HIV infection continues to be a major public health problem in sub-Saharan Africa(1). Despite increased access to antiretroviral therapy (ART) in countries in Africa, about 15–30 % of patients(Reference Liu, Spiegelman and Semu2, Reference Adal, Howe and Kassa3) attending ART clinics are malnourished due to nutritional problems associated with advanced HIV infection(Reference Paton, Castello-Branco and Jennings4). Food insecurity resulting from HIV infection may aggravate the problem, since HIV-infected patients and their families may have reduced capacity to produce or afford nutritionally adequate food(Reference Bukusuba, Kikafunda and Whitehead5).
Malnutrition, particularly wasting of lean mass (used here synonymously with fat-free mass), is associated with delayed clinical and functional recovery, and increased risk of mortality among patients starting ART(Reference Liu, Spiegelman and Semu2, Reference Mupere, Malone and Zalwango6, Reference Kotler, Tierney and Wang7). With the goal of improving nutritional rehabilitation and survival, prior randomised controlled trials have investigated the role of macronutrient and micronutrient supplementation on health and mortality of HIV-infected patients(Reference Grobler, Siegfried and Visser8, Reference Visser, Durao and Sinclair9). In some but not all macronutrient trials, these interventions have been shown to have benefits on the health of HIV patients. For example, a trial in Ethiopia among malnourished HIV-infected patients found that both whey- and soya-based supplementary foods increased fat-free mass measured by the deuterium (2H) diluted water (D2O) technique among patients with viral suppression at 3 months following these interventions(Reference Olsen, Abdissa and Kaestel10) and in Malawi malnourished HIV-infected patients receiving ready-to-use fortified spread had higher gain in fat-free mass compared with those on a corn–soya blend(Reference Ndekha, van Oosterhout and Zijlstra11). However, most of the micronutrient trials showed no, limited or only short-term benefits during HIV care. Part of the reasons for such limited effects may be that some studies were conducted in settings where malnutrition including micronutrient deficiency is not prevalent among the HIV-infected population(Reference Visser, Durao and Sinclair9).
Some discrepancies among results may also be due to different techniques used to assess body composition. The bioelectrical impedance analysis (BIA) method is simple and cheap to apply, and hence can be used in large sample sizes. Although it provides acceptable results, it has lower accuracy in individuals compared with reference methods such as air displacement plethysmography (ADP) or D2O(Reference Heyward and Wagner12, Reference Slater and Preston13) which measure tissue masses directly; however, ADP and D2O are expensive and may be difficult logistically to administer. Given contrasting strengths and limitations of these methods, using more than one method may help to better understand the effect of interventions on body composition. As potential differences between HIV-infected patients and normal healthy adults in lean tissue properties (e.g. hydration) may have an impact on direct body composition assessment, further information can be gained through assessment of grip strength, a functional indicator of fat-free mass which is reduced in malnutrition and which is inversely associated with survival(Reference Leong, Teo and Rangarajan14, Reference Filteau, PrayGod and Woodd15).
In the Nutritional Support for African Adults Starting Antiretroviral Therapy (NUSTART) trial, we found that vitamins and minerals added to lipid-based nutrient supplements (LNS-VM) increased cluster of differentiation 4 (CD4) count and some anthropometric measures by 12 weeks of ART(Reference Filteau, PrayGod and Kasonka16, Reference Rehman, Woodd and PrayGod17). In the present paper we examine the effects of LNS-VM, compared with LNS, on grip strength and body composition. We hypothesised that the intervention would have beneficial effects on handgrip strength and fat-free mass since it contained nutrients including Zn, P and Mg which are essential for repletion of muscle and organs(Reference Golden18).
Methods
Study setting
The study was conducted from August 2011 to December 2013 at the National Institute for Medical Research (NIMR), Mwanza, Tanzania and the University Teaching Hospital (UTH), Lusaka, Zambia. In Mwanza HIV-infected patients were screened at six peripheral ART clinics and recruitment was conducted at a research clinic located at the Sekou-Toure Regional Hospital. In Lusaka patients were recruited from six peripheral ART clinics and were referred to UTH for enrolment. HIV diagnosis and treatment followed local national guidelines(19, 20). The trial was registered at the Pan African Clinical Trials Registry as PACTR201106000300631 (http://www.pactr.org).
Inclusion and exclusion criteria
HIV-infected patients who were being referred for ART in Mwanza and Lusaka were included in the trial if they met the following criteria: age 18 years and above, ART-naive (except for standard short-course regimens to prevent maternal-to-child HIV transmission), undernourished (BMI <18·5 kg/m2), eligible for ART according to national criteria at the time (CD4 count <350 cells/μl or had WHO stage 3 or 4 disease), willing to undertake intensive ART follow-up in the study clinic, and provided written informed consent. Patients were not invited into the trial if they were participating in a similar study or were pregnant by self-report.
Study design and interventions
The NUSTART study was a phase III randomised controlled trial comparing in a two-stage protocol LNS-VM (intervention) v. LNS (control) given from recruitment at referral for ART until 6 weeks after starting ART(Reference Filteau, PrayGod and Kasonka16). In the first stage, from recruitment to 2 weeks after starting ART, participants in the control or treatment intervention were given 30 g/d LNS or LNS-VM, about 150 kcal (630 kJ)/d, and in the second stage, from 2–6 weeks after initiating ART, participants were given 250 g/d LNS or LNS-VM, about 1400 kcal (5860 kJ)/d. The LNS was manufactured for the trial by Nutriset (Malaunay, France) and came in ready-to-eat packets. Due to high nutrient requirements in HIV patients, the amounts of added vitamins and minerals in LNS-VM were three times the Recommended Nutrient Intake (RNI) for British women(21), but to avoid possible deleterious effects of Fe during severe infections(Reference Ashworth, Khanum and Jackson22), Fe was not included in the first stage, and in the second stage we provided one RNI only. We included bulk minerals, i.e. K, Mg and P, in both stages to address deficiencies of these minerals, correct electrolyte imbalance and promote tissue repletion. Further details of the intervention are in Supplementary Table S1 and published(Reference Filteau, PrayGod and Kasonka16).
