Introduction
Natal dispersal, the movement of subadult animals from their natal range to the place where they will breed (Howard, Reference Howard1960), is an important mechanism in the colonization of new areas. Individuals compete for local resources, and dispersers need to obtain access to unexploited resources. In a saturated population such resources are at the edge of or outside the colonized area. Hence, dispersal regulates abundance by expanding distribution (Dieckmann et al., Reference Dieckmann, O’Hara and Weisser1999). Dispersal also allows recolonization of former habitats after a population decrease (Lubina & Levin, Reference Lubina and Levin1988), and this is occurring in large carnivores in Europe and North America. Brown bears Ursus arctos, nearly extinct in the early 20th century, have recolonized large parts of Scandinavia (Swenson et al., Reference Swenson, Wabakken, Sandegren, Bjarvall, Franzén and Söderberg1995). The range outside the core areas was occupied by young bears, predominantly by males 2-4 years old, the age of most active dispersal (Swenson et al., Reference Swenson, Sandegren and Söderberg1998). Wolves Canis lupus from the Italian Apennine population recolonized the Alps, where the species had been eradicated in the 19th century (Breitenmoser, Reference Breitenmoser1998). Recolonization of former range by dispersing wolves has also been observed in Minnesota (male-biased; Mech, Reference Mech, Chepko-Sade and Halpin1987) and in the central Rocky Mountains (both sexes; Boyd & Pletscher, Reference Boyd and Pletscher1999).
The significance of dispersal for the spread of a population is less clear in felids. Long-range dispersal has been described in Eurasian lynx Lynx lynx (J. Linnell, pers. comm.), in Canadian lynx Lynx canadensis (reviewed in Mowat et al., Reference Mowat, Poole, O’Donoghue, Ruggiero, Aubry, Buskirk, Koehler, Krebs, McKelvey and Squires2000) and in puma Puma concolor (Sweanor et al., Reference Sweanor, Logan and Hornocker2000) but these were animals dispersing between or within extant populations. There is little evidence that dispersing subadult felids will colonize new territory, although this is a crucial assumption in reintroduction or recovery projects.
Eurasian lynx were reintroduced into the Swiss Alps in the early 1970s and have subsequently spread over the north-west of the Alpine arc (Breitenmoser et al., Reference Breitenmoser, Breitenmoser-Würsten and Capt1998). The expansion slowed down in the mid 1980s, despite the availability of suitable unoccupied habitat, and in the 1990s little or no evidence for lynx presence was found in some regions that had been previously colonized (Molinari-Jobin et al., Reference Molinari-Jobin, Zimmermann, Breitenmoser, Breitenmoser-Würsten and Capt2001). After 1995 an increase in lynx abundance in the north-west Swiss Alps resulted in a controversy about the return of this species. Hunters, sheep breeders and the regional authorities demanded that the lynx population in this area be reduced (Breitenmoser et al., Reference Breitenmoser, Breitenmoser-Würsten, Capt, Ryser, Zimmermann, Angst, Olsson, Baumgartner, Siegenthaler, Molinari, Laass, Burri, Jobin and Weber1999). Such permission was, however, not given at that time, as the policy of the Federal Office for the Environment was to have lynx distributed throughout the Alps, and there was a general belief that the population pressure in the north-west Swiss Alps would further the expansion of the population.
The hypothesis that population pressure will result in an expansion of the occupied area (Hell, Reference Hell1961) implies two assumptions: the population expands through natal dispersal, and dispersal is positively density dependent. Therefore both the rate and the mean distance of dispersal are greater when lynx are abundant. These assumptions have consequences for the colonization of new or former areas, and hence for the design of reintroduction projects. Furthermore, the nature of any dispersal is determined by resource availability, landscape (habitat and topography), and the social structure of the species. To assess dispersal in the north-west Swiss Alps during a period of peak lynx density (1.4-1.5 resident lynx per 100 km2; Breitenmoser-Würsten et al., Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001) we compared it with data from an earlier, similar study (Zimmermann, Reference Zimmermann1998) in the Jura Mountains where lynx density was low to average (0.7-0.8 resident lynx per 100 km2; Breitenmoser-Würsten et al., in press). To assess the potential of the population of the north-west Swiss Alps to expand we examined the dispersal rates, distances, directions, habitat and linear barriers crossed by dispersers compared to the Jura Mountains and postulated that (1) rate and distance of dispersal should be greater in the higher density population, and (2) most subadults would leave the north-west Swiss Alps and settle in neighbouring areas. Hence we assumed that the monitoring system for lynx in Switzerland (Capt et al., Reference Capt, Breitenmoser, Breitenmoser-Würsten, Ch., Rohner and Breitenmoser1998) would reveal an increase of lynx presence, after a time lag, in the areas adjacent to the north-west Swiss Alps.
Materials and Methods
Study Areas
The north-west Swiss Alps are a 2,800 km2 area more or less isolated from the rest of the Alps and fragmented both by natural and artificial barriers (for details see Zimmermann, Reference Zimmermann2004; Fig. 1). Roe deer Capreolus capreolus, lynx’s main prey (Breitenmoser & Haller, Reference Breitenmoser and Haller1987), were locally depressed during the peak of lynx density at the end of the 1990s. The number of roe deer harvested per 1 km2 forest, assumed to reflect roe deer abundance, has continuously decreased in the north-west Swiss Alps since 1994 (Fig. 2). The overall resident lynx density in the north-west Swiss Alps, based on the distribution of radio-collared animals and information about additional, untagged individuals from camera-trapping, was estimated at 1.4--1.5 per 100 km2 (Breitenmoser-Würsten et al., Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001). Areas with lynx adjacent to the north-west Swiss Alps are Valais to the south, and the west central Swiss Alps to the east.
