbinder and Ledec1995), which is degraded and threatened as a result of historical and ongoing land-use change to agriculture and forestry (Armesto et al., Reference Armesto, Manuschevich, Mora, Smith-Ramirez, Rozzi, Abarzúa and Marquet2010; Schulz et al., Reference Schulz, Cayuela, Echeverria, Salas and Rey Benayas2010). These changes include the recent expansion of avocado plantations and vineyards that is driven by high global demand for these products (Armesto et al., Reference Armesto, Manuschevich, Mora, Smith-Ramirez, Rozzi, Abarzúa and Marquet2010). Approximately 345,000 ha of sclerophyllous forest-shrublands remain, with only 2% (7,000 ha) under protection by the National System of Protected Wild Areas. The drastic loss of habitat since the late 20th century, with an average net annual deforestation rate of 1,7% (Armesto et al., Reference Armesto, Manuschevich, Mora, Smith-Ramirez, Rozzi, Abarzúa and Marquet2010; Schulz et al., Reference Schulz, Cayuela, Echeverria, Salas and Rey Benayas2010), is a major challenge for the conservation of vertebrates in central Chile, with almost 30% of all vertebrate species, including all species of carnivores, currently considered threatened with extinction (Simonetti, Reference Simonetti1999).
Protected areas in central Chile are scarce (Armesto et al., Reference Armesto, Rozzi, Smith-Ramírez and Arrollo1998; Castañeda et al., Reference Castañeda, Godoy, Manzano, Marquet and Barbosa2015), small and disconnected (Simonetti & Mella, Reference Simonetti and Mella1997; Simonetti, Reference Simonetti1999). Because carnivores require large areas of suitable habitat (Noss et al., Reference Noss, Quigley, Hornocker, Merrill and Paquet1996; Sanderson et al., Reference Sanderson, Sunquist and Iriarte2002; Thorne et al., Reference Thorne, Cameron and Quinn2006) and cannot persist solely in small protected areas (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004; Zúñiga et al., Reference Zúñiga, Muñoz-Pedreros and Fierro2009; Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013), remnant fragments of native vegetation preserved in privately owned and productive areas (e.g. within the rural or agriculture landscape) are increasingly important for their conservation. Together with less intensive agriculture and land-use changes, such habitat patches and vegetation corridors improve connectivity and facilitate movement amongst native forest remnants, thus increasing habitat availability within agricultural landscapes and supporting the conservation of carnivores (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004; Hilty & Merenlender, Reference Hilty and Merenlender2004; Pita et al., Reference Pita, Mira, Moreira, Morgado and Beja2009; Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013; Nogeire et al., Reference Nogeire, Davis, Duggan, Crooks and Boydston2013; Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017).
In the forestry landscapes of southern Chile the guiña, a habitat specialist, occurs mostly in native forests (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004; Zúñiga et al., Reference Zúñiga, Muñoz-Pedreros and Fierro2009), whereas the culpeo Lycalopex culpaeus, South American grey fox Lycalopex griseus, cougar Puma concolor and lesser grison Galictis cuja, which are all habitat generalists, are more frequently observed in open habitats and exotic pine plantations (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004; Zúñiga et al., Reference Zúñiga, Muñoz-Pedreros and Fierro2009). In temperate rainforest of southern Chile the guiña is negatively affected by forest fragmentation (Gálvez et al., Reference Gálvez, Guillera-Arroita, St. John, Schüttler, Macdonald and Davies2018), and the conservation of native forest remnants is thus important for its conservation (Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013; Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017). However, to our knowledge no research has been conducted in the Mediterranean region of central Chile, where agricultural landscapes dominate. In northern hemisphere farmlands with a Mediterranean climate that include semi-natural habitats, vegetation corridors and forest remnants support a higher diversity and abundance of carnivores compared to intensively farmed fields (Pita et al., Reference Pita, Mira, Moreira, Morgado and Beja2009).