Outcomes
The primary trial outcome was mortality between recruitment and 12 weeks post-ART initiation(Reference Filteau, PrayGod and Kasonka16). Secondary outcomes presented here include effects on handgrip strength, fat mass and fat-free mass at 6 and 12 weeks post-ART initiation and during the follow-up period. BIA results were used as our main measure of body composition, both because we had results for the greatest proportion of participants and because it is a relatively cheap and feasible technique even in fairly poorly resourced settings. BIA produces results predicted from impedance, age, sex, weight and height and we wished to compare these results with the more direct measurements given by ADP and D2O.
Randomisation, allocation concealment and blinding
Randomisation was conducted by the Data and Safety Monitoring Board (DSMB) statistician using computer-generated blocks of sixteen and stratified by country. Packages of LNS-VM and LNS, in both small- and large-dose formats, were delivered by the producer in lots designated by allocation code. Clinic pharmacists not involved in recruitment or provision of care to study participants labelled intervention packets with the study identity numbers at the time packets were dispensed. Participants were recruited by clinic nurses with no access to the code and assigned sequential identity numbers (within sites) after they were found to be eligible and had signed informed consent. Both participants and recruiting staff were not aware of the group of the dispensed supplements and intervention and control supplements packets were of equal size, colour, and similar taste. Adherence to study supplements was modest, with only 39 % of participants consuming at least 75 % of their expected number of sachets of supplement(Reference Filteau, PrayGod and Kasonka16).
Sample size justification
As we reported earlier, by the end of recruitment, we had recruited 1815 patients(Reference Filteau, PrayGod and Kasonka16). This number was sufficient to detect, at 5 % significance, 90 % power and 25 % attrition by 12 weeks due to death or loss to follow-up, differences of 0·18 of a standard deviation in secondary continuous outcomes measured at 6 and 12 weeks.
Ethics
The study was conducted according to principles laid down in the Declaration of Helsinki. Ethics committees of the London School of Hygiene & Tropical Medicine, the University of Zambia Biomedical Research Ethics Committee, and the Medical Research Coordinating Committee of NIMR, Tanzania provided ethics clearances. Patients were enrolled after providing written or thumbprint informed consent and medical care of patients was provided according to national guidelines.
Data collection
Data on demographic and socio-economic status were collected at patient enrolment. Handgrip strength and body composition data were collected at enrolment (before ART initiation), and at the 6th and 12th week post-ART initiation. The time of starting ART was determined by factors outside the investigators' control and was a median of 21 (interquartile range 15–30) d after referral for ART(Reference Filteau, PrayGod and Kasonka16). Patients who did not attend study visits were reminded by telephone or those in Mwanza were also traced to their residences and encouraged to come to clinics for follow-up measurements. Patients were asked to fast overnight and were invited for anthropometry, handgrip strength and body composition measurements in the morning. While barefoot and with minimal clothing, weight was determined to the nearest 0·1 kg using a digital scale and height (baseline only) was measured to the nearest 0·1 cm using a stadiometer fixed to the office wall. Anthropometric measurements were taken in triplicate and medians were used during analysis. We assessed body composition using BIA using Tanita instrumentation (Tanita BC418) as the primary method. In addition, we used D2O (Cortecnet) and ADP (BodPod Model 2007A; Life Measurement Instruments/COSMED) in subsamples, limited by logistic and financial constraints to Zambian participants, to supplement BIA findings. All the methods assessed fat mass (kg) and fat-free mass (kg) and, in addition, BIA also produced results on trunk and segmental fat and fat-free mass, all expressed in kg.
For the D2O technique, patients were asked to provide a 4-ml saliva sample (pre-dose saliva sample), after which they were asked to take a previously prepared dose of 30 g D2O using a straw from a 50-ml, screw-capped, leak-proof bottle. Then they drank 100 ml of drinking water from the same bottle to ensure all D2O was consumed. We collected two post-dose saliva samples at 3 and 4 h. All samples were collected into tightly capped cryogenic tubes and kept away from direct sunlight. While waiting for post-dose sample collection, patients were asked to refrain from walking, eating or drinking. On the day of collection, samples were transported in cool boxes to research laboratories at UTH, where they were stored at −20°C pending transfer to the Zambian National Institute for Scientific and Industrial Research in Lusaka for analysis.
Enrichment of D2O in saliva samples was determined by Fourier Transform Infrared Spectrophotometer (FTIR Model 8400s; Shimadzu). Post-dose enrichment used the mean of the 3- and 4-h samples except where the 4-h enrichment was appreciably higher than the 3-h, in which case only the 4-h sample was used. Using post-dose enrichment data, the dilution space and total body water (TBW) were calculated using conventional formulae(23). Fat-free mass was calculated as TBW/0·723(Reference Wang, Pierson and Heymsfield24) and fat mass was calculated as body weight minus fat-free mass.
