Clozapine, an atypical antipsychotic agent from the dibenzodiazepine group, is indicated for treatment-resistant and treatment-intolerant schizophrenia. In view of a risk of agranulocytosis all patients receiving the drug must undergo regular white cell count monitoring. In the UK and Ireland, until 2004 all patients treated with clozapine had to be registered with and monitored by the Clozaril Patient Monitoring Service (CPMS). Since 2004 generic clozapine has been available and patients receiving generic drugs are monitored by other systems. Clozapine must be immediately discontinued if a patient develops leucopenia (white cell count <3.06109/l with a satisfactory neutrophil count) or neutropenia (neutrophil count <1.56109/l) and further treatment with clozapine is subsequently contraindicated. However, rechallenge is sometimes considered, as no other antipsychotic has been found to be efficacious in treatment-resistant schizophrenia.
METHOD
The rechallenge process in the UK and Ireland
Rechallenge with clozapine is an off-label process undertaken with the assistance of the CPMS but at the discretion of the patient's psychiatrist and at the latter's responsibility. It is generally only in cases where the neutropenia or leucopenia is thought unlikely to have been related to clozapine, or in cases of ‘mild’ neutropenia or leucopenia, that clozapine rechallenge is considered. In general, patients who have experienced agranulocytosis (neutrophil count <0.56109/l), except in association with chemotherapy, are not thought suitable for rechallenge.
Study method
Patients in the UK and Ireland who, between 1998 (when a formal rechallenge process was instituted) and 2003, were rechallenged with clozapine following leucopenia or neutropenia during previous clozapine therapy were identified from the CPMS database. Patients were required to have had a break of at least 1 week between their two courses of treatment in order to be classified as rechallenge patients. Information on demographic factors (age, gender and ethnic origin), clozapine treatment and haematolgical history and outcome of these patients was gathered from the CPMS database. Clinical information, particularly information concerning any other possible causes for the blood dyscrasias, was obtained from clinician correspondence and notes made at the time by the CPMS following discussions with healthcare teams.
Statistical analysis
In the analysis of our results we considered the following three main groups of patients:
-
(a) all 53 patients who were rechallenged;
-
(b) patients who experienced a further blood dyscrasia on rechallenge;
-
(c) patients who did not develop a blood dyscrasia on rechallenge.
We further considered the group that experienced agranulocytosis on rechallenge.
Statistical analysis was carried out using SAS version 8.2 for Windows (SAS Institute Inc., Cary, North Carolina, USA). Comparisons were made between patients who experienced a second dyscrasia v. those who did not, using the Wilcoxon two-sample test or chi-squared test (categorical variables). Within-subject comparisons for characteristics of first and second dyscrasias were made using the Wilcoxon signed ranks test. Time to occurrence of dyscrasia was analysed using the Kaplan–Meier product-limit method with between-group differences tested using the log-rank test. All reported P values are two-sided.
RESULTS
Rechallenge patients: demographics and data on original dyscrasia
We identified 53 patients (16 women, 37 men) who had been rechallenged with clozapine following a confirmed blood dyscrasia. All of them had a diagnosis of treatment-resistant schizophrenia. The median age of the cohort was 34 years (range 20–61). The ethnic backgrounds of the patients were 41 (77%) White, 7 (13%) African–Caribbean, 3 (6%) ‘Asian’, 1 (2%) ‘Oriental’ and 1 (2%) mixed ethnicity. All of the three main groups of patients in our study were found to be alike demographically. Data on the first-exposure blood dyscrasia experienced by these patients are presented in the second column of Table 1. The duration of clozapine treatment at the time of the blood dyscrasias indicates that this group is not typical of patients who have experienced clozapine-induced blood dyscrasias, for whom the peak incidence occurs at 6–18 weeks (Reference Munro, O'Sullivan and AndrewsMunro et al, 1999). Twenty-eight (53%) of the patients had no alternative explanation for the blood dyscrasia. In 13 (25%) concurrent infection was reported, 9 (17%) were taking concurrent medication recognised to cause neutropenia, and 3 (6%) had both these risk factors (Table 2). In two patients the blood dyscrasias had been treated with granulocyte colony-stimulating factor (G-CSF).
Table 1 Comparison of blood dyscrasias on first exposure to clozapine
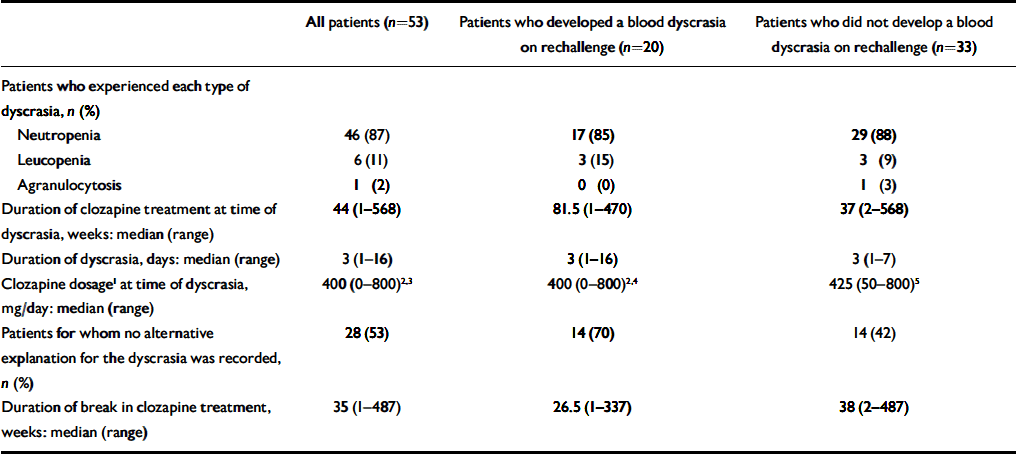
| All patients (n=53) | Patients who developed a blood dyscrasia on rechallenge (n=20) | Patients who did not develop a blood dyscrasia on rechallenge (n=33) | |
|---|---|---|---|
| Patients who experienced each type of dyscrasia, n (%) | |||
| Neutropenia | 46 (87) | 17 (85) | 29 (88) |
| Leucopenia | 6 (11) | 3 (15) | 3 (9) |
| Agranulocytosis | 1 (2) | 0 (0) | 1 (3) |
| Duration of clozapine treatment at time of dyscrasia, weeks: median (range) | 44 (1-568) | 81.5 (1-470) | 37 (2-568) |
| Duration of dyscrasia, days: median (range) | 3 (1-16) | 3 (1-16) | 3 (1-7) |
| Clozapine dosage1 at time of dyscrasia, mg/day: median (range) | 400 (0-800)2,3 | 400 (0-800)2,4 | 425 (50-800)5 |
| Patients for whom no alternative explanation for the dyscrasia was recorded, n (%) | 28 (53) | 14 (70) | 14 (42) |
| Duration of break in clozapine treatment, weeks: median (range) | 35 (1-487) | 26.5 (1-337) | 38 (2-487) |
Table 2 Possible alternative explanations for the blood dyscrasia experienced during first exposure to clozapine (n=53)
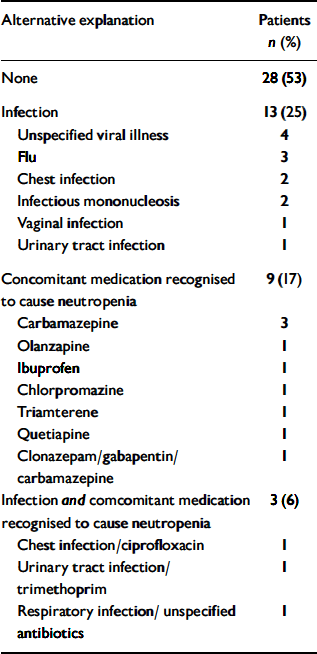
| Alternative explanation | Patients n (%) |
|---|---|
| None | 28 (53) |
| Infection | 13 (25) |
| Unspecified viral illness | 4 |
| Flu | 3 |
| Chest infection | 2 |
| Infectious mononucleosis | 2 |
| Vaginal infection | 1 |
| Urinary tract infection | 1 |
| Concomitant medication recognised to cause neutropenia | 9 (17) |
| Carbamazepine | 3 |
| Olanzapine | 1 |
| Ibuprofen | 1 |
| Chlorpromazine | 1 |
| Triamterene | 1 |
| Quetiapine | 1 |
| Clonazepam/gabapentin/carbamazepine | 1 |
| Infection and comcomitant medication recognised to cause neutropenia | 3 (6) |
| Chest infection/ciprofloxacin | 1 |
| Urinary tract infection/trimethoprim | 1 |
| Respiratory infection/ unspecified antibiotics | 1 |
Patients experiencing a further blood dyscrasia
Of the 53 patients, 20 (5 women, 15 men) (38%) experienced a further blood dyscrasia on rechallenge. The median age of these patients was 36 years (range 25–60) and the ethnicity of the group was 17 (85%) White, 2 (10%) African—Caribbean and 1 (5%) ‘Asian’. Table 1 summarises the data available for the original blood dyscrasia for the three main groups: all 53 patients, the 20 patients who developed a blood dyscrasia on rechallenge and the 33 patients who did not. There was no statistically significant difference between those who developed dyscrasia on rechallenge and those who did not in duration of clozapine treatment at the time of the original blood dyscrasia (P=0.50), number of patients in whom no alternative explanation for the original blood dyscrasia was recorded (P=0.054) or duration of clozapine treatment break (P=0.93).