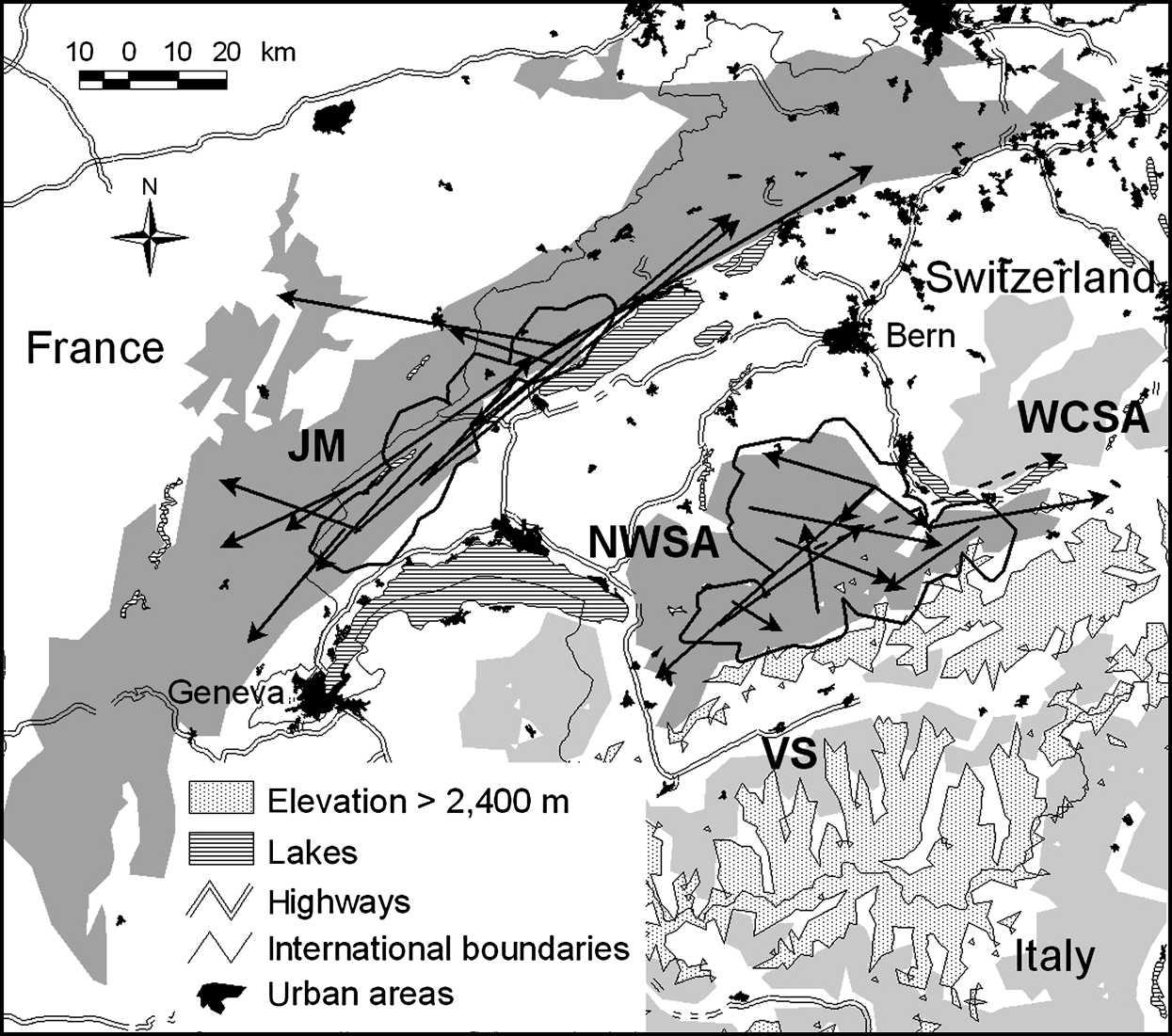
Fig. 1 Location of the study sites in Switzerland. Suitable lynx habitat patches in the north-west Swiss Alps (NWSA) and the Jura Mountains (JM) are dark grey; the adjoining patches of Valais (VS) and west central Swiss Alps (WCSA) are light grey. Thick black lines show the main study areas (see text for further details). Arrows indicate dispersal directions, distances and endpoints for 12 lynx in NWSA and 12 lynx in JM. Movements are shown as straight lines from a lynx’s natal home range centre or its capture site to its independent home range centre, mortality site or last location. All subadults settled in their natal habitat patch, except one male that left NWSA (dashed line).

Fig. 2 Roe deer hunting bag (number killed per km2 forest) from 1990 to 2002 in the north-west Swiss Alps (NWSA) and the Jura Mountains (JM). The hunting quotas are fixed each year according to estimates of population size and trend. The broken line shows the evolution of the Q1 (see text for details) signs of lynx presence in the north-west Swiss Alps.
The Jura Mountains are a secondary limestone mountain chain forming the north-west border of Switzerland with France (for details see Breitenmoser et al., Reference Breitenmoser, Kaczensky, Dötterer, Bernhart, Breitenmoser-Würsten, Capt and Liberek1993a; Fig. 1). They are less fragmented than the Alps, forming a block of contiguous suitable habitat of 6,670 km2 (Zimmermann & Breitenmoser, Reference Zimmermann and Breitenmoserin press). In contrast to the north-west Swiss Alps, prey base was not a limiting factor during the study period. The number of roe deer killed per 1 km2 of forest increased continuously since 1990 in the southern part of the Jura Mountains (Fig. 2), as well as in France (Stahl et al., Reference Stahl, Vandel, Herrenschmidt and Migot2001). The overall lynx density in the main study area remained fairly constant over the whole study period and was estimated at 0.7–0.8 residents per 100 km2 (Breitenmoser-Würsten et al., in press).
The study area in each region was defined as the area formed by the 1 km buffered 100% minimum convex polygon (MCP) of all adult resident females from which juveniles were caught and observed during dispersal (Fig. 1).
