Prediabetes is considered an intermediate state of glucose metabolism between glucose homeostasis and type 2 diabetes (T2D). This means that subjects with prediabetes have increased blood glucose levels, but they do not meet the criteria for being diagnosed with T2D( 1 ). Prediabetes is defined by the presence of isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT) or both; IFG refers to increased fasting plasma glucose (FPG) levels after 8–12 h of overnight fast, whereas IGT refers to increased postprandial plasma glucose (PG) levels after consumption of 75 g glucose during a 2-h oral glucose tolerance test (OGTT)( Reference Sequeira and Poppitt 2 ). Prediabetes has been associated with increased risk of composite CVD, CHD, stroke and all-cause mortality( Reference Huang, Cai and Mai 3 ), as well as with an increased risk of T2D incidence compared with normal glycaemia( Reference Gerstein, Santaguida and Raina 4 ).
Lifestyle modification interventions could prevent the development of T2D in subjects with prediabetes, especially those who are overweight or obese( Reference Ibrahim, Tuomilehto and Aschner 5 ). Landmark randomised intervention studies in the field of diabetes prevention through lifestyle modifications, that is, the Da Qing study (1997), the Finnish Diabetes Prevention Study (2001) and the US Diabetes Prevention Program (2002)( Reference Sénéchal, Slaght and Bharti 6 ) showed that adults with prediabetes who followed long-term lifestyle interventions had a significant reduction in T2D incidence( Reference Liu, Silvestre and Poppitt 7 ) and a sustained beneficial effect with respect to the prevention of T2D for many years after the period of active intervention( Reference Khetan and Rajagopalan 8 ). Given the fact that the duration of lifestyle intervention is an important factor that influences both anthropometric and metabolic outcomes, the Academy of Nutrition and Dietetics’ evidence-based nutrition practice guideline for the prevention of T2D recommends that individuals with prediabetes should be treated with lifestyle intervention for a minimum period of 3 months in order to significantly improve body weight, waist circumference and FPG and to prevent T2D( Reference Briggs Early and Stanley 9 ).
Nuts or tree nuts are botanically defined as dry fruits with a single seed and an ovary wall which becomes hard at maturity( Reference Ros 10 ). The most common edible tree nuts are almonds, Brazil nuts, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts( 11 ). Peanuts are of particular interest; peanuts (Arachis hypogaea) are botanically groundnuts or legumes (i.e. edible seeds enclosed in pods), and they belong to the same family as beans, lentils and peas, but they have a nutrient profile similar to tree nuts( Reference Ros 10 ). Nut consumption was studied in relation to the risk of developing T2D in several prospective studies with a mean follow-up duration of 10 years. Consumption of five or more servings (28 g/serving) of nuts per week was inversely associated with T2D incidence in the Nurses’ Health Study, compared to rare or null consumption. Similarly, consumption of four or more servings of nuts per week was inversely associated with T2D incidence in the Tehran Lipid and Glucose Study, compared to consumption of one or less servings per week. In the Physicians’ Health Study I; however, nut consumption (≥7 servings of nuts per week v. rare or never consumption) was not significantly associated with T2D either in lean or in overweight/obese participants( Reference Hernández-Alonso, Camacho-Barcia and Bulló 12 ). Finally, results from a systematic review of prospective cohort studies showed that each one serving (28 g) increase in the daily consumption of nuts was inversely associated with T2D risk( Reference Luo, Zhang and Ding 13 ).
Seed consumption is less studied in humans in relation to the T2D risk and markers of glycaemic control. Flaxseeds, pumpkin and sunflower seeds are commonly consumed seeds of plants in the Linaceae, the Cucurbitaceae and the Asteraceae family, respectively( 11 , Reference Kim, Kim and Kim 14 ). Flaxseed has a high PUFA content among seeds, and it is a particularly rich source of α-linolenic acid, an 18-carbon, essential n-3 PUFA and a bioactive compound( 15 ). The bioavailability of α-linolenic acid contained in flaxseed was found to be dependent upon the form of flaxseed consumed, being highest for the flaxseed oil, followed by the ground flaxseed( Reference Austria, Richard and Chahine 16 ). Another major component of flaxseed making it beneficial to human health is dietary fibres, mainly cellulose and lignin (insoluble fibres), and the mucilage gums (soluble fibres)( Reference Singh, Mridula and Rehal 17 ). Flaxseed mucilage, which constitutes about one-third of the dietary fibres in the flaxseed, was found to induce beneficial effects on glucose homeostasis in obese postmenopausal women( Reference Brahe, Le Chatelier and Prifti 18 ). Improvements in markers of glycaemic control (i.e. fasting blood glucose, insulin and glycated Hb (HbA1c) levels) were also found for sunflower-seed kernels among postmenopausal women with T2D( Reference Richmond, Williams and Mann 19 ).
Taking into account that nuts and seeds have a unique profile of macronutrients, micronutrients and other bioactive compounds with beneficial effects on glucose and insulin metabolism( Reference Hernández-Alonso, Camacho-Barcia and Bulló 12 , Reference Kim, Kim and Kim 14 ), we aimed to systematically review randomised controlled trials (RCT) which investigate the effects of nut and seed consumption on markers of glucose metabolism in adults with prediabetes. A secondary aim was to report other cardiometabolic effects of nut and seed consumption in the same population, according to the aims of each study.