Nine (45%) of the second blood dyscrasias were neutropenias, 2 (10%) were leucopenias and 9 (45%) were agranulocytoses. Data on these second blood dyscrasias are presented in Table 3. The median time for the blood dyscrasias to develop from the time of restarting clozapine was 5.5 weeks and in 80% (n=16) of the 20 patients the second blood dyscrasia occurred within 10 weeks of restarting clozapine (Fig. 1). Sixteen (80%) patients had no alternative explanation for this second blood dyscrasia. Two (10%) reported a concurrent infection, 1 (5%) was receiving a concomitant medication recognised to cause neutropenia and 1 (5%) had both these risk factors (Table 4). Five patients received G-CSF as treatment for the second blood dyscrasia. In all of the 20 patients the blood dyscrasia remitted when they again stopped taking clozapine; there was no fatality. None of the 20 patients subsequently restarted clozapine.
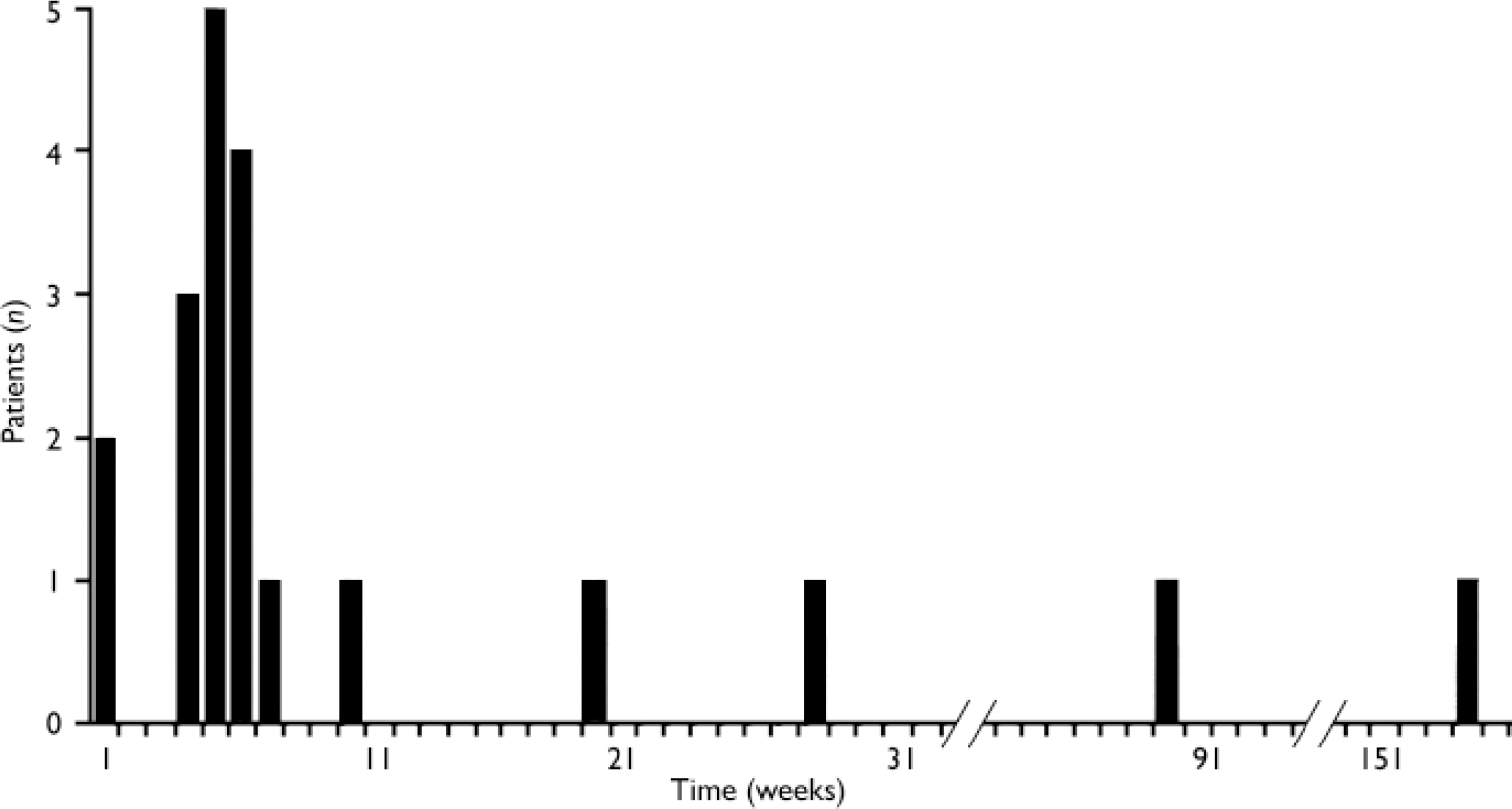
Fig. 1 Time for blood dyscrasias to develop after restarting clozapine (n=20).
Table 3 Background data on the blood dyscrasias experienced on rechallenge with clozapine (n=20)

| Variable | Median | Range |
|---|---|---|
| Time for blood dyscrasias to develop after restarting clozapine, weeks | 5.5 | 1-156 |
| Duration of blood dyscrasia, days | 7 | 1-20 |
| Clozapine dosage at time of dyscrasia, mg/day1 | 350 | 0-7002 |
Table 4 Possible alternative explanations for the blood dyscrasia experienced during rechallenge with clozapine (n=20)
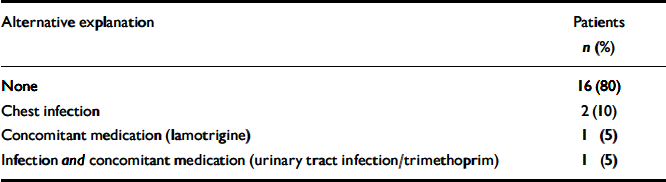
| Alternative explanation | Patients n (%) |
|---|---|
| None | 16 (80) |
| Chest infection | 2 (10) |
| Concomitant medication (lamotrigine) | 1 (5) |
| Infection and concomitant medication (urinary tract infection/trimethoprim) | 1 (5) |
Comparison of first and second blood dyscrasias
In all but 3 of the 20 patients (85%) the second blood dyscrasia occurred more quickly than the first, and overall the time to the second blood dyscrasia was much less than the time to the first (median time 5.5 weeks for the second dyscrasia compared with 81.5 weeks for the first dyscrasia in these 20 patients; P<0.001). This is strikingly illustrated by the Kaplan–Meier plot in Fig. 2. Again in all but 3 (85%) – but in different patients – the second blood dyscrasia was more severe than the first (nadir neutrophil count lower than during the first blood dyscrasia; P<0.001), and in all but 8 (60%) the second blood dyscrasia lasted longer than the first (one of the patients whose second blood dyscrasia was shorter was treated with G-CSF); P=0.0368. Figures 3, 4, 5 compare the time to onset, severity and duration of the first and second blood dyscrasias in the 20 patients who experienced a blood dyscrasia on rechallenge. The patients identified by the numbers 1–9 are those who developed agranulocytosis on rechallenge.