Field study
From 1988 to 2001 dispersal characteristics (Table 1) were obtained for 22 and 17 lynx in the north-west Swiss Alps and Jura Mountains, respectively. Dispersal data came mainly from radio-telemetry; additional information was available from kittens tagged at the den and later live-trapped or photographed by a camera trap. Details of capture, handling, radio-telemetry and number of locations are in Zimmermann et al. (Reference Zimmermann, Breitenmoser-Würsten and Breitenmoser2005). Only radio fixes with an accuracy of 1 km2 or better were considered. We located dispersing lynx almost every day when they moved through new terrain, and at least every week once home ranges were established for >1 month. After independence subadults were considered dispersers when they established a home range that overlapped by ≤5% of their natal (maternal) home range (based on a 90% MCP) or were last located outside their natal area for those that either died or lost contact before forming a home range (Sweanor et al., Reference Sweanor, Logan and Hornocker2000). Percentage overlap of total ranges and home ranges between animals A and B was calculated as
All other independent progeny (those establishing home ranges with >5% overlap with their natal range) were considered philopatric (Ph). Dispersal began when a subadult made its first move outside its natal home range without returning. Dispersers were classified as: (1) dispersers (Di) that most likely completed their natal dispersal and exhibited 6 months of site fidelity suggestive of home range establishment and/or reached sexual maturity, or (2) failed dispersers (fD) that exhibited <6 months site fidelity and/or died before they established a home range. Lynx normally reach sexual maturity at 2.75 years for males and 1.75 years for females. In one study (Kvam, Reference Kvam1991) some males and almost 50% of females reached sexual maturity one year earlier but we never observed such early maturity. A limit of 6 months was chosen for site fidelity because all dispersers, with the exception of one male that stayed for 200 days, left their transient home range after 72–114 days (median 113 days, n = 5; Zimmermann et al., Reference Zimmermann, Breitenmoser-Würsten and Breitenmoser2005).
Table 1 Characteristics and fate of juvenile lynx (M, male; F, female; FB, females ear-tagged as juveniles) followed in the north-west Swiss Alps and the Jura Mountains (Zimmermann et al., Reference Zimmermann, Breitenmoser-Würsten and Breitenmoser2005), with the maternal lines, date of first observation, method used to track each individual, and the fate of each individual.
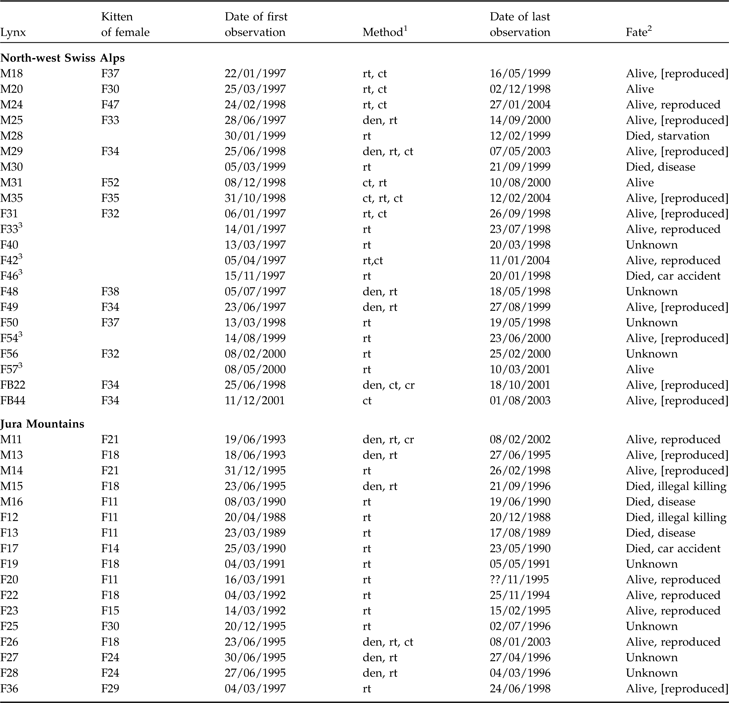
1 Den, individual ear-tagged as kitten; rt, radio-telemetry; ct, camera-trap; cr, carcass retrieved
2 Alive, survived the full year after separation from mother; reproduced, strong evidence from genetic analyses or field observations that individuals have reproduced; [reproduced], lynx reached sexual maturity (according to definition of Kvam, Reference Kvam1991) but there was no proof of reproduction
3 First observed as subadults after separation from mother
Data analysis
The dispersal direction was defined as the vector from the centre (arithmetic mean of x and y coordinates of all radio fixes) of the natal home range to the centre of the independent home range. Directional data were transformed to unimodal data and subjected to Rayleigh’s test (Zar, Reference Zar1984) to examine whether dispersal directions were distributed uniformly or not. Centroid distance (CD; Fig. 3) was the distance from the centre of a progeny’s natal home range to the centre of its independent home range. When complete dispersal information was not available, dispersal distances and directions were calculated based on one of the following combinations: centre of natal home range to mortality site or last location, or capture site to centre of independent home range, mortality site or last location. The dispersal distance was also expressed relatively as the number of sex-specific home ranges crossed during dispersal. The size of male and female home ranges, respectively, was calculated as the diameter (d) of a circle with an area equal to the average home range size (HR) for adult male and female lynx in the study areas (north-west Swiss Alps: Breitenmoser-Würsten et al., Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001; Jura Mountains: Breitenmoser-Würsten et al., in press):