From BIA, D2O and ADP fat and fat-free mass, and height measurements, fat mass index (FMI) was computed as fat mass (kg)/(height (m2)), and fat-free mass index (FFMI) as fat-free mass (kg)/(height (m2))(Reference Kyle, Schutz and Dupertuis25). We used FMI and FFMI in data management and fat and fat-free mass in evaluation of the effect of intervention.
Handgrip strength was determined to the nearest 0·1 kg using a digital dynamometer (Takei Scientific Instruments). Four measurements were taken, with the mean of the two maximum measurements (one in each hand) reported. Venous blood samples were taken for CD4 count (baseline and week 12 only)(Reference Filteau, PrayGod and Kasonka16).
Data management and statistics
Data were double entered into OpenClinica databases in Lusaka and into MySQL databases in Mwanza. Analyses were conducted in STATA version 13. The D2O technique is susceptible to chance errors resulting from incomplete dosing, sample contamination, or unrecorded fluid intake during the equilibration period. Therefore, at baseline, we excluded those with implausibly low fatness (fat mass <0 kg). We further excluded those with implausibly high fatness, given that the entire population had BMI <18·5 kg/m2; this resulted in excluding those with FMI >6 kg/m2, or FMI >4 kg/m2 if FFMI <11 kg/m2. For 6-week samples, we excluded those with implausibly low fatness (fat mass<0 kg). We also excluded those with poor agreement between BIA and ADP (difference >±7 kg TBW). For 12-week samples, we excluded those with implausibly low fatness (fat mass <0 kg), and implausibly high fatness (FMI >8 kg/m2). We also excluded those with poor agreement between BIA and ADP (difference >±7 kg TBW). Based on this we excluded fifty-six (10·5 %) at baseline, twenty (8 %) at 6 weeks and twenty (9 %) at 12 weeks.
Baseline characteristics were presented as means and standard deviations if continuous variables and percentages if categorical variables to assess comparability of treatment arms. Socio-economic status was derived using principal component analysis of a list of housing characteristics and durable assets(Reference Filteau, PrayGod and Kasonka16, Reference Filmer and Pritchett26). Outcome measures (i.e. fat mass, fat-free mass and handgrip strength) at 6 and 12 weeks post-ART were compared using linear regression with final estimate found by adjusting for baseline values, sex, age, BMI, CD4 count and socio-economic status.
In addition, since results at 6 and 12 weeks could be analysed only for patients who survived and attended 6- and 12-week visits, we also investigated treatment effects using the detailed longitudinal data collected on all patients over the course of the study. We used piece-wise mixed-effects quadratic regression models to allow inclusion of data from patients who died or were lost to follow-up up prior to 12 weeks. The additional flexibility of cubic models and cubic splines was assessed but fit was considered adequate with quadratic models(Reference Harrell27). The time axis was split at the date of starting ART, allowing two lines with differing slopes to be fitted per person while restricting these lines to join at the date of ART initiation. For presentation, the marginal predictions after starting ART were based on the median time, 21 d, spent prior to starting ART; predictions pre-ART are not graphed because of the complexity of showing different lengths of time before ART.
To assess comparability of BIA against D2O and ADP methods, we used the Bland–Altman method(Reference Bland and Altman28). Based on this approach, we analysed differences (bias) in fat and fat-free mass between BIA and the other methods and their standard deviations (error) and calculated the limits of agreement (bias ± 1·96 error) to determine if the degree to which these methods differed was within a clinically acceptable range. We further assessed the dependency of bias on the mean of fat and fat-free mass for BIA and D2O and BIA and ADP using linear regression. Among both sexes, the acceptable range of error for fat and fat-free mass is thought to be 2 to 4·5 kg for males and 1·5 to 3·6 kg for females(Reference Heyward and Wagner12).
Results
As shown in the trial flow chart (Fig. 1), of 4573 participants screened, 1876 were randomised, and 1815 included in the analysis of the primary outcome(Reference Filteau, PrayGod and Kasonka16), but only 1807 (897 allocated to LNS and 910 to LNS-VM) were included in the present analysis because they had either body composition or grip strength data or both. Of these patients, 1752 had baseline data on handgrip strength and 1461, 479 and 498 had baseline body composition data based on BIA, D2O and ADP, respectively. The mean participant age was 35·8 (range 18–78) years, 59·2 % had BMI <17 kg/m2, and 49·6 % were females. All baseline characteristics were equally distributed between treatment arms (Table 1). From baseline to 6 weeks, and 6 and 12 weeks, proportions not followed up between LNS and LNS-VM groups on participants with body composition measurements based on the three methods (i.e. BIA, D2O and ADP) were not different (47·0 v. 44·5 % (P = 0·34) and 9·5 v. 6·2 % (P = 0·12); 70·8 v. 70·8 % (P = 0·99) and 37·7 v. 32·4 % (P = 0·65; and 72·1 v. 74·1 % (P = 0·62) and 20·0 v. 17·2 % (P = 0·73), respectively). Similarly, proportions of participants with handgrip strength measurements not followed-up between baseline to 6 weeks and 6 weeks to 12 months were not different between LNS and LNS-VM groups (50·4 v. 47·9 % (P = 0·30) and 21·0 v. 21·2 % (P = 0·97), respectively). Overall, compared with patients remaining in the study, those not followed up for body composition and handgrip strength assessments at 6 and 12 weeks were more likely to be males, younger, thinner, more immunocompromised, and not on tuberculosis treatment, all factors associated with mortality in the cohort(Reference Woodd, Kelly and Koethe29).