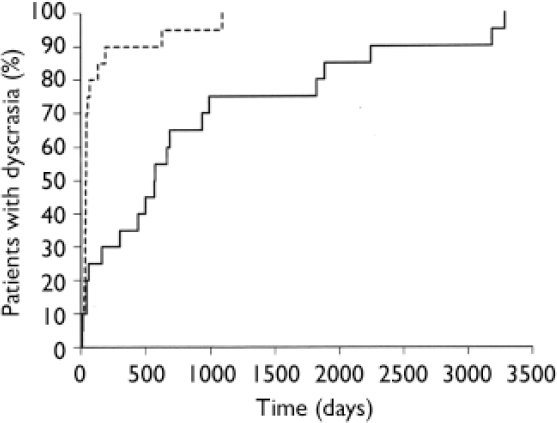
Fig. 2 Kaplan–Meier plot of time to development of first (![]() ) and second (----) blood dyscrasias (n=20).
) and second (----) blood dyscrasias (n=20).
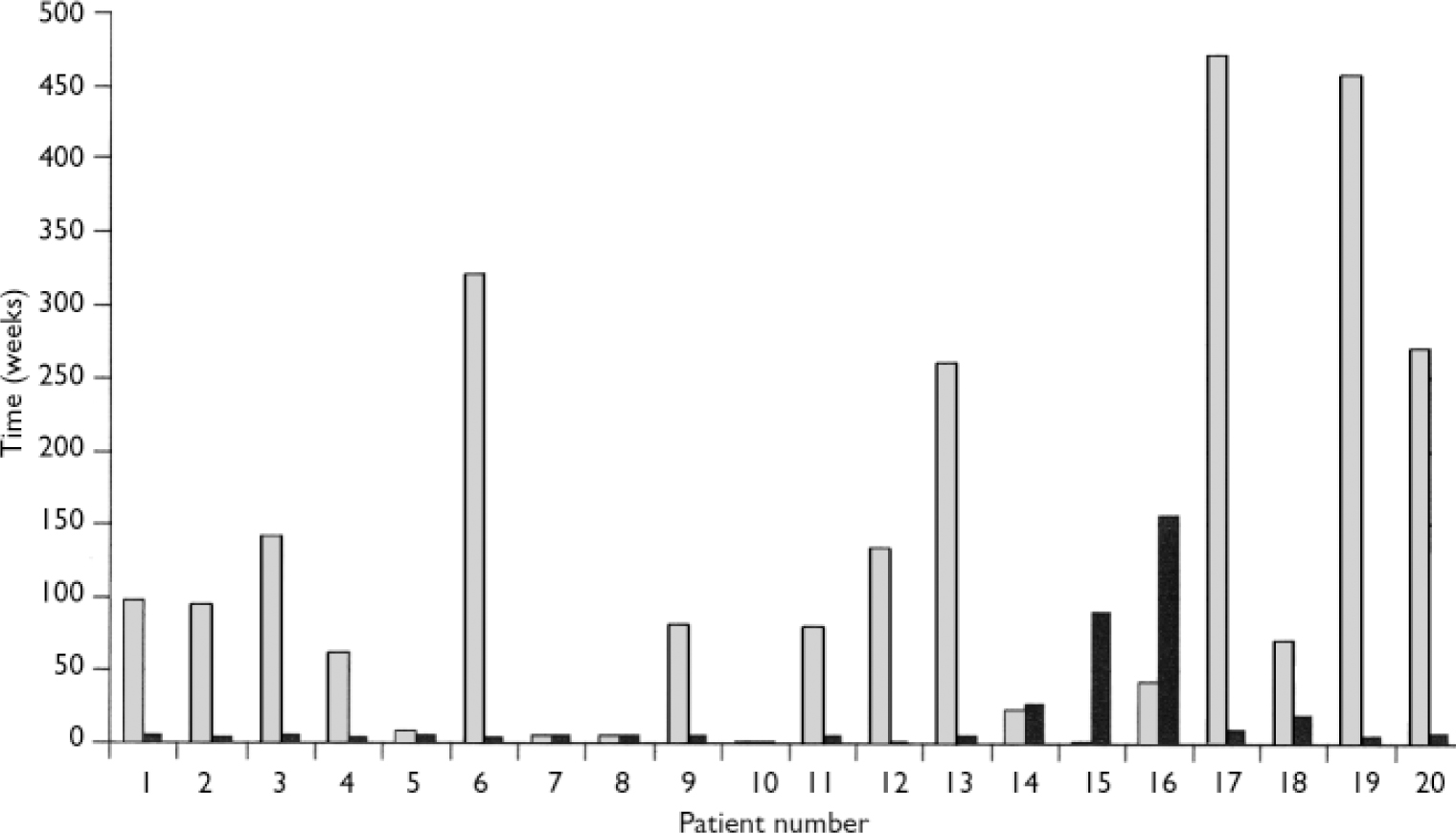
Fig. 3 Comparison of time to onset (weeks) of first (░) and second (▒) blood dyscrasias (n=20).
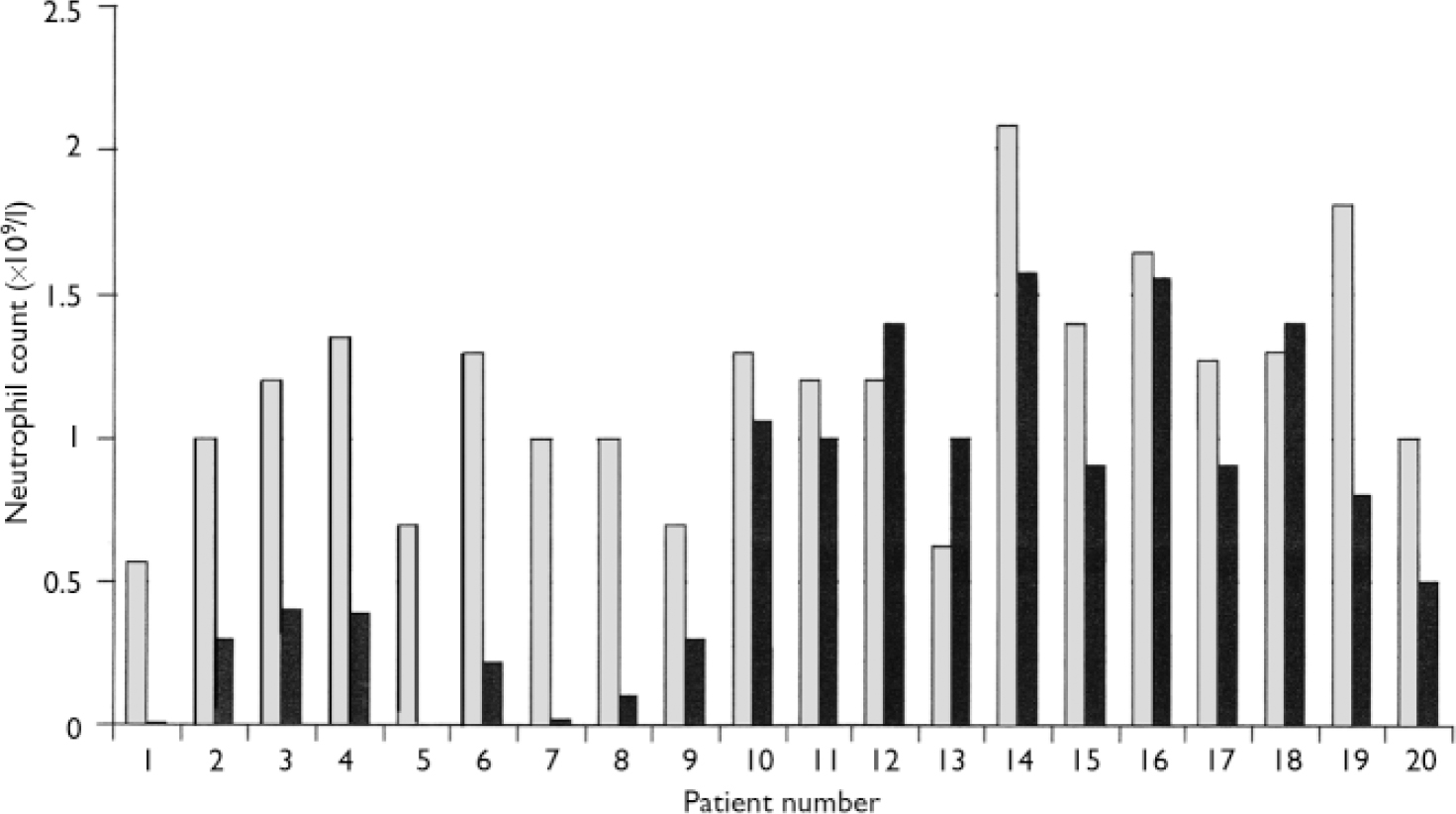
Fig. 4 Comparison of severity (nadir neutrophil count) of first (░) and second (▒) blood dyscrasias (n=20).