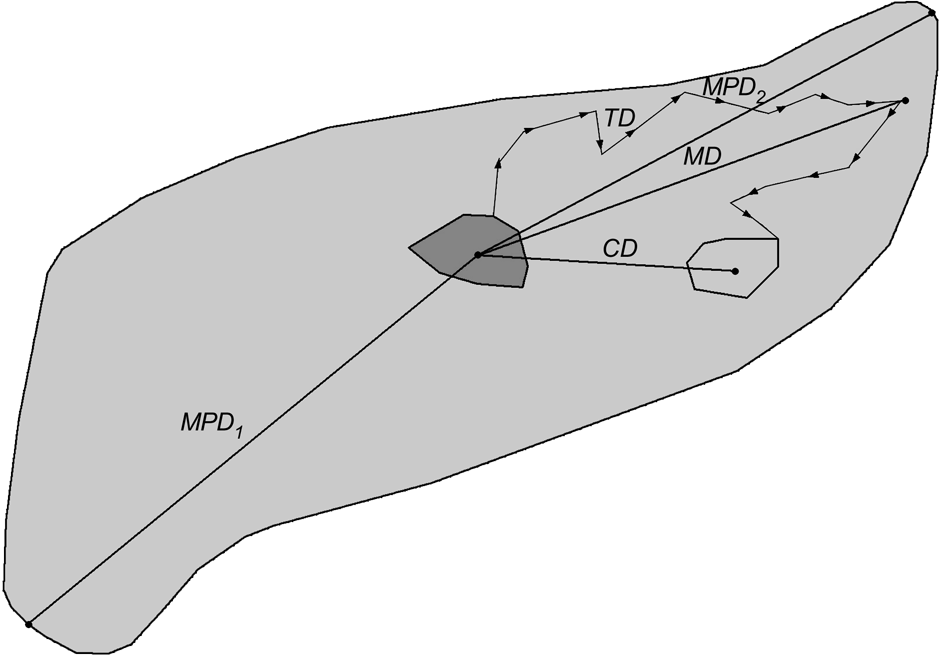
Fig. 3 Definitions of the measured dispersal distances of subadult lynx. Centroid distance (CD) is distance from arithmetic centre of natal home range (solid grey minimum convex polygon, MCP) to arithmetic centre of independent home range (light grey MCP). Total distance (TD) is sum of distances between consecutive locations during dispersal (measured from the point the subadult left the natal home range to the point when it entered its independent home range); maximum distance (MD) is longest distance a dispersing lynx was ever located from the centroid of its natal range; maximum possible distance 1 (MPD1) is distance from the centroid of the natal range to the farthest edge of good lynx habitat (light grey area); maximum possible distance 2 (MPD2) is distance from the centre of the natal home range to the most distant good lynx habitat edge in the initial dispersal direction.
The total dispersal distance (TD, Fig. 3) was the sum of distances between consecutive locations (only one radio-telemetry fix per day considered) of subadults during their dispersal. The measures were taken from the point the subadult left the natal home range to the point when entering its subsequent home range. When subadults established more than one home range, the total distances between the ranges were summed. The maximum dispersal distance (MD; Fig. 3) was the largest distance a dispersing lynx was ever located from the centre of its natal home range. We compared MD with two maximum possible dispersal distances: from the centre of the natal range to the farthest edge of good lynx habitat that could be accessed within the same area (MPD1; Fig. 3) and to the most distant good lynx habitat edge in the initial dispersal direction chosen by the respective subadult (MPD2; Fig. 3). The areas of good lynx habitat were derived from lynx habitat models developed for the Alps (Zimmermann, Reference Zimmermann2004) and the Jura Mountains (Zimmermann & Breitenmoser, Reference Zimmermann and Breitenmoserin press). We used the Mann-Whitney U-test (Zar, Reference Zar1984) to compare dispersal distances between sexes and study areas, and the Wilcoxon paired-sample test (Zar, Reference Zar1984) to compare MD with MPD1, MPD2 and CD. Comparisons were made between (i) all subadults (Ph, fD and Di), (ii) only individuals that dispersed (fD, Di) and (iii) only those that completed dispersal (Di). Centroid distance of Ph, fD and Di is equivalent to the recovery distance, with the centroid distance of Di describing the effective dispersal (Trewhella et al., Reference Trewhella, Harris and McAllister1988).
To assess the effect of increased lynx abundance in, and dispersal from, the north-west Swiss Alps on lynx presence in neighbouring areas, we used information from a standardized monitoring system (see Capt et al., Reference Capt, Breitenmoser, Breitenmoser-Würsten, Ch., Rohner and Breitenmoser1998, for details). All data were calibrated according to the criteria defined for the pan-Alpine monitoring (Molinari-Jobin et al., Reference Molinari-Jobin, Zimmermann, Breitenmoser, Breitenmoser-Würsten and Capt2001), which defines three levels of reliability (Q1, Q2 and Q3). We considered only confirmed data (Q1 and Q2) to compare lynx presence and population trends in neighbouring areas but excluded depredation reports, as livestock is not consistently available in all areas.
Results
Only two females out of 14 subadults in the Jura Mountains and one male out of 13 subadults in the north-west Swiss Alps (F23, F36 and M30; Table 2) remained philopatric, and M30 died after a short dispersal (Fig. 3). Seven out of 9 subadults from the Jura Mountains that completed dispersal left the main study area whereas three out of 12 did so in the north-west Swiss Alps. None of the subadults left the Jura Mountains whereas one (M18) left the north-west Swiss Alps.
Table 2 Dispersal type of 13 subadult lynx in the north-west Swiss Alps and 14 in the Jura Mountains with centroid, total, maximum dispersal distances, and maximum possible distances (MPD1 and MPD2), and % overlap with maternal home range. Centroid distance is also expressed as number of mean circular resident female and male home range diameters, respectively. See text and Fig. 3 for further details of the dispersal distances.