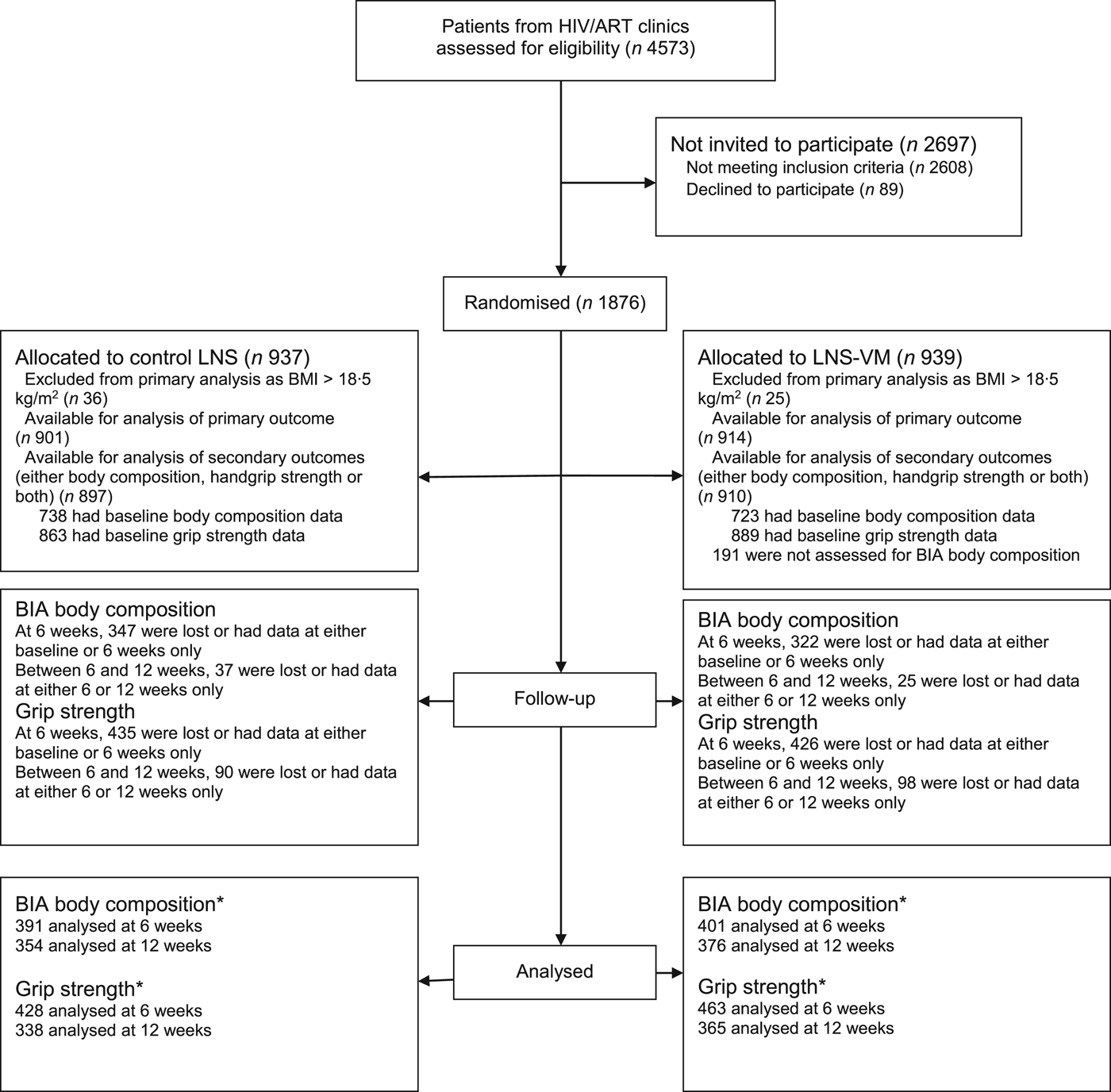
Fig. 1. Trial flow chart. * Number analysed for the effect of interventions. This may differ slightly with the actual number available at either 6 or 12 weeks because analysis of the effect of intervention included participants with data at two time points, i.e. baseline and 6 weeks and 6 weeks and 12 weeks. ART, antiretroviral therapy; LNS, lipid nutritional supplement; LNS-VM, lipid nutritional supplement with vitamins and minerals; BIA, bioelectrical impedance analysis (the main body composition assessment method for the present study).
Table 1. Baseline characteristics of patients included in the evaluation of the effect on Nutritional Support for African Adults Starting Antiretroviral Therapy (NUSTART) intervention on secondary outcomes (i.e. handgrip strength and body composition)*
(Mean values and standard deviations; numbers and percentages)

LNS, lipid nutritional supplement; LNS-VM, lipid nutritional supplement with added vitamins and minerals; CD4, cluster of differentiation 4.
* Of the participants, 1752 had baseline data on handgrip strength, and baseline body composition data were collected on 1461 using bioelectrical impedance analysis, 479 using the deuterium (2H) diluted water method and 498 using air displacement plethysmography.
Table 2 presents body composition and handgrip strength data at baseline, and at 6 and 12 weeks by treatment arm. Supplementary Table S2 presents the same information among patients with data from all three body composition assessment methods. Effects of LNS-VM v. LNS on changes in body composition between baseline and 6 weeks of ART are shown in Table 3 and similar results for changes between 6 and 12 weeks of ART are shown in Table 4. At the end of 6 weeks of ART, patients given LNS-VM tended to have higher fat mass than those randomised to LNS (0·4 (95 % CI 0·05, 0·8) kg); although the point estimate was highest for fat mass assessed by D2O, the effect was significant only for fat mass by BIA, possibly because of the larger number of participants assessed by this method. Treatment arm showed no association with change in fat-free mass. Between 6 and 12 weeks of ART there were no significant differences between treatment arms in fat mass and fat-free mass change although the point estimates suggested higher fat-free mass in the LNS-VM group. Similar results for trunk and limb composition by BIA were seen as for total body tissue masses by BIA. In a sensitivity analysis involving patients with body composition measurements by all three methods (twenty-two patients at 6 weeks and eighteen at 12 weeks), we found no effect of the intervention on fat and fat-free mass (Supplementary Tables S3 and S4).
