Overproduction of reactive oxygen species, and particularly superoxide (∙O2− ), has been implicated in the pathogenesis of various cardiovascular diseases(Reference Pechanova and Simko1–Reference Droge3). The main sources of ∙O2− include NADPH oxidase, cyclo-oxygenase, xanthine oxidase, mitochondrial oxidases and NO synthase. The NADPH oxidase enzyme system is considered a major source of ∙O2− in vascular cells as well as phagocytic polymorphonuclear neutrophils (PMN), monocytes and platelets(Reference Bedard and Krause4). The NADPH oxidase complex was originally described in phagocytes, and is comprised of the membrane-bound catalytic core NOX2 (NADPH oxidase 2) and p22phox, the small G-protein Rap1A, and the cytosolic components p47phox, p67phox and p40phox(Reference Sheppard, Kelher and Moore5). NOX2 (previously named gp91phox) homologues (NOX1, 3, 4, 5, dual oxidase (DUOX)1 and DUOX2) have been characterised in different cell types, including vascular cells(Reference Bedard and Krause4). NOX1 and NOX4 are abundantly expressed in vascular smooth muscle cells and endothelial cells, respectively(Reference Lassegue, Sorescu and Szocs6, Reference Ago, Kitazono and Ooboshi7). In contrast to phagocytic cells, vascular cells produce superoxide under basal, non-stimulated conditions but to a significantly lesser extent than phagocytes(Reference Rueckschloss, Galle and Holtz8). Recently, NADPH oxidase subunit expression and ∙O2− production have been shown to correlate with the severity of atherosclerosis(Reference Sorescu, Weiss and Lassegue9), plaque stability(Reference Azumi, Inoue and Ohashi10), oxidative stress in coronary artery disease(Reference Guzik, Sadowski and Guzik11) and plasma metalloproteinase-9 levels(Reference Zalba, Fortuño and Orbe12). Thus, it might be suggested that a reduction of these increased levels of systemic ∙O2− through the NADPH oxidase system would reduce the pathogenesis of vascular disease associated with oxidative stress.
Plant polyphenols comprise a large group of natural antioxidants that are serious candidates to explain the protective effects of vegetables and fruits against CVD and certain types of cancer(Reference Arts and Hollman13, Reference Hooper, Kroon and Rimm14). They occur naturally in foods, and are especially abundant in tea, cacao and grape products. In vitro, polyphenols have been shown to reduce NADPH oxidase activity in human neutrophils(Reference Tauber, Fay and Marletta15, Reference Poolman, Ng and Farmer16), to decrease p22phox and p67phox expression in endothelial cells(Reference Ying, Xu and Ikeda17, Reference Xu, Ikeda and Yamori18), and to reduce NADPH oxidase-dependent platelet recruitment through the inhibition of protein kinase C(Reference Pignatelli, Di Santo and Buchetti19). In animal models, polyphenols prevent angiotensin II or deoxycorticosterone acetate salt-induced expression of p22phox or p47phox associated with hypertension(Reference Sarr, Chataigneau and Martins20–Reference Sánchez, Lodi and Vera22). In addition, polyphenols reduce angiotensin II and pressure-overload-induced cardiac hypertrophy by inhibiting angiotensin II-induced NF-κB and activator protein-1 (AP-1) activation(Reference Li, Huang and Zhang23).
Consumption of grape juice, a non-alcoholic beverage containing high amounts of polyphenols, has been shown to decrease platelet aggregation(Reference Freedman, Parker and Li24), to improve flow-mediated vasodilation(Reference Chou, Keevil and Aeschlimann25, Reference Coimbra, Lage and Brandizzi26), to increase serum antioxidant capacity and to protect LDL against oxidation(Reference Day, Kemp and Bolton27, Reference O'Byrne, Devaraj and Grundy28). We recently reported that polyphenols from concentrated red grape juice (RGJ) are rapidly absorbed(Reference Dávalos, Castilla and Gómez-Cordovés29) and its daily dietary supplementation exerts hypolipidaemic, anti-inflammatory and antioxidant effects with a clear reduction of circulating oxidised LDL in different subject populations(Reference Castilla, Echarri and Dávalos30). Moreover, in haemodialysis subjects, who are at high risk of CVD due to oxidative stress(Reference Foley, Parfrey and Sarnak31, Reference Schiffrin, Lipman and Mann32), ingestion of concentrated RGJ resulted in a significant reduction of NADPH oxidase activity in circulating neutrophils(Reference Castilla, Dávalos and Teruel33). Considering that overproduction of phagocytic NADPH oxidase ∙O2− may promote LDL oxidation(Reference Rosenblat, Belinky and Vaya34, Reference Carnevale, Pignatelli and Lenti35), in the present study we have addressed the question of whether polyphenols from RGJ affect gene expression of NADPH oxidase subunits in different cell systems.
Experimental methods
Reagents
All-trans-retinoic acid, phorbol 12-myristate 13-acetate (PMA) and Krebs–Ringer phosphate buffer were purchased from Sigma (St Louis, MO, USA).