We hypothesized that (1) vineyard landscapes with higher levels of human disturbance support a lower diversity of native carnivores in remnant native vegetation compared to vineyard landscapes with more natural habitat, and (2) habitat specialists and generalists respond differentially to human disturbance at the habitat and landscape spatial scale. We therefore (1) estimated wild carnivore diversity in remnants of sclerophyllous forest-shrublands in the vineyard landscapes of central Chile, and (2) evaluated the effects of variables representing human disturbance at different spatial scales on the wild carnivores inhabiting this area.
Methods
Camera-trap survey
During 2013–2016 we installed 53 camera traps (Trophy Cam HD, Bushnell, Overland Park, USA) in 12 remnant patches of sclerophyllous forest-shrublands (4–8 cameras per site) in vineyard landscapes in the Mediterranean region of central Chile (Fig. 1). Camera traps were at a distance of < 400 m from a vineyard and baited with three pieces of fresh chicken and lynx urine (Wildlife Control Supplies, East Granby, USA) to increase the detection of carnivores (Silva-Rodríguez et al., Reference Silva-Rodríguez, Ovando, González, Zambrano, Sepúlveda and Svensson2018). We fixed cameras to tree trunks 30 cm above the ground facing south, to minimize false triggers from the rising or setting sun, with a distance of at least 300 m (mean 2,042.4 ± SD 962.8 m) between cameras at a site. Cameras recorded date and time of each picture captured, and the movement sensor was set to medium sensitivity. Cameras remained active continuously for 30 days and the total camera-trapping effort was 1,590 days (53 cameras × 30 days).
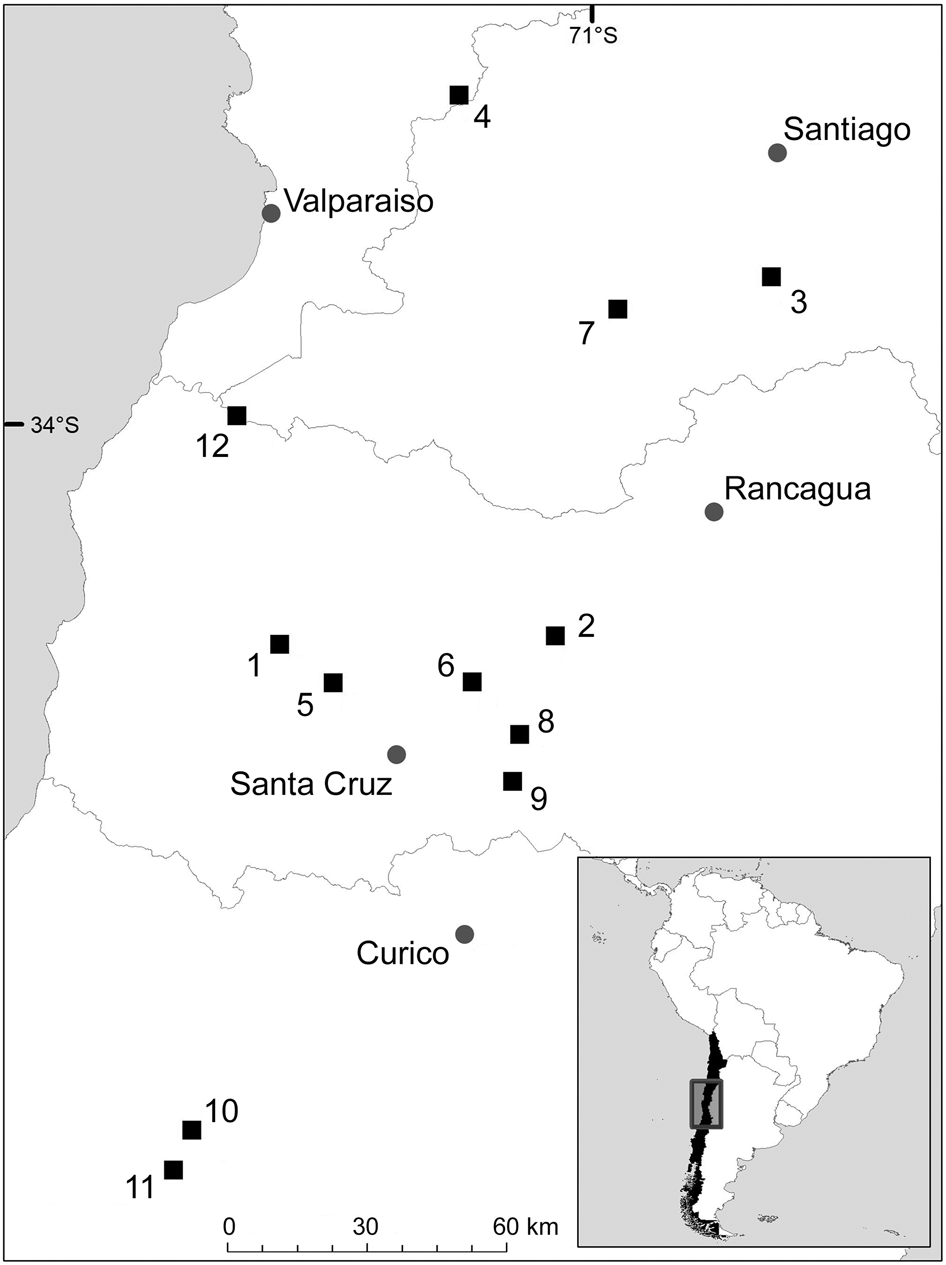
Fig. 1 Location of the 12 study sites in the vineyard landscape of central Chile.
We identified carnivores using specialized literature (Iriarte & Jaksic, Reference Iriarte and Jaksic2012) and our own field experience. We calculated the detection frequency for each species based on the number of independent photographic records on each camera trap during the 30-day sampling period. To determine the number of independent records we followed the methodology proposed by Medellín et al. (Reference Medellín, Azuara, Maffei, Zarza, Bárcenas, Cruz, Chávez