Table 2. Fat mass, fat-free mass and grip strength at baseline and at 6 and 12 weeks after starting antiretroviral therapy
(Mean values and standard deviations)

LNS, lipid nutritional supplement; LNS-VM, lipid nutritional supplement with vitamins and minerals; D2O, deuterium (2H) diluted water method; ADP, air displacement plethysmography; BIA, bioelectrical impedance analysis.
* One observation is missing.
Table 3. Effects of intervention on the change in body composition and grip strength between baseline and 6 weeks of antiretroviral therapy (ART)
(Mean values and 95 % confidence intervals)

LNS, lipid nutritional supplement; LNS-VM, lipid nutritional supplement with vitamins and minerals; D2O, deuterium (2H) diluted water method; ADP, air displacement plethysmography; BIA, bioelectrical impedance analysis; CD4, cluster of differentiation 4.
* Difference between change over time, adjusted for age and sex.
† Difference between change over time, adjusted for age, sex, baseline CD4, baseline BMI and socio-economic status.
‡ One observation missing.
Table 4. Effects of intervention on the change in body composition and grip strength between 6 and 12 weeks of antiretroviral therapy (ART)
(Mean values and 95 % confidence intervals)

LNS, lipid nutritional supplement; LNS-VM, lipid nutritional supplement with vitamins and minerals; D2O, deuterium (2H) diluted water method; ADP, air displacement plethysmography; BIA, bioelectrical impedance analysis; CD4, cluster of differentiation 4.
* Difference between change over time, adjusted for age and sex.
† Difference between change over time, adjusted for age, sex, baseline CD4, baseline BMI and socio-economic status.
‡ One observation missing.
At the end of 6 weeks post-ART, patients in the LNS-VM group had 0·72 (95 % CI −0·03, 1·5) kg greater handgrip strength regain compared with patients in the LNS arm after adjusting for sex, age, baseline CD4, baseline handgrip strength, and socio-economic status, although this was marginally significant (P = 0·06) (Table 3). However, at 12 weeks, LNS-VM intervention did not have any effect on handgrip strength (−0·28 (95 % CI −1·1, 0·5) kg; P = 0·48) (Table 4).
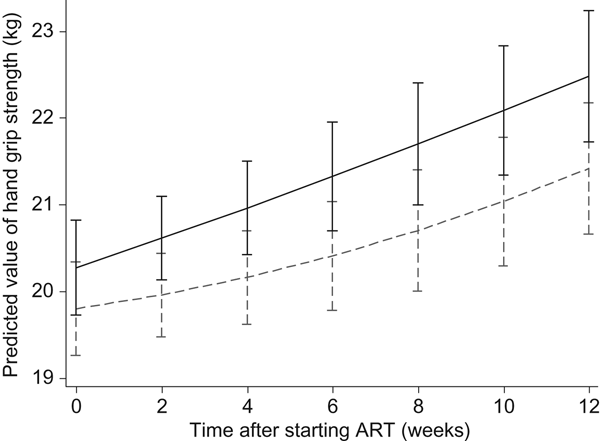
As shown in Figs 2 and 3, in the longitudinal analysis including all patients in the course of the study we found no treatment effect of the intervention on handgrip grip strength (P = 0·71 overall, P = 0·87 pre-ART and P = 0·65 post-ART), fat mass (P = 0·16 overall, P = 0·86 pre-ART and P = 0·39 post-ART), or fat-free mass (P = 0·66 overall, P = 0·43 pre-ART and P = 0·76 post-ART).