2′7′-Dichlorofluorescein diacetate and LinearFlow Green Flow Cytometry Intensity Calibration Kits were purchased from Molecular Probes (Leiden, the Netherlands); mouse monoclonal anti-p47phox and anti-p67phox antibodies were from BD Transduction Laboratories (San Jose, CA, USA); rabbit polyclonal anti-p22phox and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Concentrated RGJ from the ‘bobal’ grape variety was purchased from Dream Fruits (Quero, Toledo, Spain) containing 6·4 g total polyphenols/l (gallic acid equivalents), 124 mg anthocyanidines/l and a high concentration of flavonoids, including quercetin, myricetin and traces of ( − )-epicatechin, (+)-catechin, resveratrol and other phenolic acids; a detailed phenolic composition is described elsewhere(Reference Castilla, Echarri and Dávalos30, Reference Dávalos, Fernández-Hernando and Cerrato36). Red wine from the Tempranillo variety (kindly provided by María de Maeseneire) was used to prepare dealcoholised red wine (DRW). Alcohol was removed by vacuum pressure at room temperature and the final DRW product was 4 × concentrated from its original volume. DRW contained 5·9 g total pholyphenols/l (gallic acid equivalents) and detailed phenolic composition was determined as previously described(Reference Dávalos, Fernández-Hernando and Cerrato36). DRW contained particularly high concentrations (mg/l) of: delphinidin-3-glucoside (20·5), cyanidin-3-glucoside (3·27), petunidin-3-glucoside (26·3), peonidin-3-glucoside (14·7), malvidin-3-glucoside (159), gallic acid (68·7), ethyl gallate (23·2), syringic acid (11·3), trans-caftaric acid (5·8), (+)-catechin (31·9), ( − )-epicatechin (20·7), procyanidin B1 (24·6), quercetin (25·1), quercetin-3-O-glucuronide (13·5), myricetin (23·6) and trans-resveratrol-3-O-glucoside (2·44). Quercetin, (+)-catechin, ( − )-epicatechin, myricetin and ascorbic acid were purchased from Sigma (St Louis, MO, USA). Polyphenols were used at a concentration of 5 μmol/l, which is reachable in plasma after flavonoid-rich food ingestion(Reference Manach, Williamson and Morand37).
Cell lines
The human promyelocytic leukaemia cell line HL-60 (ECACC 98070106) was obtained from the European Collection of Cell Cultures (Salisbury, Wilts, UK) and cultured in Roswell Park Memorial Institute (RPMI) 1640 media (Life Technologies) supplemented with 10 % fetal bovine serum, l-glutamine and antibiotics in a humidified atmosphere containing 5 % CO2 at 37°C. HL-60 cells were subcultured (3 × 105/ml) in fresh medium and induced to differentiate toward neutrophils with 1 μm-all-trans-retinoic acid for 72 h before experiments. The human endothelial cell line EA.hy926 (kindly provided by Dr S. Lamas, CIB-CSIC, Madrid, Spain) was cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 20 % fetal bovine serum, antibiotics and HAT supplement (hypoxanthine, aminopterin and thymidine, Gibco; Invitrogen) at 37°C in a humidified atmosphere of 5 % CO2.
Leucocyte separation
PMN and mononuclear circulating cells were obtained from blood samples from healthy volunteers in the morning, after overnight fasting. Blood was collected in sodium heparin-containing tubes and immediately subjected to a gradient separation. Briefly, 15 ml Histopaque-1119 was placed in the bottom of a 50 ml conical sterile tube and 15 ml Histopaque-1077 was layered carefully on top. Then, 15 ml of blood diluted 1:1 in Ca2+-and Mg2+-free PBS was carefully layered over the Histopaque-1077. Centrifugation was performed at 700 g for 30 min. The mononuclear and polymorphonuclear cells layers separately were carefully aspirated with a sterile Pasteur pipette and transferred to different sterile tubes. The cell solutions were diluted with isotonic PBS supplemented with 5 mm-glucose and centrifuged at 250 g for 10 min at 4°C. The cell pellet was washed gently with PBS glucose and centrifuged again. PMN and mononuclear cells were re-suspended either in RPMI 1640 complete medium for culturing or Krebs–Ringer phosphate buffer for NADPH oxidase activity.
Assay of superoxide anion generation
Polymorphonuclear neutrophils
Isolated neutrophils were re-suspended in Krebs–Ringer phosphate buffer containing glucose (1800 mg/l) and supplemented with Ca2+ and Mg2+ (Krebs–Ringer glucose). To evaluate the effect of RGJ or pure polyphenols, PMN were incubated (1 × 106/ml) in the presence or absence of different concentrations of RGJ or pure polyphenols for 30 min at 37°C in an atmosphere of 5 % CO2. Then, cells were extensively washed with cold PBS glucose. Next, neutrophils were re-suspended in Krebs–Ringer glucose and superoxide generation was assayed using the method described by Bass et al. (Reference Bass, Parce and Dechatelet38), with minor modifications. For this, cells were pre-incubated (1 × 106 cells/ml) with 5 μm-2′7′-dichlorofluorescein diacetate for 15 min at 37°C. Then, cells were incubated in the absence (resting) or the presence (activated) of PMA (50 ng/ml) and incubated for 45 min at 37°C in an atmosphere of 5 % CO2. Immediately after incubation, cells were analysed by flow cytometry (FACScalibur; Becton Dickinson, San Jose, CA, USA). Superoxide production was also measured in the presence of diphenylene iodonium (DPI; 5 μmol/l), a flavoprotein inhibitor, and apocynin (250 μmol/l), a specific intracellular inhibitor of NADPH oxidase assembly(Reference Castilla, Dávalos and Teruel33). With each volunteer's samples a set of polystyrene-bead standards were run in order to standardise the fluorescence activated cell sorting fluorescence response measurements. This was carried out using the LinearFlow Green Flow Cytometry Intensity Calibration Kit (Molecular Probes, Invitrogen). NADPH oxidase activity was performed in duplicate (two each, resting and activated) and in at least four different volunteers. Data were analysed using Cell Quest software (Becton Dickinson) and the median intensity of fluorescence was used to evaluate the fluorescence of each sample.