and Ceballos2006), which has been applied in several studies (Monroy-Vilchis et al., Reference Monroy-Vilchis, Zarco-González and Rodríguez-Soto2011; Lira-Torres & Briones-Salas, Reference Lira-Torres and Briones-Salas2012). Consecutive photographs were counted as independent records if they showed different identifiable individuals (distinguished by marks such as fur colouring pattern or scars), or after an interval of 24 hours when we could not determine whether a series of consecutive photographs of the same species showed the same individual. Non-consecutive photographs of the same species were also counted as independent records. We considered the number of records to represent animal activity and a proxy for animal abundance (sensu Silva-Rodríguez et al., Reference Silva-Rodríguez, Ovando, González, Zambrano, Sepúlveda and Svensson2018). We used previous literature to categorize carnivore species as habitat generalists or specialists.
Carnivore richness
Using the independent photographic records of each camera trap, we estimated species richness (i.e. the total number of species detected) and detection frequency of each species. We assessed dissimilarity of carnivore richness between sites using the Morisita–Horn index (Wolda, Reference Wolda1981) with functions available in package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and McGlinn2016) for R 3.4.4 (R Core Team, 2018).
Habitat characterization
We evaluated the effect of habitat characteristics on carnivores at two spatial scales: (1) at the local habitat level, defined as the area within a circular plot with a radius of 200 m around each camera trap (i.e. within sites), and (2) at the landscape level, defined as the area within circular plots of 1, 5 and 10 km radius from the centroid of the camera traps at each site. At each scale the land-use type of the surrounding vineyard landscape was characterized using the naturalness evaluation index (NEI; Baiamonte et al., Reference Baiamonte, Domina, Raimondo and Bazan2015), which assesses different land uses according to the degree of human disturbance, using the formula:
where C0 is an area with high levels of human disturbance, C1 is an agricultural area, C2 is a semi-natural and C3 is a natural area.