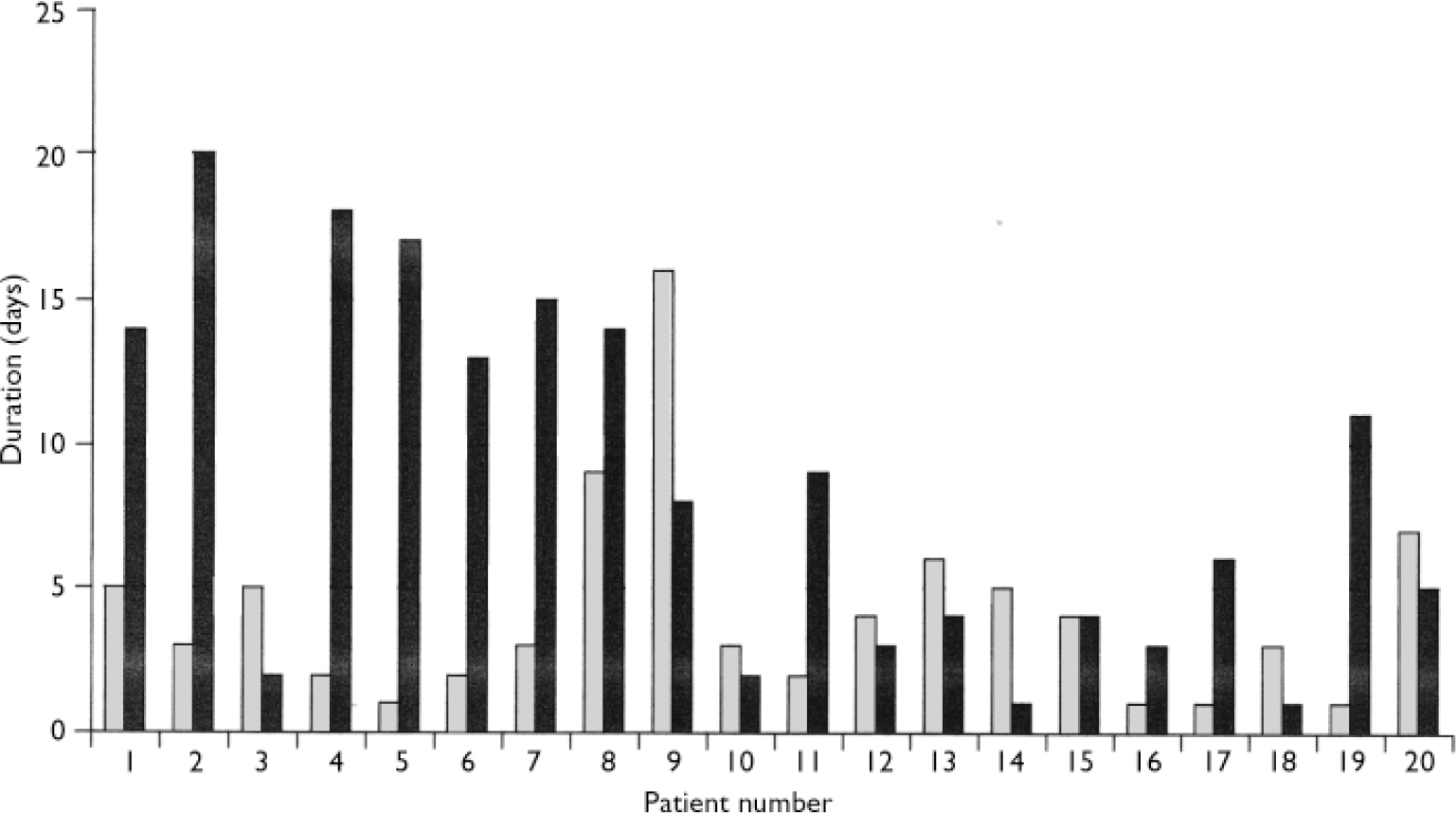
Fig. 5 Comparison of duration of first (░) and second (▒) blood dyscrasias (n=20)
Patients who developed agraunulocytosis
Table 5 lists the nine patients who developed agranulocytosis on rechallenge. It provides background data including factors that can be considered likely to predict agranulocytosis on rechallenge. Both men and women are represented and the median age is 38 years (range 27–59). All of these patients were White and all experienced a neutropenia on first exposure. The median clozapine dosage at the time of the first blood dyscrasia for these patients was 400 mg per day (range 0–800). None of the patients received G-CSF as treatment for their first blood dyscrasia. The duration of the first exposure demonstrates that six of the first blood dyscrasias in this group could be considered atypical of clozapine-induced dyscrasias as they occurred after a year of treatment, well outside the peak risk period. However, there were potential risk factors for rechallenge in some of the patients, as three of the first blood dyscrasias occurred during the peak risk period, four had a duration of more than 3 days and three had a nadir neutrophil count below 1.0 × 109/l. However, no one patient demonstrated all three of these potential risk factors and patients 2, 4 and 6 demonstrated none of them. The median duration of break in clozapine treatment for these nine patients was 14 weeks (range 5–337).