Mononuclear cells
As infiltrated monocytes/macrophages contribute to ∙O2− production in the vessel wall(Reference Sorescu, Weiss and Lassegue9), NADPH oxidase activity in mononuclear cells was also assayed. Measurement of ∙O2− production was assessed using the lucigenin luminescence assay as described(Reference Zalba, Beloqui and San José39). Briefly, after 8 or 20 h treatment, mononuclear cells were extensively washed with PBS. Then 1 × 106 mononuclear cells were incubated at 37°C for 30 min in the presence or absence of PMA (50 ng/ml). After addition of lucigenin (5 μmol/l), luminescence was measured every 11 s for 5 min in a SIRIUS Luminometer tube (Berthold Technologies GmbH and Co. KG, Bad Wildbad, Germany). The PMA-stimulated ∙O2− production was also measured in the presence of DPI (5 μmol/l), apocynin (250 μmol/l) and superoxide dismutase (SOD; 1000 U/ml). Data are expressed as relative light units produced per s and at least three independent experiments in duplicate were performed for each determination.
EA.hy926 endothelial cells
Since certain differences exist in the NADPH oxidase system in different tissues, and endothelial cells along with fibroblast and smooth muscle cells contribute to NADPH oxidase ∙O2− production in the pathogenesis of atherosclerosis and coronary artery disease(Reference Sorescu, Weiss and Lassegue9, Reference Guzik, Sadowski and Guzik11, Reference Guzik, Sadowski and Kapelak40, Reference Silver, Beske and Christou41), superoxide generation in endothelial cells was assessed. After treatment with RGJ, quercetin or vehicle for 20 h, cells were washed four times with cold PBS and harvested. Cell lysates were prepared and NADPH oxidase activity was measured by lucigenin chemiluminescence assay in a 50 mm-phosphate buffer (pH 7·4), containing lucigenin (5 μmol/l) and 100 μm-NADPH as substrate, as described(Reference Jaimes, DeMaster and Tian42). Superoxide measurement was initiated by adding 20 μl cell lysate (100 μg protein) and chemiluminescence was measured every 2 min for 60 min in a 1450 MicroBeta Trilux luminescence counter (EG&G Wallac, Turku, Finland). The ∙O2− production was also measured in the presence of DPI (5 μmol/l), apocynin (250 μmol/l), superoxide dismutase (1000 U/ml), allopurinol (100 μmol/l), l-nitro-arginine methyl ester (100 μmol/l), indomethacine (10 μmol/l) and rotenone (10 μmol/l). Data are expressed as relative light units produced/s per mg cell protein to evaluate ∙O2− production. At least three independent experiments in quadruplicate were performed for each determination.
Quantitative real-time reverse transcriptase polymerase chain reaction assay
Total RNA was extracted with Trizol reagent. Total RNA (2 μg) was reverse-transcribed using the Maloney murine leukaemia virus reverse transcriptase (M-MLV RT) enzyme (Promega, Madison, WI, USA). Real-time PCR amplification was performed on a Lightcycler 1.5 (Roche Diagnostics GmbH, Mannheim, Germany) using FastStart DNA Master SYBR Green I (Roche Diagnostics GmbH). The primers used were the following: ribosomal protein, large, P0 (PRLP0), forward (sequence 5′-3′) CCT CAT ATC CGG GGG AAT GTG, reverse GCA GCA GCT GGC ACC TTA TTG; p22phox, forward 5′TTG GTG CCT ACT CCA TTG TG, reverse CGG CCC GAA CAT AGT AAT TC; p47phox, forward CTG AGC CCA ACT ATG CAG GT, reverse TGA CGT CGT CTT TCC TGA TG; p67phox, forward AAG CTG TTT GCC TGT GAG GT, reverse CTG CTT CCA GAC ACA CTC CA; NOX2/gp91phox, forward AGG ATT GCC TGA AGG GTT CT, reverse AGG GCT AGC TGG AGA AGA CC. Amplification cycles used were 95°C for 10 s, 60°C for 20 s, and 72°C for 20 s. Melting curves were evaluated for each gene and PCR reaction products were separated on a 2 % agarose gel and stained with ethidium bromide to assure a single product. Relative mRNA levels were determined using GADPH or PRLP0 as reference. The relative expression ratio for each target gene was calculated considering the reaction efficiency and crossing point of the unknown sample v. the control (LC relative quantification software, 2001; Roche Diagnostics GmbH). At least three independent experiments in duplicate were performed for each determination.
Western blotting
For Western blot analysis 10 × 106 HL-60-derived neutrophils were subjected to treatment. Cells were harvested and washed with cold PBS and then treated with lysis buffer. Equal amounts of extracts (50 μg protein) were separated by SDS-PAGE (10 %) and transferred to polyvinylidene difluoride membranes (Hybond-P; Amersham Biosciences, Piscataway NJ, USA). Membranes were blocked with 5 % non-fat milk powder in 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-buffered saline (TBS) containing 0·1 % Tween 20, and incubated overnight at 4°C with different antibodies. Then, the membranes were washed with TBS containing 0·1 % Tween 20 and incubated for 1 h with a horseradish peroxidase conjugate secondary antibody, and detected with a chemiluminescence assay system (Amersham Biosciences). At least four independent experiments were performed for each determination and Quantity One 4.5.2 1D Imaging Software (Bio-Rad Laboratories, Hercules, CA, USA) was used to evaluate the images.
Statistical analysis
All values are presented as mean values with their standard errors. NADPH oxidase activity data were compared using one-way repeated-measures ANOVA and pairwise comparisons by the Tukey test. Effects on gene or protein expression were analysed by ANOVA and comparisons between each experimental condition and the control were made by the confidence interval method. A P value of less than 0·05 was considered statistically significant. Calculations were performed using the SigmaStat Statistical Analysis, System v.1.00 (Jandel Corporation, San Rafael, CA, USA) and Statgraphics Plus v5.0 (Statistical Graphics, Herndon, VA, USA).