For this index we defined different levels of anthropogenic modification as (1) areas with high human impact such as urban and industrial areas, (2) agricultural areas such as arable farms, vineyards and forest plantations, (3) semi-natural areas such as scrubland dominated by wild exotic species (e.g. Acacia dealbata, Teline monspessulana), and (4) natural areas such as native forest-shrublands and wetlands.
We assessed land use at the landscape level using the Native Forest Inventory of the National Forestry Corporation of Chile (CONAF, 2016), including the regions of Valparaiso, O'Higgins, El Maule and the Metropolitan Region. We analysed the data using ArcGIS 10.1 (Esri, Redlands, USA). At the habitat level we analysed land use with a Quickbird 2014 satellite image from Google Earth Pro (Google, 2016).
At each site we assessed the human influence on carnivore richness using the variables human population (the number of inhabitants per district; data from INE, 2017), minimum distance to urban areas or roads, and distance to the nearest protected area. Additionally, at the habitat scale (i.e. within sites) we evaluated the effects of the detection frequency of domestic dogs and habitat complexity, the latter estimated by the Shannon–Wiener index derived from the land-use data associated with each camera trap.
Statistical analysis
To assess the effect of human disturbance on species richness at the landscape and habitat scales we used a generalized linear mixed model (Burnham & Anderson, Reference Burnham and Anderson2002). Predictor variables included factors measured at the landscape level, factors common to each site and factors measured at the habitat level (i.e. at the level of individual camera traps). The generalized linear mixed model was fit assuming a Poisson distribution of errors, because the response variable (i.e. number of species detected) is a count. Additionally, we evaluated the effect of human disturbance on the detection frequency of habitat specialist and generalist native carnivores. We considered both fox species (culpeo and grey fox) and the grison habitat generalists, and the guiña, pampas cat Leopardus colocolo and Molina's hog-nosed skunk Conepatus chinga habitat specialists (Acosta-Jamentt & Simonetti, Reference Acosta-Jamett and Simonetti2004; Guzmán-Sandoval et al., Reference Guzmán-Sandoval, Sielfeld and Ferru2007; Zúñiga et al., Reference Zúñiga, Muñoz-Pedreros and Fierro2009). We only considered species with sufficiently high detection frequencies to support model fitting, excluding species with < 10 records (pampas cat, Molina's hog-nosed skunk and lesser grison).
The fixed model factors measured at the landscape level were the naturalness evaluation indices estimated for the 1, 5 and 10 km radius buffers around the centre of camera-trap sites, human population of the district in which the site was located, minimum distance to urban areas, minimum distance to roads and minimum distance to the nearest protected area. At the habitat level, the fixed factors were the naturalness evaluation index estimated within a 200 m radius around each camera trap, the detection frequency of domestic dogs and habitat complexity. Random factors of the mixed model were the vineyard near which the camera trap was located (i.e. the site) and the survey year.
Fixed effects were z-standardized because they were measured in different units and a common scale of analysis facilitates comparison of the relative contribution of each predictor variable in the model (Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017). To initialize the model selection procedure, we specified a global model containing all possible predictors for the fixed part of the mixed model. The best model was then selected from a large number of subset models generated using the dredge function in the R package MuMIn (Barton, Reference Barton2018). Using an Akaike information criterion (AIC) approach, we selected the best model based on the lowest AIC value.
We evaluated the collinearity between the predictor variables using variance inflation factors (accepted threshold < 3), with the R packages lme4 (Bates et al., Reference Bates, Maechle, Bolker and Walker2015) and MuMIn (Barton, Reference Barton2018), and evaluated the variance inflation factors function based on Zuur et al. (Reference Zuur, Ieno, Walker, Saveliev and Smith2009).
Finally, we determined the effects of human disturbance on carnivore composition at the site level (i.e. each vineyard) through PERMANOVA analysis using Adonis 2 (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and McGlinn2016) with 999 permutations. The response variable was the matrix of distances generated using the Horn method based on a matrix of carnivore detection frequencies, including native and exotic species. The co-variables used in this analysis were the naturalness evaluation indices estimated at 1, 5 and 10 km, and the human population of the district in which the site was located. This analysis was carried out using the R package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre and McGlinn2016).