Table 5 Patients who developed agranulocytosis on rechallenge with clozapine (n=9): background data including factors that could predict agranulocytosis on rechallenge
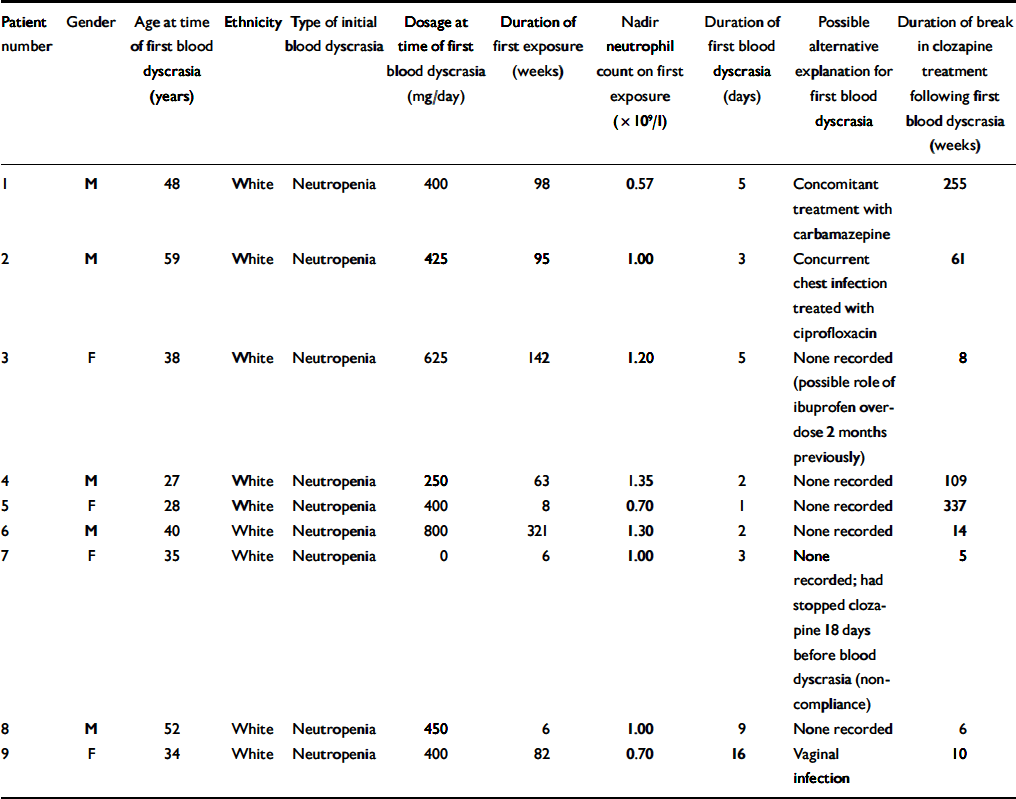
| Patient number | Gender | Age at time of first blood dyscrasia (years) | Ethnicity | Type of initial blood dyscrasia | Dosage at time of first blood dyscrasia (mg/day) | Duration of first exposure (weeks) | Nadir neutrophil count on first exposure (× 109/l) | Duration of first blood dyscrasia (days) | Possible alternative explanation for first blood dyscrasia | Duration of break in clozapine treatment following first blood dyscrasia (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | White | Neutropenia | 400 | 98 | 0.57 | 5 | Concomitant treatment with carbamazepine | 255 |
| 2 | M | 59 | White | Neutropenia | 425 | 95 | 1.00 | 3 | Concurrent chest infection treated with ciprofloxacin | 61 |
| 3 | F | 38 | White | Neutropenia | 625 | 142 | 1.20 | 5 | None recorded (possible role of ibuprofen overdose 2 months previously) | 8 |
| 4 | M | 27 | White | Neutropenia | 250 | 63 | 1.35 | 2 | None recorded | 109 |
| 5 | F | 28 | White | Neutropenia | 400 | 8 | 0.70 | 1 | None recorded | 337 |
| 6 | M | 40 | White | Neutropenia | 800 | 321 | 1.30 | 2 | None recorded | 14 |
| 7 | F | 35 | White | Neutropenia | 0 | 6 | 1.00 | 3 | None recorded; had stopped clozapine 18 days before blood dyscrasia (noncompliance) | 5 |
| 8 | M | 52 | White | Neutropenia | 450 | 6 | 1.00 | 9 | None recorded | 6 |
| 9 | F | 34 | White | Neutropenia | 400 | 82 | 0.70 | 16 | Vaginal infection | 10 |
Table 6 provides the data available for the repeat-exposure blood dyscrasia for the same nine patients. The median age is still 38 years (range 29–60). The speed of onset (all dyscrasias occurred 4–6 weeks after rechallenge), duration (in all but one case the dyscrasia lasted more than 3 days) and severity of the blood dyscrasias are notable. The median dosage at the time of the second blood dyscrasia was 300 mg per day (range 0–700); for 2 patients dosage was not recorded.
Table 6 Patients who experienced agranulocytosis on rechallenge with clozapine (n=9): available data for blood dyscrasia on rechallenge
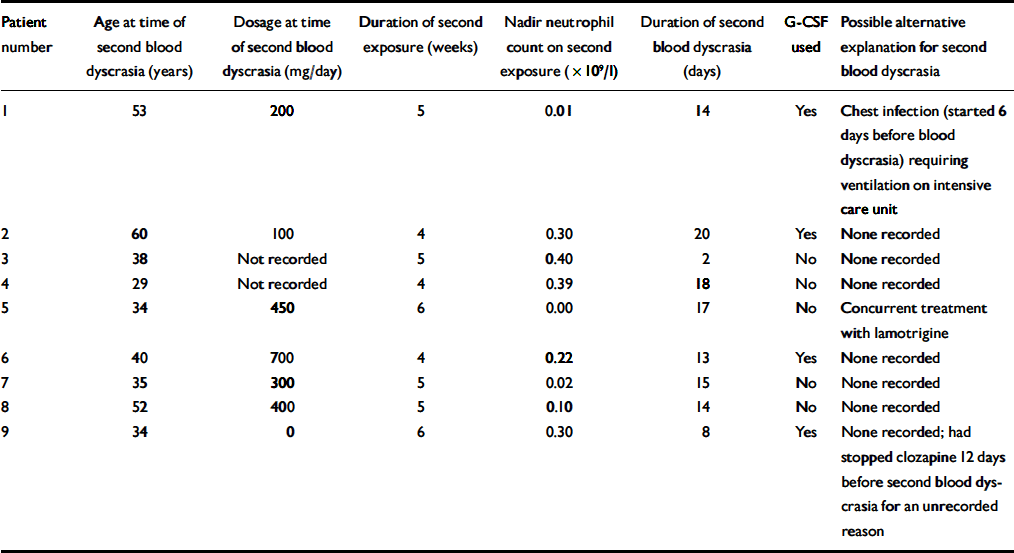
| Patient number | Age at time of second blood dyscrasia (years) | Dosage at time of second blood dyscrasia (mg/day) | Duration of second exposure (weeks) | Nadir neutrophil count on second exposure (× 109/l) | Duration of second blood dyscrasia (days) | G-CSF used | Possible alternative explanation for second blood dyscrasia |
|---|---|---|---|---|---|---|---|
| 1 | 53 | 200 | 5 | 0.01 | 14 | Yes | Chest infection (started 6 days before blood dyscrasia) requiring ventilation on intensive care unit |
| 2 | 60 | 100 | 4 | 0.30 | 20 | Yes | None recorded |
| 3 | 38 | Not recorded | 5 | 0.40 | 2 | No | None recorded |
| 4 | 29 | Not recorded | 4 | 0.39 | 18 | No | None recorded |
| 5 | 34 | 450 | 6 | 0.00 | 17 | No | Concurrent treatment with lamotrigine |
| 6 | 40 | 700 | 4 | 0.22 | 13 | Yes | None recorded |
| 7 | 35 | 300 | 5 | 0.02 | 15 | No | None recorded |
| 8 | 52 | 400 | 5 | 0.10 | 14 | No | None recorded |
| 9 | 34 | 0 | 6 | 0.30 | 8 | Yes | None recorded; had stopped clozapine 12 days before second blood dyscrasia for an unrecorded reason |
Patient rechallenged after agranulocytosis during first treatment
The patient who had an agranulocytosis on first exposure did not develop a blood dyscrasia on second exposure during the period of data collection. However, he developed neutropenia, which resolved when clozapine administration was stopped, after the cut-off point for data collection, 4 weeks after he restarted clozapine. At the time of this second blood dyscrasia the patient had a cough, a shadow on chest X-ray, and was prescribed antibiotics.
Patients who did not develop a blood dyscrasia on rechallenge
Thirty-three patients (62%) (11 women, 22 men) did not develop a second blood dyscrasia during rechallenge with clozapine. The median age of these patients was 33 years (range 20–61), and the ethnicity of this group was 24 (73%) White, 5 (15%) African–Caribbean, 2 (6%) ‘Asian’, 1 (3%) ‘Oriental’ and 1 (3%) mixed ethnicity. The median daily dosage of clozapine at the study cut-off point or at the time the patient discontinued clozapine was 400 mg (range 200–800); in 3 patients dosage was not recorded. Twenty-nine (88%) of these 33 patients were still receiving clozapine at the time of writing. The median duration of the second exposure in these 29 patients was 24 months (range 1–58); Fig. 6. Of the four patients no longer receiving clozapine, two died: the first 2.5 months after restarting clozapine, from chronic obstructive airways disease and ischaemic heart disease due to coronary artery atheroma; the second 6.5 months after restarting clozapine, from acute pulmonary oedema, bilateral pleural effusions and ascites, presumed toxic effect of pneumococcal pneumonia, glomerulosclerosis or pseudomembranous colitis. One patient discontinued clozapine owing to the occurrence of chest pain 29 months after restarting, and the fourth patient stopped clozapine 1 month after restarting in view of an overdue blood test. Thus, of the original 53 patients, more than half (55%; n=29) were still receiving clozapine in February 2005.