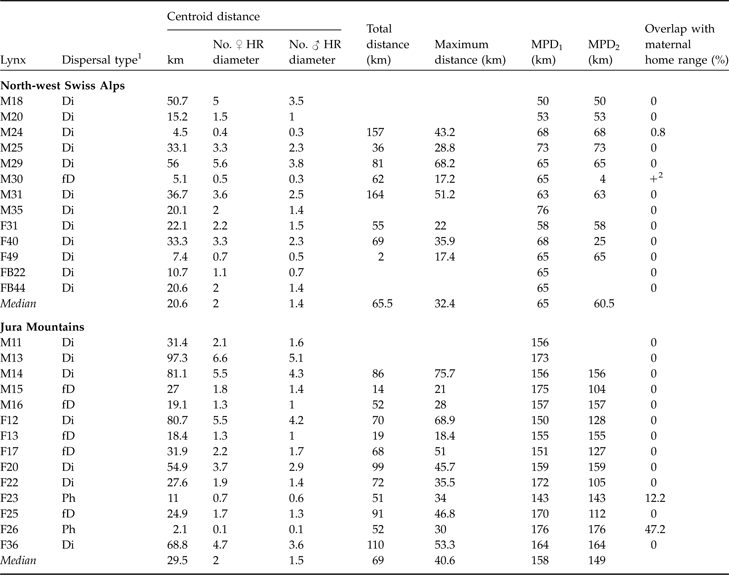
1 Ph, remained philopatric; Di, individuals that most likely completed their dispersal (6 months of site fidelity suggestive of home range establishment and/or surveyed until sexual maturity); fD, failed dispersal (<6 months site fidelity and/or died before establishing a home range)
2 Overlap, but maternal home range not known exactly
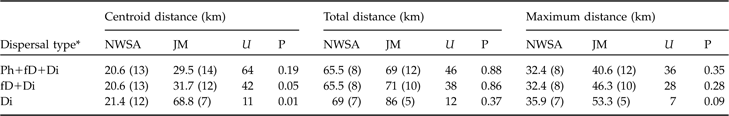
In both areas no difference between sexes was observed for centroid, total or maximum dispersal distances whichever dispersal category was considered (all P >0.05). Males and females were therefore pooled for all subsequent analyses. Centroid distances were 4.5–56.0 km in the north-west Swiss Alps and 2.1–97.3 km in the Jura Mountains (Table 2). Subadult lynx that completed and/or had a fatal dispersal dispersed further in the Jura Mountains than in the north-west Swiss Alps: centroid distance was higher in the Jura Mountains than in the north-west Swiss Alps when either both fatal (fD) and completed dispersal (Di) or when only completed dispersal (Di) was considered but did not differ when all individuals (Ph+fD+Di) were included (Table 3).
Table 3 Statistical comparison between the centroid, total and maximum dispersal distances (medians with sample size in parentheses) of subadult lynx in the north-west Swiss Alps (NWSA) and the Jura Mountains (JM), using the Mann-Whitney U statistic. See text and Fig. 3 for further details of the three distances.
* See footnote to Table 2
Median recovery distance (CD of Ph, fD and Di) in the north-west Swiss Alps was 2.0 (range 0.4–5.6) times the mean circular resident female’s home range diameter and 1.4 (range 0.3–3.8) times the mean circular resident male’s home range diameter. In the Jura Mountains, it was 2.0 (range 0.1–6.6) and 1.5 (range 0.1–5.1) times the respective means. Median effective dispersal distance (CD of Di) was 2.1 (range 0.4–5.6) times female home range diameter and 1.5 (range 0.3–3.8) times the mean resident male’s home range diameter in the north-west Swiss Alps. In the Jura Mountains it was 4.7 (range 0.1–6.6) and 3.6 (range 0.1–5.1) times the respective means.
Dispersal directions of individuals that either failed or completed dispersal (Table 2) were randomly distributed in the north-west Swiss Alps, but not in the Jura Mountains (Rayleigh test; north-west Swiss Alps: Z = 2.1, n = 12, P >0.1; Jura Mountains: Z = 5.5, n = 12, 0.002 < P < 0.005). The main dispersal direction in the Jura Mountains was to the south-west and north-east, corresponding approximately to the orientation of the predominant ridgelines of this mountain range (Fig. 1). The total and maximum dispersal distances were 2.0–164.0 km and 17.2–68.2 km, respectively, in the north-west Swiss Alps and 14.0–110.0 km and 21.0–75.7 km, respectively, in the Jura Mountains (Table 2). Total and maximum dispersal distances did not differ significantly between the two areas whichever dispersal category was considered (Table 3).
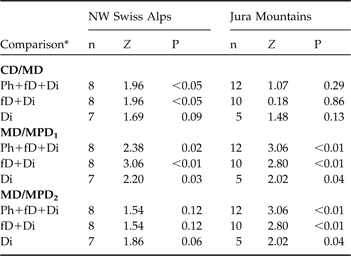
In the north-west Swiss Alps CD was smaller than MD, indicating a circular dispersal, when all individuals, and only individuals that exhibited fatal or completed dispersal, were compared (Table 4), but did not differ for individuals that completed dispersal. No difference between CD and MD was observed in the Jura Mountains (Table 4).
Table 4 Statistical comparison between the centroid distance (CD) and maximum distance (MD) and between the maximum distance (MD) and maximum possible distances (MPD1 and MPD2) of dispersing subadult lynx in the north-west Swiss Alps and the Jura Mountains using a Wilcoxon paired-sample test (Z). See text and Fig. 3 for further details of CD, MD, MPD1 and MPD2.
* See footnote to Table 2
In both areas the maximum distance was smaller than MPD1 whichever dispersal category was considered but was significantly smaller than MPD2 for all comparisons in the Jura Mountains but did not differ in the north-west Swiss Alps, indicating that in the latter most of the subadults reached the edge of suitable habitat during their dispersal (Table 4). The suitable habitat patch in the Jura Mountains is larger than in the north-west Swiss Alps as MPD1 and MPD2 were significantly higher in the former (all P <0.05).
In the north-west Swiss Alps four out of nine subadults, all males, went beyond the edge of good lynx habitat while dispersing. Only M18, however, reached the neighbouring area of the west central Swiss Alps (Fig. 1). The three other males (M24, M29 and M30) returned after having spent a few days in the vicinity of a highway. Two of them (M24 and M30) turned back close to the place where they initially started their dispersal. M30 in the north-west Swiss Alps (Fig. 4) and M16 in the Jura Mountains, covered a considerable distance through sparsely wooded areas of the Swiss Plateau before returning to their respective mountain ranges.