Results
We obtained 406 independent photographic records of carnivores from a total of c. 25,000 camera-trap photographs. We identified eight carnivore species, six of which were native and two exotic. Amongst the native carnivores, the guiña is considered Vulnerable, the pampas cat Near Threatened, Molina's hog-nosed skunk Rare, and the two foxes and the grison are of Least Concern according to national categorization (Ministerio del Medio Ambiente, 2012), which is based on the IUCN Red List categories and the Chilean hunting law (Ministerio de Agricultura, 1998; Table 1).
Table 1 Origin, conservation status and detection frequency (%) of carnivores recorded in the sampled vineyards of central Chile.

1According to Ministerio del Medio Ambiente (2012).
The South American grey fox was the most frequently detected species (33% of all records), followed by the culpeo with 24%, the exotic domestic dog Canis lupus familiaris with 20% and the guiña with 17% (Table 1). The native pampas cat, Molina's hog-nosed skunk and grison, and the exotic domestic cat Felis catus together accounted for < 5.5% of all records (Table 1), with the pampas cat being the least frequently recorded species, with only a single record (Supplementary Table 1).
The generalized linear mixed models indicated that human population of the district in which the site was located has a negative influence on native carnivore richness (Table 2), whereas the random effect variances for the grouping factors vineyard and year were close to zero.
Table 2 Results of linear regression analysis for the relationship between the number of carnivore species detected in vineyard landscapes (12 surveyed sites) in central Chile. Predictors are variables selected after applying a best subset model routine to a larger number of candidate variables.

The detection frequencies of both habitat generalists (foxes) and specialists (the guiña) were negatively influenced by an increased presence of domestic dogs (Table 3). Human population and a higher degree of naturalness of the landscape at the 10-km scale also negatively influenced the detection frequency of the guiña, whereas a higher naturalness index on a 5-km scale had a small but significant positive influence (Table 3).
Table 3 Results of generalized linear mixed model regression analysis for the relationship between the frequency of occurrence of native habitat generalist (foxes L. griseus and L. culpaeus) and specialist (guiña L. guigna) carnivores in surveyed vineyard landscapes of central Chile. The table shows predictors that remained after applying a subset selection routine over a larger number of potential explanatory variables.

1Random effect for habitat generalist (SD): Vineyard = 0.5077; Year < 0.0001.
2Random effect for habitat specialist (SD): Vineyard = 0.6824836; Year = 0.0002575.
3NEI, naturalness evaluation index.
The PERMANOVA analysis showed that none of the parameters significantly influenced the composition of carnivore communities between different vineyards (Table 4).
Table 4 Results of PERMANOVA analysis, using the Morisita–Horn index, of dissimilarity of carnivore composition between different sites in vineyard landscapes in central Chile.

1NEI, naturalness evaluation index.
Discussion
Our camera traps recorded almost all wild carnivore species occurring in the Mediterranean region of Chile (Iriarte & Jaksic, Reference Iriarte and Jaksic2012), except for the cougar, in the vicinity of vineyards. This highlights the importance of remnants of native vegetation for carnivore conservation in agricultural landscapes.
The most frequently recorded species were the culpeo and South American grey fox. Their presence in human-dominated landscapes could be related to their omnivorous diets (Guzmán-Sandoval et al., Reference Guzmán-Sandoval, Sielfeld and Ferru2007). The guiña was the most frequently observed felid, whereas the pampas cat was recorded only once, probably because it is principally a montane species (Iriarte & Jaksic, Reference Iriarte and Jaksic2012). Contrary to our findings, an approach using species distribution models (Guillera-Arroita et al., Reference Guillera-Arroita, Lahoz-Monfort, Elith, Gordon, Kujala and Lentini2015) reported a low probability of guiña occurrence in the sclerophyllous forest-shrublands of central Chile (Cuyckens et al., Reference Cuyckens, Morales and Tognelli2015). It is possible that the relatively high detection frequency of guiñas, which are forest specialists (Acosta-Jamett & Simonetti, Reference Acosta-Jamett and Simonetti2004), in our study was a result of their movements through remnant forest strips along vineyard borders within rural areas (Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017).