Results
Red grape juice polyphenols reduce NADPH oxidase activity in human peripheral blood neutrophils
In agreement with previous studies(Reference Dodd-O and Pearse43), PMA-induced superoxide and other reactive oxygen species production was inhibited by DPI (>85 %) and apocynin, thus indicating that the enzymic source of superoxide and other reactive oxygen species in neutrophils is the NADPH oxidase system. To study the effects of RGJ polyphenols, cells were treated for either 30 min or 20 h and then washed out before the activity assay. Treatment of neutrophils with different concentrations of RGJ produced a dose-dependent reduction of NADPH oxidase activity (Fig. 1(a)), as observed at both the 30 min and the 20 h treatment, indicating that the effect produced by RGJ was sustained. Next, to confirm that the effect on NADPH oxidase activity was mediated by polyphenols, experiments were performed using the pure flavonoids quercetin, ( − )-epicatechin, (+)-catechin and myricetin, all of which are present in RGJ. As analysed by simple regression, all the tested flavonoids decreased NADPH oxidase activity in a dose-dependent manner, with quercetin being the most active (Fig. 1(b)). These results suggest that polyphenols may be responsible for the reduction of NADPH oxidase activity produced by RGJ.
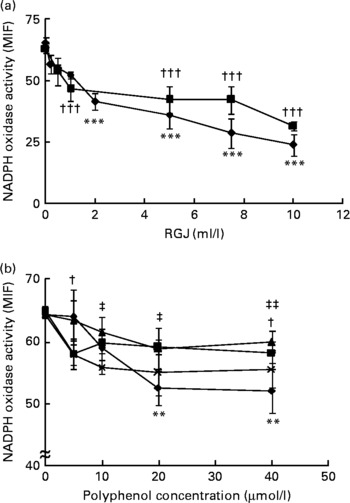
Fig. 1 Red grape juice (RGJ) and pure polyphenols reduce neutrophil NADPH oxidase activity. (a) Human peripheral blood neutrophils were treated for 30 min (–♦–) or 20 h (–■–) with different concentrations of RGJ before superoxide anion determination. The NADPH oxidase activity was evaluated by flow cytometry and the median intensity of fluorescence (MIF) was calculated. Values are means (n ≥ 4), with standard errors represented by vertical bars. *** Mean value for the 30 min treatment was significantly different from that at 0 ml/l (P < 0·001). ††† Mean value for the 20 h treatment was significantly different from that at concentration 0 ml/l (P < 0·001). (b) Neutrophils were incubated with different pure polyphenols (quercetin (–♦–), ( − )-epicatechin (–■–), (+)-catechin (–▲–), myricetin (– × –)) before NADPH oxidase activity evaluation. Values are means (n ≥ 4), with standard errors represented by vertical bars. ** Mean value for quercetin was significantly different from that at concentration 0 μmol/l (P < 0·01). † Mean value for ( − )-epicatechin was significantly different from that at concentration 0 μmol/l (P < 0·05). Mean value for myricetin was significantly different from that at concentration 0 μmol/l: ‡ P < 0·05, ‡‡ P < 0·01.
To further confirm these effects of RGJ polyphenols on NADPH oxidase activity, we used all-trans-retinoic acid-differentiated HL-60 cells. These neutrophil-like cells exhibited the NADPH oxidase components and superoxide-producing activity (Fig. 2(a)). As shown in Fig. 2(a), PMA-stimulated ∙O2− production in HL-60-derived neutrophils was clearly inhibited by both DPI (10 μmol/l) and apocynin (250 μmol/l), indicating that it involved the NADPH oxidase system. Similar to circulating neutrophils, both RGJ (Fig. 2(b)) and pure polyphenols (Fig. 2(c)) reduced NADPH oxidase activity in HL-60-derived neutrophils, with quercetin having the highest activity. These results confirm the previous finding indicating that polyphenols are potent inhibitors of phagocyte NADPH oxidase activity.
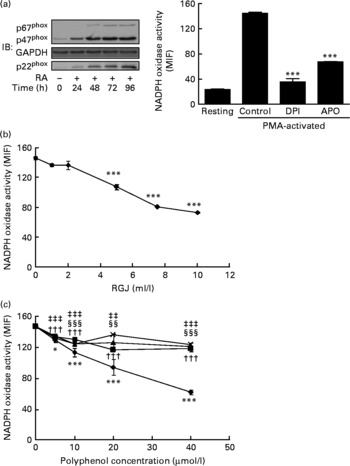
Fig. 2 Red grape juice (RGJ) and pure polyphenols reduce NADPH oxidase activity in HL-60-derived neutrophils. The HL-60 cell line was differentiated to neutrophils with all-trans-retinoic acid (RA; 1 μmol/l) for 72 h before treatment (20 h). (a) Representative immunoblots of NADPH oxidase neutrophil-like protein expression and response to phorbol 12-myristate 13-acetate (PMA) activation and NADPH oxidase inhibitors. IB, immunoblotting; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MIF, median intensity of fluorescence; DPI, diphenylene iodonium; APO, apocynin. Values are means (n ≥ 4), with standard errors represented by vertical bars. *** Mean value was significantly different from that of the control (P < 0·001). (b) Effect of different concentrations of RGJ on NADPH oxidase activity, evaluated in PMA-activated cells. Values are means (n ≥ 4), with standard errors represented by vertical bars. *** Mean value was significantly different from that at concentration 0 ml/l (P < 0·001). (c) Effect of pure polyphenols (quercetin (–♦–), ( − )-epicatechin (–■–), (+)-catechin (–▲–), myricetin (– × –)) on NADPH oxidase activity, evaluated in PMA-activated cells. Values are means (n ≥ 4), with standard errors represented by vertical bars. Mean value for quercetin was significantly different from that at concentration 0 μmol/l: * P < 0·05, *** P < 0·001. ††† Mean value for ( − )-epicatechin was significantly different from that at concentration 0 μmol/l (P < 0·001). Mean value for myricetin was significantly different from that at concentration 0 μmol/l: ‡‡ P < 0·01, ‡‡‡ P < 0·001. Mean value for (+)-catechin was significantly different from that at concentration 0 μmol/l: §§ P < 0·01, §§§ P < 0·001.