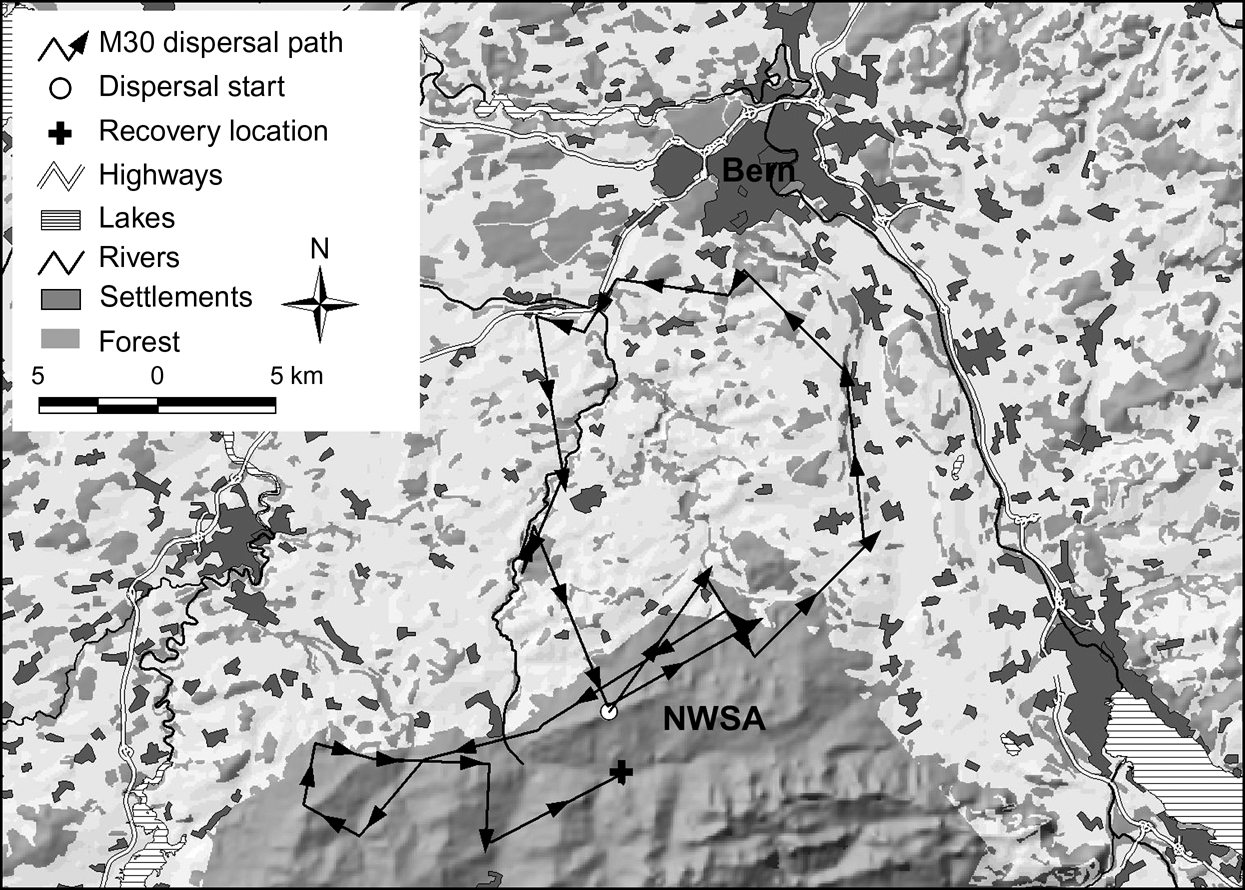
Fig. 4 Dispersal route of lynx M30. He traversed a sparsely wooded part of the Swiss Plateau and moved north until he reached the surroundings of Bern where he turned west. After moving into an area 8 km west of Bern he followed a fenced highway for >4.5 km and spent a week in the vicinity of both the highway and a railway. He returned to the north-west Swiss Alps (NWSA) by moving along a river course.
The monitoring data showed a significant increase in lynx abundance in the north-west Swiss Alps from the middle to the end of the 1990s followed by a decrease (Fig. 5b). No significant increase in lynx abundance could, however, be observed in the neighbouring areas of Valais and the west central Swiss Alps, even after a time lag of 3 years (Fig. 5a,c).
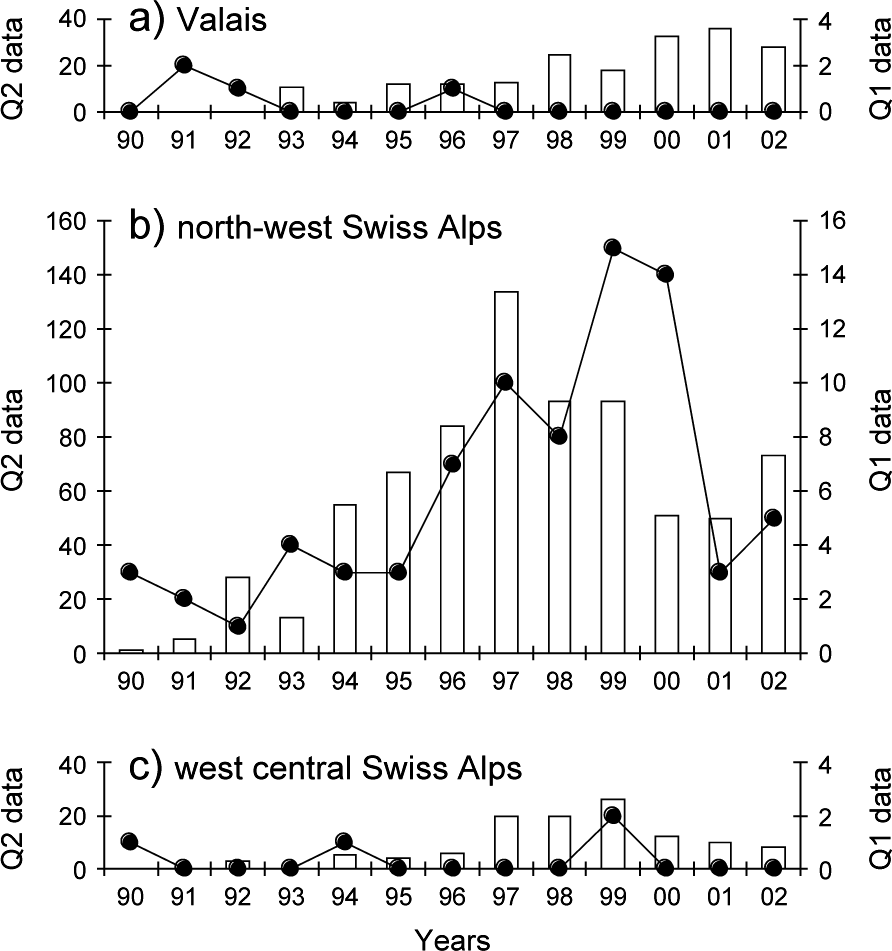
Fig. 5 Reported signs of lynx presence over 1990–2002 in (a) the Valais, (b) the north-west Swiss Alps and (c) the west central Swiss Alps. Reports of lynx killed or found dead, or young orphaned lynx caught and taken into captivity (Quality 1, continuous line, right y-axis), records of wild prey remains, tracks, scats, sightings, and vocalizations confirmed by trained personnel (Quality 2, columns, left y-axis).