In agreement with our first hypothesis, we found that species richness was higher in landscapes with less anthropogenic pressure: higher human population had a negative influence on carnivore richness. However, none of the anthropogenic variables influenced carnivore composition between different vineyard landscapes. This is probably a result of the overall low number of recorded species, and the high detection frequency of only a few of them.
The detection frequency of domestic dogs negatively affected the detection of both habitat generalists (foxes) and specialists (the guiña) in vineyard landscapes. This corroborates previous research in Chile, which found a negative correlation between the abundance of domestic dogs and the abundance of the South American grey fox and Darwin's fox Lycalopex fulvipes (Silva-Rodríguez et al., Reference Silva-Rodríguez, Ortega-Solís and Jiménez2010, Reference Silva-Rodríguez, Ovando, González, Zambrano, Sepúlveda and Svensson2018; Moreira-Arce et al., Reference Moreira-Arce, Vergara and Boutin2015), and highlights the importance of managing feral domestic animals in Chile (Bonacic et al., Reference Bonacic, Almuna and Ibarra2019). The negative relationship between domestic and wild canids could be explained by their phylogenetic closeness, which may lead to the transmission of infectious diseases from dogs to foxes (Acosta-Jamett et al., Reference Acosta-Jamett, Chalmers, Cunningham, Cleaveland, Handel and Bronsvoort2011), in addition to competition for prey (Silva-Rodríguez & Sieving, Reference Silva-Rodríguez and Sieving2012). Domestic dogs may thus have cumulative effects on native carnivores through resource competition and interference (Vanak & Gompper, Reference Vanak and Gompper2009). However, although there is documented evidence of negative effects of dogs on foxes, few studies have reported any mortality of the guiña attributable directly to domestic dogs (Silva-Rodríguez et al., Reference Silva-Rodríguez, Ortega-Solís and Jimenez2007).
Previous studies suggest human disturbance negatively affects native carnivores in southern Chile (Sanderson et al., Reference Sanderson, Sunquist and Iriarte2002; Silva-Rodríguez et al., Reference Silva-Rodríguez, Ortega-Solís, Jiménez and Soto-Gamboa2009, Reference Silva-Rodríguez, Ovando, González, Zambrano, Sepúlveda and Svensson2018; Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013; Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017). The guiña does not avoid human-dominated areas and can fall victim to ecological traps when attracted to human areas by food (Schüttler et al., Reference Schüttler, Klenke, Galuppo, Castro, Bonacic, Laker and Henle2017). Threats to the guiña in human-dominated areas include collisions with vehicles and hunting in retaliation for poultry depredation (Sanderson et al., Reference Sanderson, Sunquist and Iriarte2002; Silva-Rodríguez et al., Reference Silva-Rodríguez, Ortega-Solís, Jiménez and Soto-Gamboa2009; Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013).
The proportion of natural areas in the landscape affected the guiña, as shown by the small positive influence of higher naturalness at the 5-km scale on its detection frequency. However, at the 10-km scale we detected a significantly negative influence of this variable. These contrasting findings can be attributed to a bias in the naturalness index that did not consider the degree of fragmentation of the sampled natural areas or any physical barriers to animal movement across private lands (e.g. fences). The guiña is affected by habitat fragmentation resulting from the subdivision of large farms into smaller properties (Gálvez et al., Reference Gálvez, Guillera-Arroita, St. John, Schüttler, Macdonald and Davies2018). Further studies of rural landscapes in central Chile should thus consider parcelling of land as a form of habitat fragmentation. In addition, corridors and continuous areas of native vegetation should be retained or created as a common practice to avoid habitat fragmentation in agricultural landscapes.