Red grape juice polyphenols reduce gene transcription of NADPH oxidase subunits in neutrophils
In order to explore whether RGJ affects NADPH oxidase at the transcription level, we examined the expression of NADPH oxidase subunits. Since circulating neutrophils are not reliable for this type of assay, HL-60-derived neutrophils were used. RGJ reduced mRNA levels of p22phox, p47phox and NOX2/gp91phox in a dose-dependent manner, while leaving p67phox unaffected (Fig. 3(a)). To confirm whether the reduced mRNA levels resulted in a decrease in protein expression, p22phox, p47phox and p67phox levels were assessed by immunoblotting. RGJ reduced protein levels of p22phox and p47phox, while p67phox remained unchanged (Fig. 3(b)), which is in accordance with the observed mRNA expression. To provide evidence that polyphenols present in RGJ are responsible for these effects, we next evaluated the effect of pure polyphenols on NADPH oxidase subunit expression. Among the flavonoids tested, quercetin and epicatechin exhibited the strongest effect reducing p47phox mRNA expression. The other flavonoids were less active (Table 1). These findings indicate that the effect of RGJ on neutrophil NADPH oxidase activity is due, at least in part, to the reduction of NADPH oxidase subunit expression exerted by polyphenols.
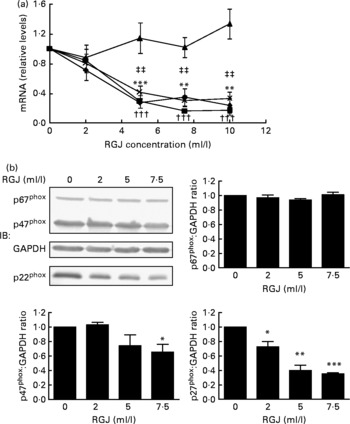
Fig. 3 Effects of red grape juice (RGJ) on NADPH oxidase subunit expression in HL-60-derived neutrophils. (a) Cells were incubated with different concentrations of RGJ for 20 h and NADPH oxidase subunit mRNA expression (p22phox (–♦–), p47phox (–■–), p67phox (–▲–), NOX2 (– × –)) was evaluated by real-time RT-PCR. Values are means for four or more independent experiments, with standard errors represented by vertical bars. Mean value for p22phox was significantly different from that at concentration 0 ml/l: * P < 0·01, *** P < 0·001. ††† Mean value for p47phox was significantly different from that at concentration 0 ml/l (P < 0·001). ‡‡ Mean value for NOX2 was significantly different from that at concentration 0 ml/l (P < 0·01). (b) Protein levels were determined by Western blotting. Densitometric band values were normalised to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data are expressed taking the control as reference (equal to 1). IB, immunoblotting. Values are means for four or more independent experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Table 1 NADPH oxidase subunit gene expression profile in HL-60-derived neutrophils incubated with different pure polyphenols (5 μmol/l) for 20 h
(Mean values with their standard errors for three or more independent experiments)
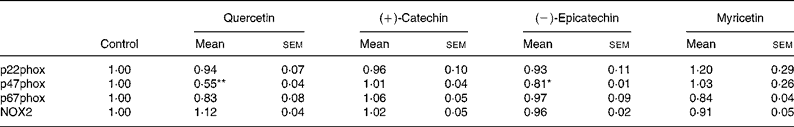
NOX2, NADPH oxidase 2.
Mean value was significantly different from that of the control: * P < 0·05, ** P < 0·01.
Dealcoholised red wine reduces NADPH oxidase activity and gene transcription in HL-60-derived neutrophils
To evaluate whether polyphenols from red wine exert similar effects as polyphenols from RGJ, a DRW was prepared. As with RGJ, incubation of HL-60-derived neutrophils with different concentrations of DRW for 20 h produced a dose-dependent reduction of NADPH oxidase activity (Fig. 4(a)). These results confirm that polyphenols from grape origin exhibit inhibitory effects on phagocyte NADPH oxidase activity. Moreover, incubation of HL-60-derived neutrophils with DRW (5 ml/l) caused a reduction of mRNA expression of p22phox, p47phox and NOX2/gp91phox, while the level of p67phox expression remained unchanged (Fig. 4(b)). This finding confirms that polyphenols from red wine also have the ability to inhibit phagocyte NADPH oxidase subunit expression.