Discussion
Subadults from the north-west Swiss Alps and the Jura Mountains appeared to have the same dispersal potential as there were no observed differences between the two areas in the total and maximum distances dispersed. Contrary to our expectation, centroid dispersal distances did not differ between the two areas when all individuals were considered, and were only significantly smaller in the north-west Swiss Alps than in the Jura Mountains when individuals that most likely completed dispersal were considered. However, a larger proportion of individuals in the north-west Swiss Alps, all males, moved through unfavourable habitat but all stopped at fenced highways and turned back, except M18 which left the area. Two of the individuals settled in, or in the vicinity of, their maternal home range. Subadult lynx appear, therefore, to have a low capability to move through unfavourable habitat and to cross linear barriers such as fenced highways. Observations from adult radio-tagged lynx, which sometimes roam far outside their home ranges show, however, that lynx can cross such obstacles (Breitenmoser-Würsten et al., Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001; Ryser et al., Reference Ryser, von Wattenwyl, Ryser-Degiorgis, Willisch, Zimmermann and Breitenmoser2004).
The apparent reduced ability of subadults to cross barriers led to circular dispersal in the case of two males in the north-west Swiss Alps. Similarly, severe habitat restriction led to philopatry in male cougar Puma concolor coryi in Florida, where they returned to the vicinity of their natal areas after unfruitful dispersal attempts (Maehr et al., Reference Maehr, Land, Shindle, Bass and Hoctor2002). A similar process was described for the Iberian lynx Lynx pardinus (Ferreras et al., Reference Ferreras, Delibes, Palomares, Fedriani, Calzada and Revilla2004). Habitat quality and barriers also shaped dispersal directions. In the north-west Swiss Alps, with no parallel ridgelines, dispersal directions were oriented randomly but the ridges of the Jura Mountains run south-west to north-east and this appeared to shape the movements of dispersing subadult lynx. The lack of south-east dispersal in the Jura Mountains may indicate hesitance to leave the continuous forest and travel over open agricultural areas. Similar behaviour has been reported in American black bears Ursus americanus in west Virginia that used the predominant ridgelines of the Appalachian Mountains as corridors (Lee & Vaughan, Reference Lee and Vaughan2003), and in red fox Vulpes vulpes in North Dakota where dispersal directions were altered by a 4-lane interstate highway (Allen & Sargeant, Reference Allen and Sargeant1993).
The most important outcome of our analysis, however, was that we failed to detect any positive density-dependent effects in lynx dispersal and hence cannot confirm the hypothesis that high population density will encourage the expansion of the population. Contrary to expectation a high proportion of subadults also dispersed in the Jura Mountains, an area with a lower lynx density and relatively high prey availability compared to the north-west Swiss Alps. Only one out of 12 individual lynx that completed dispersal actually left the north-west Swiss Alps, and the proportion of individuals leaving was smaller than in the Jura Mountains. As a consequence of the low rate of successful dispersals, the population pressure in the north-west Swiss Alps did not trigger an increase in lynx abundance in the two neighbouring areas (Fig. 5).
High lynx abundance can depress locally the numbers of roe deer and chamois Rupicapra rupicapra, lead to an increase in depredation on livestock, and ultimately diminish the acceptance of lynx by local people (Breitenmoser et al., Reference Breitenmoser, Breitenmoser-Würsten, Capt, Ryser, Zimmermann, Angst, Olsson, Baumgartner, Siegenthaler, Molinari, Laass, Burri, Jobin and Weber1999). In 2000 at least eight individuals were known to have been killed illegally (Breitenmoser-Würsten et al., Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001) and four animals were removed as stock raiders during 1997–2001. In addition, six lynx were taken from the north-west Swiss Alps in 2001 for a translocation programme into the eastern Swiss Alps (Molinari-Jobin et al., Reference Molinari-Jobin, Zimmermann, Breitenmoser, Breitenmoser-Würsten and Capt2001). All of these removals lead to a considerable reduction in abundance (Fig. 5).
Generally, there is good evidence in natural populations that dispersal rate increases with increasing competition for limited resources (Lambin et al., Reference Lambin, Aars, Piertney, Clobert, Danchin, Dhondt and Nichols2001) but dispersal rates have also been reported to be negatively density dependent, with a smaller fraction of individuals dispersing at higher densities (Wolff, Reference Wolff1997; Lambin et al., Reference Lambin, Aars, Piertney, Clobert, Danchin, Dhondt and Nichols2001). Furthermore, those that disperse may only move relatively short distances (McCarty, Reference McCarty1997). This pattern has been reported for Townsend’s voles Microtus townsendii (Lambin, Reference Lambin1994), red foxes (Trewhella et al., Reference Trewhella, Harris and McAllister1988), and Canadian lynx (Breitenmoser et al., Reference Breitenmoser, Slough and Breitenmoser-Würsten1993b). The little data available (Schmidt et al., Reference Schmidt, Jedrzejewski and Okarma1997; Sunde et al., Reference Sunde, Kvam, Moa, Negård and Overskaug2000) indicate that median recovery distance may be negatively correlated with lynx density and positively with mean male and female home range diameter. With the exception of the hunted Norwegian population, lynx dispersed roughly 1.5 male or 2.0 female home range diameters when recovery distances were considered. If dispersal is negatively correlated with density, the longest dispersal distances should be observed in areas where the lynx density is low and the home ranges large. The data (Table 5) seem to confirm this. However, central and northern Europe (where densities are generally much lower) are not necessarily comparable.