Our second hypothesis was that the spatial scale of human disturbance affects habitat specialists and generalists carnivores differently. We found that habitat generalists (foxes) were not influenced by the naturalness of the landscape, whereas habitat specialists (the guiña) were affected. These findings corroborate a previous study that found the scale of spatial analysis influenced the impact of human activity and domestic dogs on native carnivores in southern Chile (Moreira-Arce et al., Reference Moreira-Arce, Vergara and Boutin2015), suggesting there is an urgent need to control domestic dogs in rural areas to conserve native carnivores (Bonacic et al., Reference Bonacic, Almuna and Ibarra2019).
There is debate regarding the use of photographic detection frequencies as a proxy for abundance (Rovero & Marshall, Reference Rovero and Marshall2009), and occupancy models are being used to account for detection probability. This is a limitation of our methodology, because detection rates reflect activity levels rather than abundances. However, our criteria for identifying independent records were more restrictive than those used in previous studies on carnivores (O'Brien et al., Reference O'Brien, Kinnaird and Wibisono2003; Rovero & Marshall, Reference Rovero and Marshall2009; Silva-Rodríguez et al., Reference Silva-Rodríguez, Ovando, González, Zambrano, Sepúlveda and Svensson2018), which considered photographs as independent events if separated by at least 60 minutes, with the detection frequency termed relative abundance index and used as a proxy for animal abundance (O'Brien et al., Reference O'Brien, Kinnaird and Wibisono2003). Despite the limitations of the method, to our knowledge this is the first study to assess native carnivore richness and activity in the agricultural area of central Chile. Our results highlight the importance of remnant native forest fragments in vineyard landscapes as potential areas for carnivore conservation, and provide an example of how industries, by preserving natural habitats within their properties, can support biodiversity conservation in productive areas.
Our findings also support the conclusion of a previous study on the importance of remnant patches of native southern temperate rainforest in human-dominated landscapes for the conservation of the guiña (Gálvez et al., Reference Gálvez, Hernández, Laker, Gilabert, Petitpas and Bonacic2013). Preservation of sclerophyllous forest-shrublands in rural landscapes of central Chile may not only benefit wildlife populations, but also provide other ecosystem services such as pollination, pest control and water regulation to agricultural areas (Power, Reference Power2010; Liss et al., Reference Liss, Mitchell, Macdonald, Mahajan, Méthot and Jacob2013). Farming landscapes are essential for a growing human population, both for the provision of food and the conservation of biodiversity, because they occupy large expanses of land, are often adjacent to critical wildlife habitats and depend on ecosystem services (Viers et al., Reference Viers, Williams, Nicholas, Barbosa, Kotzé and Spence2013). Vineyards can benefit from ecosystems services and contribute to biodiversity, particularly as wine producers and consumers place increasing value on the environmental impact of these productive areas (Viers et al., Reference Viers, Williams, Nicholas, Barbosa, Kotzé and Spence2013). Wine producers should thus evaluate and implement land management strategies that support biodiversity conservation. Protecting existing forest remnants and increasing the number and width of biological corridors (Hilty & Merenlender, Reference Hilty and Merenlender2004) could help safeguard the biodiversity of sclerophyllous forest-shrublands. Vintners play an important role in biodiversity conservation: their efforts to preserve and expand native forest remnants on their properties are vital for the conservation of the native fauna of central Chile.
Acknowledgements
This study is a contribution to the Wine, Climate Change and Biodiversity Program of the Institute of Ecology and Biodiversity. The study was partially supported by PUCV-DI 039.407/19 and ACT192027 to JLC-D. JLC-D is an affiliated researcher with the Institute of Ecology and Biodiversity, Chile. SA was funded by FONDECYT 1170995. We thank the vineyards Concha y Toro, Veramonte, Santa Rita, San Pedro de Tarapacá, Viu Mannent, Emiliana and Luis Felipe Edwards for their support.
Author contributions
Study design JLC-D, JDF, GLS; fieldwork: GLS, CB, MIU; data analysis: CBG, KG, SA, JLC-D, GLS; writing: JLC-D, CBG, SA, OB, JDF, AN.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards.