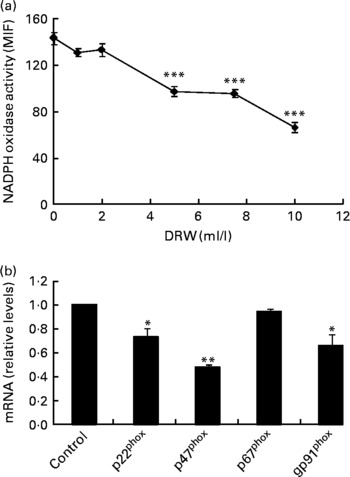
Fig. 4 Effect of dealcoholised red wine (DRW) on NADPH oxidase activity in HL-60-derived neutrophils (a) and mRNA expression (b). HL-60-derived neutrophils were treated without or with DRW for 20 h, and NADPH oxidase activity was assayed by flow cytometry (the median intensity of fluorescence (MIF) was calculated) and NADPH oxidase subunit mRNA expression was evaluated by real-time RT-PCR. Gene expression data are expressed taking the control as reference (equal to 1). Values are means for three or more independent experiments, with standard errors represented by vertical bars. Mean value was significantly different from that at concentration 0 ml/l or from the control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Red grape juice reduces NADPH oxidase activity and NADPH subunit expression in mononuclear and endothelial cells
To determine whether the observed effects of RGJ in PMN extended to other cells, mononuclear cells were isolated from peripheral blood and the NADPH oxidase-dependent ∙O2− production was assayed. Both DPI (5 μmol/l) and apocynin (250 μmol/l) greatly reduced (>90 %), whereas superoxide dismutase (1000 U/ml), an enzymic scavenger of ∙O2− , completely abolished the PMA-stimulated chemiluminescence, thus verifying its specificity for ∙O2− generation (Fig. 5(a)). As in the case of PMN, both RGJ (5 ml/l) and its representative flavonoid component quercetin (5 μmol/l) reduced NADPH oxidase activity in mononuclear cells treated for 8 or 20 h (Fig. 5(b)). In these cells, DRW (5 ml/l) also caused a reduction in ∙O2− production, thus confirming that polyphenols from red wine also exhibit this activity. In contrast, the common antioxidant ascorbic acid (100 μmol/l) showed no significant effect in reducing mononuclear NADPH oxidase activity (Fig. 5(b)). To determine whether the reduction of ∙O2− production was accompanied by changes in NADPH oxidase subunit expression, mononuclear cells were treated for 8 and 20 h in the presence of RGJ and quercetin separately, and subjected to mRNA expression analysis. As shown in Table 2, RGJ (5 ml/l) consistently reduced the expression of p22phox, gp91phox, the components of cytochrome b558, and p47phox, whereas pure quercetin (5 μmol/l) only significantly reduced the expression of p47phox, the key mediator of NADPH oxidase activation by PMA. These results suggest that in mononuclear cells, like in HL-60-derived neutrophils, RGJ flavonoids reduce NADPH oxidase activity by decreasing the transcription of NADPH oxidase subunits. In contrast, NADPH oxidase subunit expression was not affected by ascorbic acid (100 μmol/l) either at 8 or 20 h treatment (Table 2). Therefore, this effect of RGJ polyphenols may not be solely explained by their intrinsic antioxidant activity.
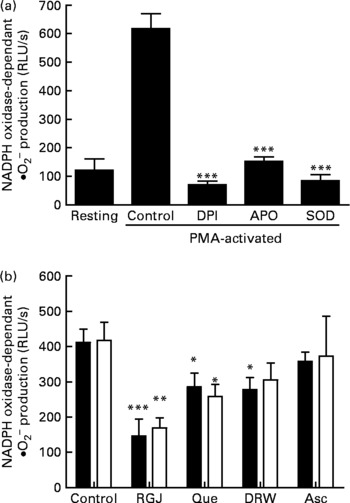
Fig. 5 Effect of red grape juice (RGJ), quercetin (Que), dealcoholised red wine (DRW) and arcorbic acid (Asc) on phorbol 12-myristate 13-acetate (PMA)-stimulated NADPH oxidase-dependent superoxide generation in mononuclear cells. (a) Mononuclear cells were preincubated for 15 min with diphenylene iodonium (DPI; 5 μmol/l), apocynin (APO; 250 μmol/l) or superoxide dismutase (SOD; 1000 U/ml) and stimulated for an additional 30 min with PMA (50 ng/ml) before lucigenin was added (5 μmol/l). (b) Cells were incubated with RGJ (5 ml/l), quercetin (5 μmol/l), DRW (5 ml/l) or ascorbic acid (100 μmol/l) for 8 h (■) or 20 h (□) before PMA-stimulated NADPH oxidase-dependent superoxide evaluation. Data are expressed as relative light units (RLU)/s. Values are means for three or more independent experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Table 2 NADPH oxidase subunit gene expression profile in human circulating mononuclear cells incubated with red grape juice (RGJ), quercetin or ascorbic acid for 8 and 20 h
(Mean values with their standard errors for four or more independent experiments)
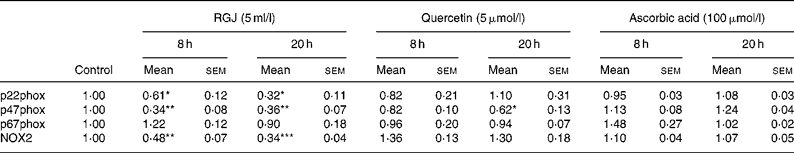
NOX2, NADPH oxidase 2.
Mean value was significantly different from that of the control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Finally, we decided to assess whether RGJ polyphenols could also affect NADPH oxidase activity in EA.hy926 cells. In this case, ∙O2− production was determined in cell lysates as a measure of NADPH oxidase activity. First we confirmed that ∙O2− production was reduced by adding DPI or apocynin to the cell lysates, whereas it was abolished by superoxide dismutase (Fig. 6(a)). These results confirm the involvement of NADPH oxidase activity. Although the xanthine oxidase enzymic system inhibitor allopurinol slightly reduced ∙O2− production, the NO synthase inhibitor l-nitro-arginine methyl ester, the cyclo-oxygenase inhibitor indomethacin and the mitochondrial respiratory complex inhibitor rotenone had little or no effect on ∙O2− production in EA.hy926 cell lysates (Fig. 6(a)). However, incubation of EA.hy926 cells with RGJ significantly reduced NADPH oxidase activity as measured in cell lysates (Fig. 6(b)). Similar effects were observed with pure quercetin and DRW as an alternative source of grape polyphenols. To further explore whether grape polyphenols affect NADPH oxidase subunit expression in endothelial cells, like in leucocytes, mRNA levels were evaluated. RGJ reduced the mRNA expression of p47phox (Fig. 6(c)), while p22phox, p67phox and NOX2/gp91phox were unchanged (data not shown). Similar effects on p47phox expression were observed with quercetin (5 μmol/l) and DRW (5 ml/l), indicating that p47phox expression is also sensitive to grape polyphenols in endothelial cells.