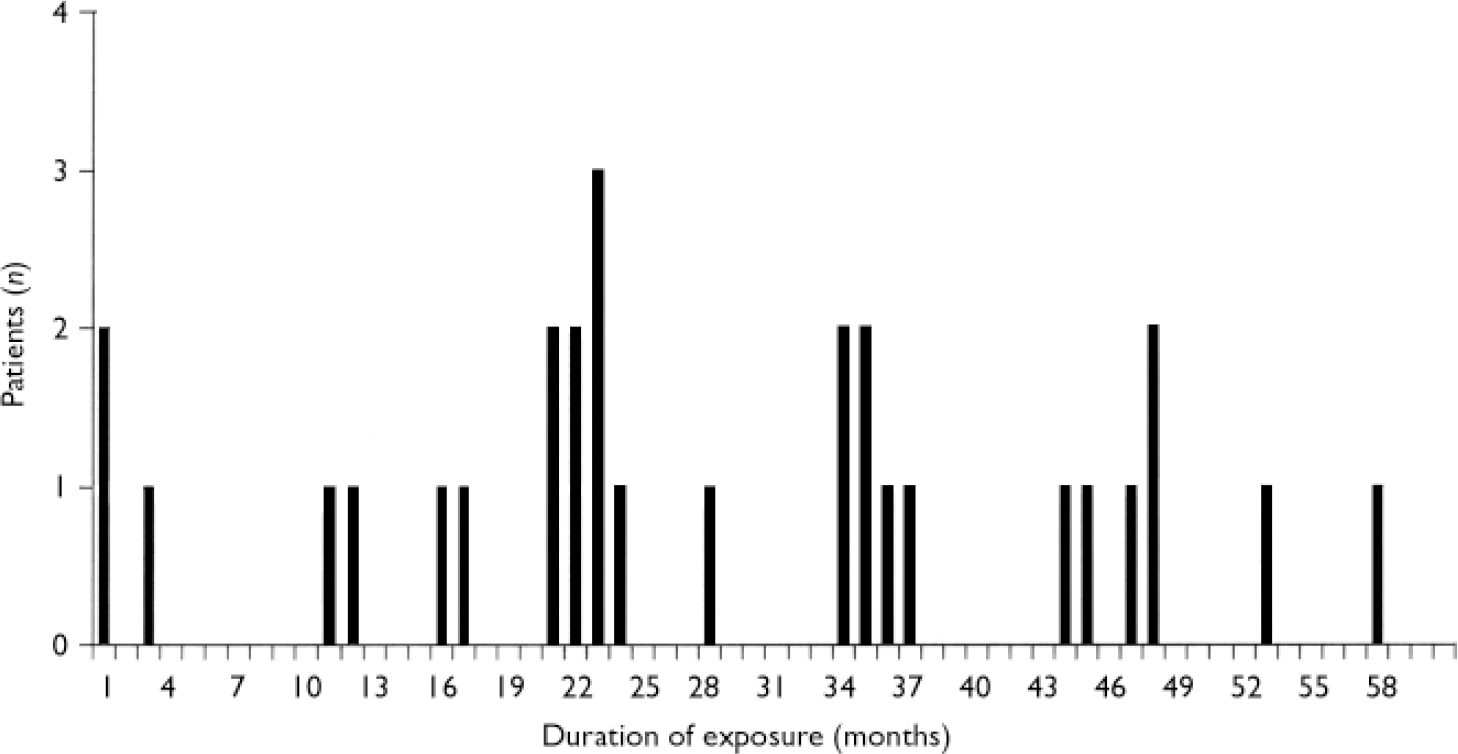
Fig 6 Duration of second exposure in patients still receiving clozapine (n=29).
DISCUSSION
Since the grant of the clozapine licence in the UK in 1989, and in Ireland in 1993, until 1 December 2003, a total of 38 106 patients in these countries received clozapine. Within this cohort there have been 688 (1.8%) cases of neutropenia (neutrophil count 0.5–1.5 × 109/l), 72 (0.19%) cases of leucopenia and 298 (0.78%) cases of agranulocytosis. There have been three deaths attributed to agranulocytosis (in one the white cell count had begun to rise before the patient died; Reference Mangan and ToalMangan & Toal, 1994). The last of these deaths was in 1998.
Clozapine-induced agranulocytosis
Agranulocytosis associated with clozapine is an idiosyncratic (type B) reaction and is not dose-related, as previous analysis of the CPMS cohort has shown (Reference Munro, O'Sullivan and AndrewsMunro et al, 1999). The toxicity mainly affects the myeloid (neutrophil) precursor cells, although the mature neutrophil may also be affected simultaneously (Reference Pirmohamed and ParkPirmohamed & Park, 1997). Seventy per cent of cases occur 6–18 weeks after the start of treatment (Reference Munro, O'Sullivan and AndrewsMunro et al, 1999). Although the risk of agranulocytosis decreases with time, some cases are reported after a number of years of continued therapy. The mechanism of clozapine-induced agranulocytosis is not known but some evidence favours an immune-mediated mechanism that may involve a toxic metabolite (Reference Pirmohamed and ParkPirmohamed & Park, 1997). The disorder is reversible in the vast majority of cases if clozapine is withdrawn promptly. Supportive care, such as reverse isolation and treatment with G-CSF or granulocyte–macrophage colony-stimulating factor (GM-CSF), antibiotics and antifungals as appropriate, may be required. The CPMS data indicate an increased risk of agranulocytosis with increasing age and Asian ethnicity (Reference Munro, O'Sullivan and AndrewsMunro et al, 1999). Additionally, some authors have suggested an increased risk in women (Reference Alvir and LiebermanAlvir & Lieberman, 1994), although this was not seen in the UK CPMS cohort, and in people of Ashkenazi Jewish descent with the haplotype HLA-B38, DR4, DQw3 (Reference Lieberman, Yunis and EgeaLieberman et al, 1990). Concomitant treatment with other drugs reported to cause leucopenia may also increase the risk of agranulocytosis (Reference Idänpään-Heikkilä, Alhava and OlkinuoraIdänpään-Heikkilä et al, 1977; Reference Povlsen, Noring and FogPovlsen et al, 1985; Reference LindstromLindström, 1988; Reference Gerson, Lieberman and FriedenbergGerson et al, 1991; Reference Gerson and MeltzerGerson & Meltzer, 1992; Reference Junghan, Albers and WoggonJunghan et al, 1993; Reference Valevski, Modai and LabavValevski et al, 1993; Reference Chengappa, Gopalani and HaughtChengappa et al, 1996; Reference Sénéchal, Landry and DeschampsSénéchal et al, 2002), as perhaps does a history of drug-induced blood dyscrasias (Reference Idänpään-Heikkilä, Alhava and OlkinuoraIdänpään-Heikkilä et al, 1977; Reference Valevski, Modai and LabavValevski et al, 1993).