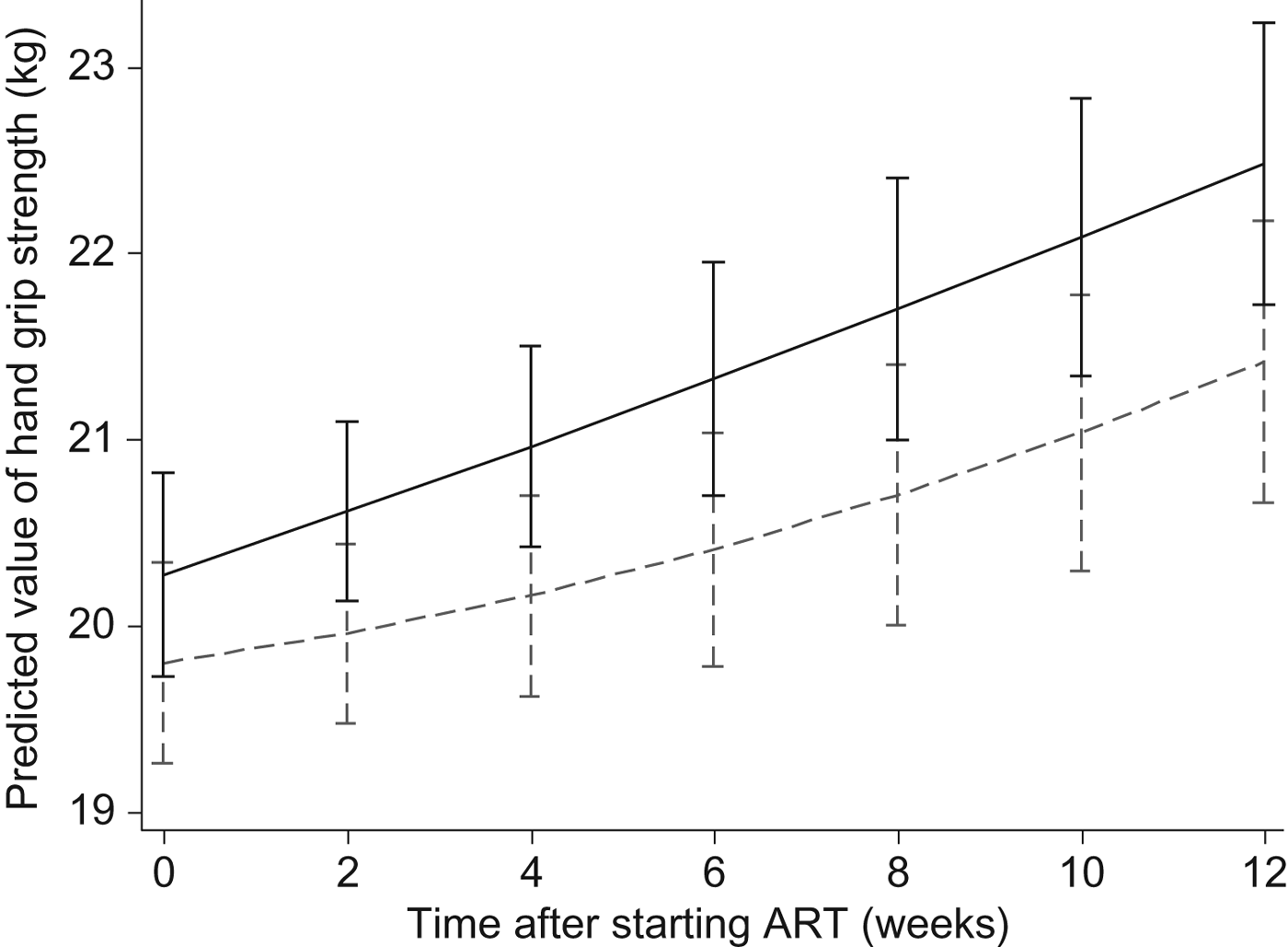
Fig. 2. Effects of the Nutritional Support for African Adults Starting Antiretroviral Therapy (NUSTART) intervention (lipid-based nutritional supplement (LNS; ----) or LNS with added vitamins and minerals (LNS-VM; –––) on handgrip strength during antiretroviral therapy (ART). Values are means, with standard deviations represented by vertical bars. Difference between LNS-VM and LNS: P=0·71.

Fig. 3. Effects of the Nutritional Support for African Adults Starting Antiretroviral Therapy (NUSTART) intervention (lipid-based nutritional supplement (LNS; ----) or LNS with added vitamins and minerals (LNS-VM; –––) on body composition changes during antiretroviral therapy (ART). Values are means, with standard deviations represented by vertical bars. (a) Effects of NuSTART intervention on fat mass during ART. Difference between LNS-VM and LNS: P=0·16. (b) Effects of NUSTART intervention on fat-free mass during ART. Difference between LNS-VM and LNS: P=0·66.
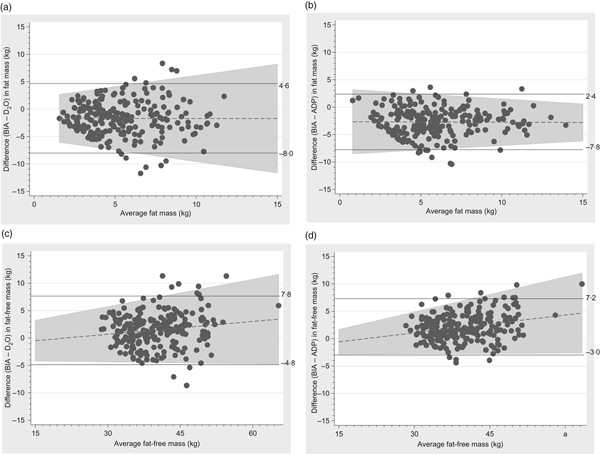
Fat mass measured by BIA tended to be lower than when measured by ADP or D2O and fat-free mass tended to be correspondingly higher and the limit of agreements were −8·0 to 4·6 kg and −7·8 to 2·4 kg for fat mass and −4·8 to 7·8 kg and −3·0 to 7·2 kg kg for fat-free mass, respectively (Fig. 4).

Fig. 4. Bland–Altman plots of fat mass and fat-free mass comparing bioelectrical impedance analysis (BIA) with the deuterium (2H) diluted water (D2O) method and air displacement plethysmography (ADP) technique. (a) Plot with regression line of difference v. mean fat mass measured by BIA and D2O. The bias was –1·7 kg (±3·2) and the limit of agreement was between –8·0 and 4·6 kg. (b) Plot with regression line of difference v. mean fat mass measured by BIA and ADP. The bias was –2·7 kg (±2·5) and the limit of agreement was between –7·8 and 2·4 kg. (c) Plot with regression line of difference v. mean fat-free mass measured by BIA and D2O. The bias was 1·5 kg (±3·2) and the limit of agreement was between –4·8 and 7·8 kg. (d) Plot with regression line of difference v. mean fat-free mass measured by BIA and ADP. The bias was 2·1 kg (±2·6) and the limit of agreement was between –3·0 and 7·2 kg.