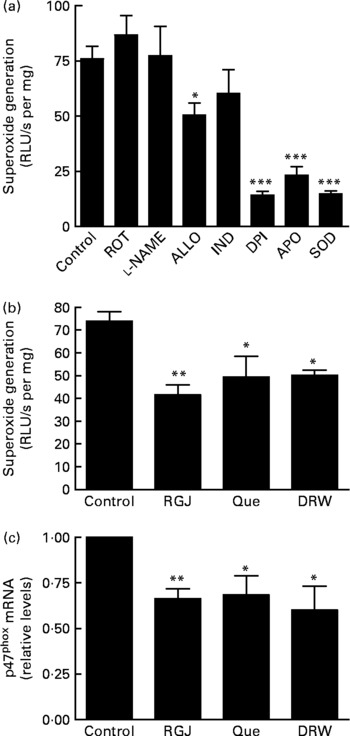
Fig. 6 Effect of red grape juice (RGJ), quercetin (Que) and dealcoholised red wine (DRW) on NADPH oxidase-dependent superoxide generation and mRNA expression in endothelial cells. (a) Effect of different inhibitors on endothelial EA.hy926 cell lysates. In the presence of NADPH (100 μmol/l), cell lysate (100 μg) was incubated with the vehicle (control), rotenone (ROT; 10 μmol/l), l-nitro-arginine methyl ester (l-NAME; 100 μmol/l), allopurinol (ALLO; 100 μmol/l), indomethacine (IND; 10 μmol/l), diphenylene iodonium (DPI; 5 μmol/l), apocynin (APO; 250 μmol/l) or superoxide dismutase (SOD; 1000 U/ml) before lucigenin was added (5 μmol/l). (b) EA.hy926 cells were cultured in the presence or absence of RGJ, quercetin or DRW for 20 h, and superoxide was produced in the presence of NADPH (100 μmol/l), and luminescense was recorded after lucigenin was added (5 μmol/l). Data are expressed as relative light units (RLU)/s per mg cell lysate. (c) After 20 h treatment, p47phox mRNA expression was evaluated by real-time RT-PCR. Values are means for three or more independent experiments, with standard errors represented by vertical bars. Mean value was significantly different from that of the control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Discussion
We recently reported that dietary supplementation with concentrated RGJ decreases NADPH oxidase-dependent ∙O2− production in circulating neutrophils(Reference Castilla, Dávalos and Teruel33). In the present study we investigated the effect of this polyphenol-rich RGJ concentrate on the activity of phagocytic NADPH oxidase-dependent superoxide production in different human cells in vitro and the mechanism accounting for this effect. We show that both RGJ concentrate and different pure polyphenols contained in RGJ dose-dependently decrease the activity of NADPH oxidase in neutrophils in vitro. This is in accordance with previous studies by others showing that different pure polyphenols such as quercetin, morin or resveratrol inhibit phagocytic NADPH oxidase activity(Reference Tauber, Fay and Marletta15, Reference Poolman, Ng and Farmer16). The reduction of NADPH oxidase activity was sustained after the polyphenols were washed out from the medium, suggesting that this effect was due either to the free radical-scavenging action of flavonoids remaining intracellular or to regulatory actions or both. Thus, we investigated whether grape polyphenols affected NADPH oxidase subunit expression in unstimulated cells. Since circulating neutrophils are not reliable for gene transcription studies, we used HL-60-derived neutrophils and peripheral blood mononuclear cells. We found that the RGJ concentrate consistently reduced mRNA levels of p22phox, NOX2/gp91phox and p47phox, in a dose-dependent manner. Similar results were found with peripheral blood mononuclear cells, showing important reductions in the mRNA levels of p22phox, gp91phox and p47phox by the effect of RGJ. These effects appeared to be selective, as the expression of p67phox remained unaffected in cells treated with grape polyphenols. Notably, these effects on gene transcription were accompanied by parallel changes in protein levels, as demonstrated for both p22phox and p47phox in HL-60-derived neutrophils. The effects of RGJ were not restricted to phagocytic cells, since grape polyphenols also reduced p47phox mRNA levels in unstimulated EA.hy926 endothelial cells. Moreover, pure flavonoids, such as quercetin, also decreased NADPH oxidase subunit expression in different cell lines when used at relevant concentrations (5 μmol/l). In NADPH-stimulated cells, other authors reported that different polyphenols have the ability to inhibit protein kinase C activity in platelets(Reference Pignatelli, Di Santo and Buchetti19, Reference Nardini, Scaccini and Packer44), which phosphorylates p47phox, a necessary step to trigger NADPH oxidase activation in phagocytes(Reference el Benna, Faust and Babior45). Thus, strong evidence is available to support that grape polyphenols selectively decrease the expression of both the NADPH oxidase activator p47phox and, particularly in phagocytic cells, the constitutive cytochrome b558 components, p22phox and gp91phox.
DRW, another polyphenol-rich, grape-derived beverage, also inhibited superoxide generation in both HL-60-derived neutrophils and endothelial cells, and reduced NADPH oxidase subunit expression, thus indicating that polyphenols are the active compounds. However, the effects of pure flavonoids were much lower than those reached with either RGJ or DRW. Considering that the content of total quercetin in RGJ concentrate was about 70 μmol/l(Reference Dávalos, Fernández-Hernando and Cerrato36), the expected concentration of quercetin in the medium of cells supplemented with RGJ concentrate at 5 ml/l was only 0·35 μmol/l. In vivo, the sum of flavanols at tissue levels after 5 d supplementation with tea, containing about 200 mg total flavanol, has been found to be about 200 pmol/g tissue(Reference Henning, Aronson and Niu46). In vitro, we have previously shown in other cell type that quercetin from RGJ was effectively taken up and reached intracellular concentration at about 25 pmol/mg total cell protein(Reference Dávalos, Fernández-Hernando and Cerrato36). This could be extended to the other flavanols and flavonols present in RGJ and DRW. Therefore, the effect of the concentrates should be attributed either to a not tested single compound or to the combined effect of the different polyphenols present in the concentrates. In contrast, the potent antioxidant ascorbic acid did not affect NADPH oxidase gene expression in phagocytic mononuclear cells, indicating that the effects of RGJ polyphenols on gene expression are not explained, at least solely, by antioxidant action. In this regard, it has been recently shown that the ability of different flavonoid metabolites to inhibit NADPH oxidase, as measured in cell extracts by using an enzyme assay, is unrelated to their superoxide-scavenging properties(Reference Steffen, Gruber and Schewe47).