Table 5 Data on dispersal distances of Eurasian lynx in four European areas, sorted from low to high density, with mean resident male and female home range diameters (HR ø) and population densities (number of resident lynx 100 km−2), and median recovery distances and effective dispersal distances (km; see text for further details), with median number of mean circular resident female and male home range diameters crossed.
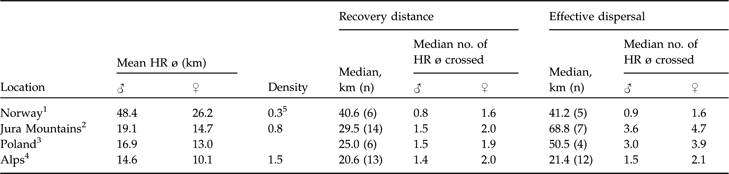
1 Sunde et al. (Reference Sunde, Kvam, Moa, Negård and Overskaug2000)
2 Breitenmoser-Würsten et al. (in press)
3 Schmidt et al. (Reference Schmidt, Jedrzejewski and Okarma1997)
4 Breitenmoser-Würsten et al. (Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001)
5 Managed population
In contrast to wolves and bears, which can disperse over long distances (Swenson et al., Reference Swenson, Sandegren and Söderberg1998; Merrill & Mech, Reference Merrill and Mech2000; Stratman et al., Reference Stratman, Alden, Pelton and Sunquist2001), subadult lynx are conservative dispersers because of their life history and land tenure system and therefore maintain close contact to conspecifics (Zimmermann et al., Reference Zimmermann, Breitenmoser-Würsten and Breitenmoser2005). Their low ability to cross major barriers such as fenced highways hampers the colonization of a fragmented landscape such as the Alps. In the long-term the population may yet disperse but because of prey depression and conflicts with local people high population densities are not maintained over long periods.
Natural spread of the species could potentially be increased by the use of wildlife crossings but the type of crossings that lynx will use have yet to be determined. Riparian vegetation may serve as natural corridors, as shown by the dispersal path of M30 (Fig. 4). Connectivity may be enhanced if wildlife crossings are established in the direction of predominant ridgelines or other landscape level features such as forests and riparian vegetation that may aid or direct dispersing lynx.
Our findings may have consequences for the design of carnivore reintroductions (Breitenmoser et al., Reference Breitenmoser, Breitenmoser-Würsten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001) or recovery programmes in fragmented landscapes. Rather than creating one population, population nuclei could be founded in several neighbouring patches through reintroductions or artificial transfer of individuals to neighbouring areas. Considering that dispersal may be inversely density dependent, such an approach may be a better release strategy for reintroduction, as dispersing animals may encounter conspecifics when moving away from the release site. In a reintroduction project in Austria lynx dispersed in all directions from the site of release (Gossow & Honsig-Erlenburg, Reference Gossow, Honsig-Erlenburg, Miller and Everett1986) without founding a population.
To compensate for the lack of expansion of the Swiss populations six lynx from the north-west Swiss Alps and three from the Jura Mountains were translocated to the eastern part of Switzerland during the winters of 2000/2001 and 2002/2003. This allowed reduction of locally high abundance in the north-west Swiss Alps and accelerated the desired spread of the species. Paradoxically, it is possible that the intervention in the north-west Swiss Alps and the resulting reduction of the population will now lead to an increased dispersal rate. Even though there are more vacant territories for youngsters close to their birth place in controlled populations there is no evidence that dispersal is less common (Macdonald & Johnson, Reference Macdonald, Johnson, Clobert, Danchin, Dhondt and Nichols2001). If anything, control seems only to increase dispersal (Frank & Woodroffe, Reference Frank, Woodroffe, Gittleman, Funk, Macdonald and Wayne2001). Exchange between established neighbouring populations will almost certainly take place later on, as resident adult lynx, especially males, show a higher propensity to cross barriers than subadults, particularly during the mating season. This could help to improve genetic exchange between established subpopulations.
Acknowledgements
We thank C. Angst, A. Burri, S. Capt, J. Laass, P. Molinari, A. Molinari-Jobin, A. Ryser and many other colleagues for gathering data over the past 20 years, and P. Sunde who provided dispersal data for Norway. Hunting statistics were kindly provided by P. Demierre, P. Juesy, and S. Sachot. We also appreciated the assistance of J. Hausser with analysis and P. Jackson with English. We thank M. von Arx for valuable discussions, and P. Wandeler and D. Hetherington for providing helpful comments on a previous version of this manuscript. The study was funded by the FOEN, the Canton of Vaud, Bern and Fribourg, Pro Natura, WWF Switzerland, the Stotzer-Kästli Foundation, and the Dr. Berthold Suhner Foundation. Sources of the digital geographical database were the Federal Office of Topography and Swiss Federal Statistical Office GEOSTAT.
Biographical sketches
Fridolin Zimmermann is a wildlife biologist with KORA, which coordinates research projects for the conservation and management of carnivores of Switzerland. His research interests include habitat modelling, dispersal, mark-recapture analyses and the transfer of scientific results to practical conservation and management measures.
Christine Breitenmoser-Würsten is a wildlife biologist who has worked on various large carnivore issues over the past 20 years, including a study of conservation and population genetics of lynx in Europe. She is currently a project coordinator at KORA.
Urs Breitenmoser has been involved in lynx conservation issues since 1981. He is a co-director of KORA and a senior researcher at the University of Bern, specializing in carnivore ecology and rabies epidemiology.