Discussion
In the NUSTART trial, we found benefits of the vitamin and mineral supplementation for CD4 count and some anthropometric measures: calf and mid-upper arm circumferences and triceps skinfold(Reference Filteau, PrayGod and Kasonka16, Reference Rehman, Woodd and PrayGod17). Here we investigated the effect of the vitamin and mineral intervention on body composition assessed by three different methods as well as handgrip strength. We found that the LNS-VM intervention led to an increase in BIA-assessed fat mass at 6 but not 12 weeks. ADP and D2O measures of fat mass showed similar point estimates of the changes but were not statistically significant, possibly due to lower sample size. The intervention had no effect on fat-free mass, an outcome measure associated with improved physical functions(Reference Mostert, Goris and Weling-Scheepers30) and reduced mortality(Reference Kotler, Tierney and Wang7). The intervention had a borderline significant effect on handgrip strength at 6 weeks but this was not sustained at 12 weeks. Based on longitudinal analysis, the intervention did not alter any outcome measures throughout the follow-up period.
Several possible mechanisms could have mediated the effect on fat mass found with BIA, including treatment group differences in food intake or physical activity, neither of which was directly measured in our study. In theory, there could have been an increase in food intake in the LNS-VM group as a result of increase in appetite secondary to higher intake of vitamins and minerals as has been shown previously in HIV-infected South African children(Reference Mda, van Raaij and Macintyre31). However, in NUSTART we found no effect of the intervention on appetite(Reference Rehman, Woodd and PrayGod17). A higher intake of vitamins and minerals leading to improved muscle functions, as supported by our data on grip strength, should have resulted in increased rather than reduced energy expenditure, so effects on fat mediated by differential physical activity seem unlikely. As an additional potential mechanism, the persistence of inflammation could have favoured fat accretion over fat-free tissue accretion as seen following severe infections(Reference Macallan, McNurlan and Kurpad32, Reference Schwenk, Hodgson and Wright33). We have shown that patients in this cohort had severe inflammation which had not appreciably decreased by 6 weeks of ART and which may have limited fat-free mass but not fat mass regain(Reference PrayGod, Blevins and Woodd34).
The acute-phase response due to severe inflammation may be a major factor explaining the lack of tissue repletion during nutritional trials of HIV patients(Reference PrayGod, Friis and Filteau35). Despite high vitamin and mineral intake, the acute-phase response to infection may have depressed the serum or plasma concentrations of vitamins A, C and E and folate, and Zn and Se(Reference Tomkins36, Reference Friis, Kaestel and Nielsen37), possibly reducing their availability for tissue synthesis in the first weeks of ART initiation when the acute-phase response remains high. Results from previous nutritional trials provide support for this possible explanation. Among tuberculosis patients, a population with as severe inflammation as HIV patients, nutritional supplementation did not lead to full nutritional recovery due to impaired anabolism during treatment(Reference Macallan, McNurlan and Kurpad32, Reference Schwenk, Hodgson and Wright33). Among apparently healthy HIV-infected individuals, a multi-micronutrient supplement given for 3 months did not result in increased plasma Fe and Zn among those who had inflammation, but led to increased levels among those who had no inflammation(Reference Mburu, Thurnham and Mwaniki38, Reference Mburu, Thurnham and Mwaniki39). In a nutritional supplementation trial in Ethiopia, at 3 months of follow-up there was a higher increase in fat-free mass in a subgroup of patients with viral suppression(Reference Olsen, Abdissa and Kaestel10), compared with those without viral suppression, suggesting that reduction of inflammation may have contributed to the accrual of fat-free mass in this subgroup.
Other factors limiting accrual of fat-free mass in the present study may include low compliance with interventions(Reference Filteau, PrayGod and Kasonka16) and poor nutrient absorption. Although the supplement contained high amounts of growth nutrients including Zn, phosphate and Mg, bioavailability may have been limited since in the study area maize meal and legumes are the staple foods(Reference Malenganisho, Magnussen and Vennervald40) and have high content of phytates which tend to chelate metals like Zn, Ca and Mg resulting in insoluble complexes which are poorly absorbed(Reference Gibson and Ferguson41, Reference Coulibaly, Kouakou and Chen42). We did not study nutrient absorption properties in this study. Lastly, although the supplement contained many essential micronutrients in high amounts, the amounts of growth nutrients including Zn provided may have been inadequate to support additional synthesis of tissues at the time of very high demand.
In the NUSTART study we had hypothesised that the LNS-VM would have led to significant repletion of fat-free mass and help improve physical functions and work capacity(Reference Mostert, Goris and Weling-Scheepers30) and reduce mortality(Reference Kotler, Tierney and Wang7). In contrast, the intervention led to an increment in adipose tissue rather than fat-free mass and to no mortality reduction(Reference Filteau, PrayGod and Kasonka16). It is possible that at the time of ART initiation when the inflammation is severe, regaining fat mass rather than fat-free mass is a more appropriate short-term survival strategy in malnourished HIV patients starting ART since fat mass provides energy needed to sustain inflammation(Reference Lord43), a process aimed at modulating HIV infection. This is consistent with recent findings from a systematic review which found that patients starting ART with CD4 counts <350 cells/mm3 have greater risk of developing obesity during treatment in comparison with patients starting ART with higher CD4 counts(Reference Nduka, Uthman and Kimani44). Adiposity is characterised by a trade-off between short-term benefits and long-term risks(Reference Wells45). Thus, in the long run, patients starting ART when malnourished may have increased risk for developing non-communicable diseases including diabetes and hypertension. In fact, several large cohorts have shown that HIV-infected patients have higher risk of diabetes and hypertension partly explained by nutritional recovery during ART(Reference Hernandez-Romieu, Garg and Rosenberg46–Reference Han, Jiamsakul and Kiertiburanakul48).