Increased vascular superoxide production through the NADPH oxidase system has been implicated as an important factor in the pathogenesis of atherosclerosis and coronary artery disease(Reference Sorescu, Weiss and Lassegue9, Reference Guzik, Sadowski and Guzik11, Reference Jaimes, DeMaster and Tian42, Reference Vendrov, Hakim and Madamanchi48). Recently, an increase in vascular endothelial expression of p47phox has been shown in overweight and obese adults, which has been related to the alterations in vascular biology accompanying this pathology(Reference Silver, Beske and Christou41). We are reporting the reduction of NADPH oxidase activity and expression of p47phox through the effects of RGJ, DRW and pure quercetin in monocytes and endothelial cells in vitro. Tea polyphenols have been shown to down-regulate the expression of NADPH oxidase subunits p22phox and p67phox in endothelial cells(Reference Ying, Xu and Ikeda17). Others reported that administration of red wine polyphenols to rats decreases the expression of p22phox and NOX1 in aortic tissue(Reference Sarr, Chataigneau and Martins20) and that quercetin reduces vascular p47phox expression in animal models of induced hypertension(Reference Sánchez, Lodi and Vera22). Taken together, the effects of polyphenols on NADPH oxidase subunit expression represent an additional mechanism contributing to the reduction of superoxide anion production, and could be considered among the beneficial effects of polyphenols on vascular biology. The present study has been circumscribed to RGJ polyphenols and a limited number of pure flavonoids. Based on the rapid metabolisation of these compounds once absorbed by the intestine and the distinct actions of aglycones as compared with conjugated flavonoids on NADPH oxidase activity recently observed(Reference Steffen, Gruber and Schewe47, Reference Schewe, Steffen and Sies49), the action of the different flavonoid metabolites on gene expression deserves further investigation.
Neutrophil NADPH oxidase plays a key role in host defence and inflammation, and the deficiency of ∙O2− production leads to a marked increase in the susceptibility of infections as demonstrated in chronic granulomatous disease(Reference Holmes, Page and Good50). On the other hand, overproduction of phagocytic NADPH oxidase superoxide may promote LDL oxidation(Reference Rosenblat, Belinky and Vaya34, Reference Carnevale, Pignatelli and Lenti35), which may be indirectly implicated in atherosclerosis. In fact, increased NADPH oxidase activity has been detected in circulating neutrophils from patients subjected to haemodialysis(Reference Castilla, Dávalos and Teruel33), as well as in mononuclear cells from patients with chronic kidney disease(Reference Fortuño, Beloqui and San José51) and hypertensive patients(Reference Fortuño, Oliván and Beloqui52). Both circulating neutrophils from haemodialysis patients(Reference Morena, Delbosc and Dupuy53) and synovial neutrophils from rheumatoid arthritis patients(Reference Dang, Stensballe and Boussetta54) have been shown to be primed and thus highly susceptible to activation. Perhaps the most important clinical relevance of our finding that RGJ polyphenols reduce the expression of NADPH oxidase components in phagocytic and non-phagocytic cells is that consumption of RGJ polyphenols may restrain the exaggerated neutrophil responses usually found in inflammatory environments, such as in patients subjected to haemodialysis or affected by arthritis(Reference Morena, Delbosc and Dupuy53, Reference Dang, Stensballe and Boussetta54), whilst preserving the physiological ability to release large amounts of superoxide and other reactive oxygen species required for host defence. Consistent with this, we have recently shown that oral supplementation with concentrated RGJ is well tolerated and leads to the reduction of neutrophil NADPH oxidase ∙O2− production in patients with end-stage renal disease(Reference Castilla, Dávalos and Teruel33). Interestingly, this decrease is correlated with the reduction of oxidised LDL plasma concentration(Reference Castilla, Dávalos and Teruel33), which is considered a risk factor for CVD(Reference Holvoet, Lee and Steffes55). Thus, our present findings provide an explanation that accounts for the beneficial effects of dietary polyphenols, decreasing phagocyte NADPH oxidase subunit expression and superoxide overproduction.
Acknowledgements
We thank Lorena Crespo for excellent technical assistance, Clotilde Blanco and María S. Paraíso for blood extraction, Dr Víctor Abraira for his help in statistical analyses, María de Maeseneire for providing the red wine for dealcoholisation, Dra. Ma Carmen Vidal-Aragón for preparing DRW. The study was supported by grants from the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (VIN03-027), Fondos FEDER, Ministerio de Educación y Ciencia (AGL2004-07075 and SAF2005-07308) and Ministerio de Ciencia e Innovación (SAF2008-01104), Spain. A. D. is a recipient of a postdoctoral fellowship from the Instituto de Salud Carlos III, Spain. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of the Instituto de Salud Carlos III, Spain. The authors declare no conflicts of interest.
A. D. performed the experiments and the NADPH oxidase activity and gene expression determinations. G. de la P. isolated leucocytes from blood and maintained the cell lines in culture. C. C. S.-M. participated in the analysis of NADPH oxidase subunit expression. M. T. G. prepared the DRW. B. B. analysed the polyphenol composition of the concentrates. M. A. L. designed and supervised the study. A. D. and M. A. L. wrote the manuscript, which was reviewed by all the authors.