Previous rechallenge experience
We identified 12 published reports (37 patients) providing information on outcome in patients rechallenged with clozapine after previous leucopenias, neutropenias or agranulocytoses during clozapine treatment (Reference Idänpään-Heikkilä, Alhava and OlkinuoraIdänpään-Heikkilä et al, 1977; Reference Povlsen, Noring and FogPovlsen et al, 1985; Reference LindstromLindström, 1988; Reference Grohmann, Schmidt and Spiess-KieferGrohmann et al, 1989; Safferman et al, Reference Safferman, Lieberman and Alvir1992, Reference Safferman, Lieberman and Zeman1993; Reference Frankenberg, Stormberg and GersonFrankenberg et al, 1994; Reference Barrons, Johnson and NynkowskiBarrons et al, 1996; Reference Chengappa, Gopalani and HaughtChengappa et al, 1996; Reference Sperner-Unterweger, Czeipek and GagglSperner-Unterweger et al, 1998; Reference Sénéchal, Landry and DeschampsSénéchal et al, 2002; Reference Ahn, Jeong and JangAhn et al, 2004). Five reports (14 patients) described unsuccessful clozapine rechallenge. We are defining successful rechallenge as re-exposure that does not result in leucopenia, neutropenia or agranulocytosis or re-exposure where the patient is maintained on clozapine and ultimately has a normal white cell and neutrophil count. Five of the eight reports of successful rechallenge were of unconventional procedures: one described a clozapine overdose after clozapine discontinuation, and in four clozapine was not discontinued despite low white cell or neutrophil counts. All 12 reports are summarised in Table 7.
Table 7 Published reports of rechallenge with clozapine
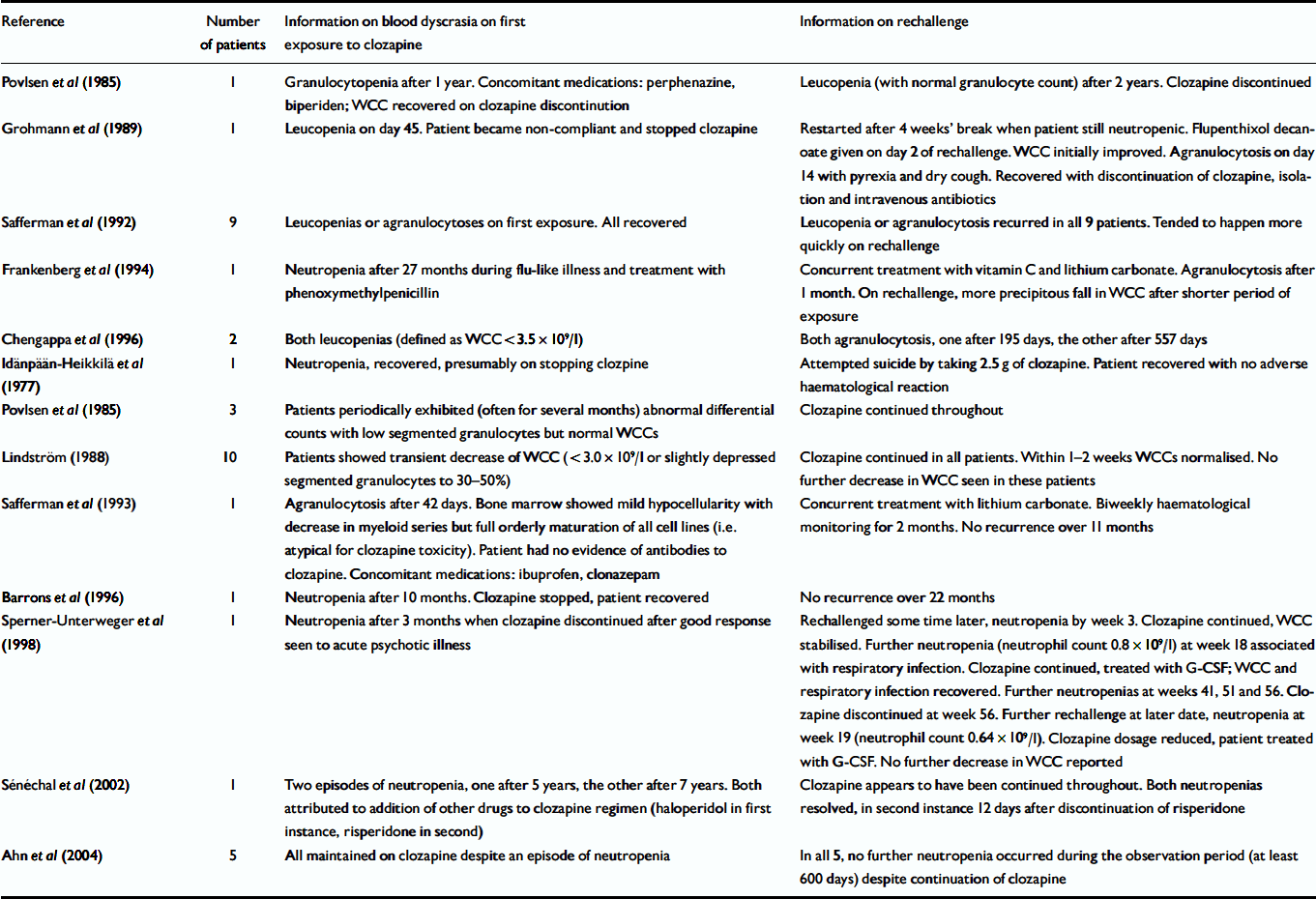
| Reference | Number of patients | Information on blood dyscrasia on first exposure to clozapine | Information on rechallenge |
|---|---|---|---|
| Povlsen et al (Reference Povlsen, Noring and Fog1985) | 1 | Granulocytopenia after 1 year. Concomitant medications: perphenazine, biperiden; WCC recovered on clozapine discontinution | Leucopenia (with normal granulocyte count) after 2 years. Clozapine discontinued |
| Grohmann et al (Reference Grohmann, Schmidt and Spiess-Kiefer1989) | 1 | Leucopenia on day 45. Patient became non-compliant and stopped clozapine | Restarted after 4 weeks' break when patient still neutropenic. Flupenthixol decanoate given on day 2 of rechallenge. WCC initially improved. Agranulocytosis on day 14 with pyrexia and dry cough. Recovered with discontinuation of clozapine, isolation and intravenous antibiotics |
| Safferman et al (Reference Safferman, Lieberman and Alvir1992) | 9 | Leucopenias or agranulocytoses on first exposure. All recovered | Leucopenia or agranulocytosis recurred in all 9 patients. Tended to happen more quickly on rechallenge |
| Frankenberg et al (Reference Frankenberg, Stormberg and Gerson1994) | 1 | Neutropenia after 27 months during flu-like illness and treatment with phenoxymethylpenicillin | Concurrent treatment with vitamin C and lithium carbonate. Agranulocytosis after 1 month. On rechallenge, more precipitous fall in WCC after shorter period of exposure |
| Chengappa et al (Reference Chengappa, Gopalani and Haught1996) | 2 | Both leucopenias (defined as WCC < 3.5 × 109/l) | Both agranulocytosis, one after 195 days, the other after 557 days |
| Idänpään-Heikkilä et al (Reference Idänpään-Heikkilä, Alhava and Olkinuora1977) | 1 | Neutropenia, recovered, presumably on stopping clozpine | Attempted suicide by taking 2.5 g of clozapine. Patient recovered with no adverse haematological reaction |
| Povlsen et al (Reference Povlsen, Noring and Fog1985) | 3 | Patients periodically exhibited (often for several months) abnormal differential counts with low segmented granulocytes but normal WCCs | Clozapine continued throughout |
| Lindström (Reference Lindstrom1988) | 10 | Patients showed transient decrease of WCC (< 3.0 × 109/l or slightly depressed segmented granulocytes to 30-50%) | Clozapine continued in all patients. Within 1-2 weeks WCCs normalised. No further decrease in WCC seen in these patients |
| Safferman et al (Reference Safferman, Lieberman and Zeman1993) | 1 | Agranulocytosis after 42 days. Bone marrow showed mild hypocellularity with decrease in myeloid series but full orderly maturation of all cell lines (i.e. atypical for clozapine toxicity). Patient had no evidence of antibodies to clozapine. Concomitant medications: ibuprofen, clonazepam | Concurrent treatment with lithium carbonate. Biweekly haematological monitoring for 2 months. No recurrence over 11 months |
| Barrons et al (Reference Barrons, Johnson and Nynkowski1996) | 1 | Neutropenia after 10 months. Clozapine stopped, patient recovered | No recurrence over 22 months |
| Sperner-Unterweger et al (Reference Sperner-Unterweger, Czeipek and Gaggl1998) | 1 | Neutropenia after 3 months when clozapine discontinued after good response seen to acute psychotic illness | Rechallenged some time later, neutropenia by week 3. Clozapine continued, WCC stabilised. Further neutropenia (neutrophil count 0.8 × 109/l) at week 18 associated with respiratory infection. Clozapine continued, treated with G-CSF; WCC and respiratory infection recovered. Further neutropenias at weeks 41, 51 and 56. Clozapine discontinued at week 56. Further rechallenge at later date, neutropenia at week 19 (neutrophil count 0.64 × 109/l). Clozapine dosage reduced, patient treated with G-CSF. No further decrease in WCC reported |
| Sénéchal et al (Reference Sénéchal, Landry and Deschamps2002) | 1 | Two episodes of neutropenia, one after 5 years, the other after 7 years. Both attributed to addition of other drugs to clozapine regimen (haloperidol in first instance, risperidone in second) | Clozapine appears to have been continued throughout. Both neutropenias resolved, in second instance 12 days after discontinuation of risperidone |
| Ahn et al (Reference Ahn, Jeong and Jang2004) | 5 | All maintained on clozapine despite an episode of neutropenia | In all 5, no further neutropenia occurred during the observation period (at least 600 days) despite continuation of clozapine |
Two types of clozapine-induced neutropenia?