The beneficial effect on handgrip strength of about 700 g, although only marginally significant, may be of clinical and survival importance(Reference Leong, Teo and Rangarajan14, Reference Cooper, Kuh and Hardy49). The increase may reflect improvements in muscle functions rather than muscle size since we did not see any effect on fat-free mass. Irrespective of the mechanisms involved, this seemingly positive effect on grip strength in the present and other trials(Reference Olsen, Abdissa and Kaestel10, Reference PrayGod50) suggests that nutritional interventions with added vitamins and minerals may help increase physical strength and work capacity of HIV-infected patients(Reference Leong, Teo and Rangarajan14).
Our results for the different body composition methods showed that BIA tended to give lower values for fat mass than D2O or ADP but that this did not appreciably affect the group differences over time. Although limits of agreement were wide, the biases and errors were within acceptable ranges(Reference Heyward and Wagner12). In an Ethiopian study there was no overall difference between BIA and D2O but BIA tended to overestimate fat-free mass at low fat-free mass(Reference Hegelund, Wells and Girma51). Among Senegalese HIV-infected adults, the bias between BIA and D2O methods was considered relatively small such that BIA, the simpler and cheaper method, was acceptable(Reference Diouf, Gartner and Dossou52). Similarly, BIA, when compared with D2O, was found to be acceptable for HIV-infected and uninfected breastfeeding South African women(Reference Papathakis, Rollins and Brown53). Overall, it appears that, in spite of concerns, BIA is a useful tool for measuring body composition even in seriously ill, malnourished HIV-infected patients and particularly in low-income settings where resources may be a major limitation in using superior methods. The findings in the present analysis and previous studies(Reference Visser, Durao and Sinclair9) suggest that further research is required to understand what should be provided to HIV patients in order to achieve optimal nutritional recovery.
The strengths of the present trial were that it was randomised and double-blinded, had large sample size and body composition was assessed using BIA and other better methods rather than anthropometry alone. However, we did not obtain direct measures of food intake and energy expenditure and compliance with nutritional intervention was modest and assessed using reported information. This limited the scope of our data interpretation. In addition, the study was conducted 5 years ago and during this period changes in ART regimens and eligibility criteria have occurred which could limit the external validity of our study findings. Future studies should include mechanistic substudies to be able to better explain the findings.
Conclusions
Added high doses of vitamins and minerals led to a higher regain of fat mass but not fat-free mass and borderline significant effect on handgrip strength at 6 weeks post-ART. Taken together, these findings suggest the need for a well-considered approach in providing nutritional supplements high in vitamins and minerals to malnourished HIV-infected patients starting ART in sub-Saharan Africa. Further research on appropriate intervention timing, micronutrients requirements, optimal regimens and uptake monitoring is needed to guide the design of future nutritional intervention trials in HIV patients.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2019.15
Acknowledgements
This paper was prepared from data arising from the NUSTART trial. We are therefore grateful to the NUSTART study team which includes: principal investigator: Suzanne Filteau; senior investigators: Aase Bengaard Andersen, John Changalucha, Henrik Friis, Douglas C. Heimburger, Lackson Kasonka, Paul Kelly; statisticians and other senior research fellows: John R. Koethe, Daniela Manno, Natasha Larke, Andrea M. Rehman, Susannah Woodd, Daniel Yilma; steering group: David Thurnham, Andrew Tomkins; Mwanza trial manager: George PrayGod; Lusaka trial managers: Molly Chisenga, Joshua Siame; Mwanza senior clinic team: Jeremiah Kidola, Denna Michael, Kelvin Mussa, Charles Masilingi, Elizabeth Fue, Eva Masesa, Neema Mpandachalo; Lusaka senior clinic team: Anne Kanunga, Likando Munalula, Brenda Kapinda, Nellie Sikanyika; laboratory technicians: Julius Mngara, George Ogweno, Piu Ikigo, Mutinta Muchimba, Memory Samwinga, Leo Beacroft, Harry Black, Celeste Gregg Smith; postgraduate students: Caroline Chisenga, Marlene Hebie, Derek Munkombwe, Gemma Sampson; administrators and data managers: Yolanda Fernandez, Gunda Wandore, Aswile Jonas, Hildah Banda Mabuda, Wakwoya Adugna; pharmacists: Stephen Makandilo, Mwangana Mubita, Jessy Mulenga. We are grateful also to nurses, data entry clerks, drivers and other support staff at both NUSTART sites. We thank Grace Munthali, National Institute for Scientific and Industrial Research, Lusaka, Zambia, for measuring D2O.
This study was funded by European and Developing Countries Clinical Trials Partnership grant no. IP.2009·33011·004. Trial foods were prepared and supplied by Nutriset, Malaunay, France. Funds for measuring D2O were provided by the International Atomic Energy Agency. None of the funders had any role in the design, conduct or interpretation of the study and decision to publish results.
S. F., H. F., D. C. H., J. C., L. K., J. C. K. W. and J. R. K. designed the study. G. P., M. C., J. S., S. W. and K. J. collected data. G. P. and A. M. R. analysed data. G. P. wrote the first draft and all authors critically reviewed subsequent drafts and approved the final version for submission.
J. C. K. W. has previously (more than 10 years ago) received two BIA instruments gratis and some funding from Tanita UK. This donor had no influence on the design, funding, conduct or interpretation of the present study. All other authors declare no conflicts of interest.