It has been postulated that clozapine can induce two clinically distinct types of neutropenia (Gerson, Reference Gerson1993, Reference Gerson1994); the first type is a mild to moderate neutropenia (neutrophil count below 1.5 × 109/l but not lower than 0.5 × 109/l), which occurs in 1.8% of treated patients. When clozapine is discontinued, recovery is rapid (2–8 days). The second type of neutropenia is more severe with a neutrophil count below 0.5 × 109/l (agranulocytosis) and an incidence of 0.78%. In the second type, even if clozapine is stopped when the neutrophil count is just below 1.5 × 109/l, agranulocytosis none the less develops in some patients, usually within 2–5 days and generally lasting for 14–21 days. In such patients, monitoring allows the early detection and treatment but not the prevention of neutrophil suppression. Bone marrow from these patients demonstrates an absence of myeloid precursors, only occasional promyelocytes and myeloblasts, and relative erythroid hyperplasia (Reference Gerson and MeltzerGerson & Meltzer, 1992). In contrast, in the milder clozapine-induced neutropenia, it has been reported that there is evidence of myeloid maturation in the bone marrow, suggesting peripheral destruction of neutrophils (Reference GersonGerson, 1993). The theory is compatible with the fact that, despite a considerable decrease in the incidence of fatal clozapine-induced agranulocytosis since the onset of white cell count monitoring – from 0.26% in Finland in 1975 (Reference Idänpään-Heikkilä, Alhava and OlkinuoraIdänpään-Heikkilä et al, 1977) to 0.01% in the UK and Ireland with monitoring – the incidence of agranulocytosis has remained fairly constant and has not decreased: a cautious estimate of 0.5% in Finland in 1975 (Reference Idänpään-Heikkilä, Alhava and OlkinuoraIdänpään-Heikkilä et al, 1977; as there was no routine monitoring some cases are likely to have missed identification), 0.78% in the UK and Ireland with monitoring. However, it does not seem compatible with the results we have presented, as one would not expect patients who had experienced only neutropenia or leucopenia on first exposure to clozapine to develop agranulocytosis on second exposure, unless they had somehow become more sensitive to clozapine bone-marrow toxicity. It also implies that patients who develop only neutropenia or leucopenia on first exposure to clozapine can be relatively safely rechallenged, and our results demonstrate that this is not the case, with nine patients who experienced a neutropenia on first exposure developing agranulocytosis on repeat exposure.
Mechanism of clozapine-induced agranulocytosis
The exact mechanism of clozapine-induced agranulocytosis is unknown; it could be immune-mediated or involve a toxic mechanism, or even a combination of both. In the group of nine patients unsuccessfully rechallenged with clozapine by Safferman et al (Reference Safferman, Lieberman and Alvir1992), blood dyscrasias recurred after a number of weeks, with an average time to onset of 14.6 weeks. These authors concluded that their data favoured an immune-mediated mechanism, because the blood dyscrasias recurred in all nine patients and did so more quickly on repeat exposure. The single case report by Frankenberg et al (Reference Frankenberg, Stormberg and Gerson1994) also documents a more rapid blood dyscrasia on rechallenge. Our findings differ from those of Safferman et al (Reference Safferman, Lieberman and Alvir1992) as blood dyscrasias recurred in only 38% of the 53 patients rechallenged in our study, and we found that the median time to onset of the rechallenge dyscrasia was 5.5 weeks, with 80% of cases, including all the cases of agranulocytosis, occurring before 10 weeks. However, we did find that when blood dyscrasias occurred on rechallenge they tended to happen more quickly, last longer and be more severe. Our results, therefore, also suggest an immune-mediated mechanism, but as the blood dyscrasias occurred weeks rather than days after rechallenge, a simple immunological toxicity cannot be the mechanism. It might be that a certain concentration of metabolite needs to accumulate in order to stimulate an immunologically mediated toxicity. Whatever the mechanism, it is clear that extreme caution is required when rechallenging a patient with clozapine.
Implications of our findings
The results we have described represent the first published report of a well-documented cohort of patients who have been rechallenged with a good degree of success. This is due in part to the careful selection of patients on the basis that their initial blood dyscrasia might well have had a cause other than clozapine and/or that they had experienced a ‘mild’ episode. Despite this, 38% (n=20) of our patients did experience a second blood dyscrasia and 45% (n=9) of these patients developed agranulocytosis, which represents a 22-fold increase in risk of agranulocytosis in our rechallenge group compared with clozapine-naïve patients (in clozapine-naïve patients, 2.8% of patients would be expected to develop a blood dyscrasia, of whom 28% would be expected to develop agranulocytosis). Furthermore, in the majority of our unsuccessfully rechallenged patients, the second blood dyscrasia occurred more rapidly, lasted longer and/or was more severe than the first blood dyscrasia. Our data demonstrate that even patients in whom the indications are that clozapine was not causative in their blood dyscrasia (e.g. patients who develop blood dyscrasias after lengthy treatment) can experience agranulocytosis on rechallenge. Our results demonstrate how difficult it is to identify with any certainty patients whose blood dyscrasias are unrelated to clozapine, and the need for extreme vigilance when rechallenging any patient. However, we also demonstrated that patients who do not develop a blood dyscrasia on rechallenge are likely to continue with clozapine treatment: indeed, the majority of all the rechallenged patients continued clozapine treatment, many for a number of years. Therefore, in a carefully selected group rechallenge may well be justified if it is considered that the risks of withholding treatment are greater than the risks of rechallenge.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Clozapine rechallenge should not be undertaken lightly, because patients rechallenged with clozapine are considerably more likely to develop a further blood dyscrasia, and specifically are 22 times more likely to develop an agranulocytosis, than clozapine-naïve patients.
-
▪ The first 10 weeks of rechallenge are critical as most blood dyscrasias are likely to become apparent within this time.
-
▪ In a carefully selected group rechallenge may well be justified if it is considered that the risks of withholding treatment are greater than the risks of rechallenge.
LIMITATIONS
-
▪ Some patient data may not be complete as the Clozaril Patient Monitoring Service does not have complete records on clozapine dosage, and concomitant medications and illnesses experienced by the patient are only recorded if the treating team discusses them with the monitoring service.
-
▪ Although adherence to the blood monitoring schedule is an indicator of compliance with clozapine, this was not verified directly.
-
▪ The data, excluding some haematological data, have not been verified at source.
Acknowledgements
We thank Novartis Australia for support and Novartis UK for support and the provision of data.
















eLetters
No eLetters have been published for this article.