Methods
The present systematic review was based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses Statement( Reference Liberati, Altman and Tetzlaff 20 , Reference Beller, Glasziou and Altman 21 ).
Eligibility criteria
Published reports with abstract, written in English, were considered eligible. No publication date limitations were imposed. To comply with the aims of the present review, we were only interested in interventional studies. The details of the study eligibility criteria are provided herein: (1) study design: studies with a RCT design were considered eligible; (2) type of participants: participants aged 18 years or older with prediabetes were considered. Prediabetes was defined as presence of isolated IFG, isolated IGT, IFG and IGT or elevated HbA1c, based on established criteria (e.g. WHO or American Diabetes Association criteria( Reference Yip, Sequeira and Plank 22 , 23 )); (3) Type of intervention and control: Studies comparing the effects of a diet containing nuts or seeds (whole or ground/milled) against a diet without nuts or seeds were considered eligible. All types and doses of nuts and seeds were considered; (4) type of primary outcome measures: studies evaluating markers of glucose metabolism were considered eligible. These include, but are not limited to FPG, 2-h PG during OGTT and HbA1c levels; (5) length of intervention: consumption of nuts or seeds for at least 3 consecutive months was considered.
Information sources and study selection
Reports were identified by searching PubMed and Scopus electronic databases all years to February 2018. An update literature search was performed from February 2018 onwards. Search on PubMed was performed without any limitations, whereas only one limitation was applied during search on Scopus (i.e. document type should be article or review). The search was developed and conducted by A. N. and T. N. Firstly, we chose the keywords for the exposure and the outcome; (1) keywords for the exposure: almonds, Brazil nuts, cashew nuts, hazelnuts, macadamia nuts, pecans, pine nuts, pistachios, walnuts, peanuts, tree nuts, sunflower seeds, pumpkin seeds, Cucurbitaceae seeds and flaxseeds; (2) keywords for the primary outcome: prediabetes, impaired fasting glucose, impaired glucose tolerance, glucose intolerance, glycated haemoglobin and insulin resistance. Secondly, keywords were turned into search terms in order to include as much information as possible. We applied two strategies to search databases. In the first strategy, we entered all search terms for the exposure at once and we separated one term from another with the Boolean operator ‘OR’. We did the same with respect to terms for the outcome. We used parentheses to nest terms for the exposure, as well as terms for the outcome in order to be processed as two different units. Finally, we combined these units with the Boolean operator ‘AND’. The second strategy differed with respect to terms for the exposure; we entered one term at a time (online Supplementary Appendix S1).
Identified records were checked for duplicates and subjected to a three-step screening process. In the preliminary screening (first screening), records with no abstract available, written in other language than English or being any type of record except research article or review were excluded. After screening the titles, abstracts and authors’ keywords of the remaining records (second screening), records reporting in vitro studies or animal studies or human studies among a different age group than adults and records which were irrelevant based on the keywords for the exposure and/or the outcome were excluded. All records which passed the second screening process were considered potentially relevant. In the third screening, we checked the full texts and the reference lists of the potentially relevant records to identify records reporting on intervention studies. All other studies were discarded. The full text of records reporting on intervention studies, identified by searching electronic databases and checking full texts and reference lists of relevant records, was subsequently examined in detail in order to assess eligibility. The preliminary screening step was performed by reviewer A. N. The subsequent screening steps of the study selection and the eligibility assessment were performed by two independent reviewers, A. N. and T. N. Disagreements were resolved through discussion with a third reviewer, S. A.
Data extraction
Data were extracted from each article included in the present systematic review: first author, year of publication, country of origin, funding source, conflict of interest, aim, inclusion criteria, setting of recruitment, study design, intervention and control treatments, attrition rates, statistical analysis, baseline characteristics of participants, parameters measured or estimated and outcomes (i.e. changes between end and beginning of each treatment and difference in changes between treatments). Data extraction was carried out by reviewer A. N. and was verified by two independent reviewers S. A. and T. N. During the verification process, trial registries were used to provide missing data and to cross-check information.
Risk of bias assessment
All reports included in the present systematic review were assessed for risk of bias using the Cochrane Collaboration’s tool( Reference Higgins, Altman and Sterne 24 ). The full text of published reports and trial registries provided information to support a judgement about the risk of bias. The assessment was carried out by reviewer A. N. and was verified by reviewer T. N. Disagreements were resolved through discussion with a third reviewer, S. A.
Finally, studies were not sufficiently homogeneous in terms of design and intervention treatment/exposure. Thus, we did not conduct meta-analysis.
Results
Search and study selection
Both search strategies provided 293 records on PubMed and 459 records on Scopus, that is, 752 records in total. After adjusting for duplicates, 587 records remained. After the preliminary screening, twenty-six records were discarded for the following reasons; seven had no abstract available, ten were written in other language than English and nine were not research articles or reviews (one commentary paper, one paper in proceedings, two letters to the editor, one author reply, one editorial and three recommendations/guideline papers). After the second screening of the remaining records (n 561), 461 records were discarded for the following reasons: twenty-five reported in vitro studies, 136 reported animal studies, seven addressed children and/or adolescents and 293 were irrelevant to the keywords for the exposure and/or the outcome. In contrast, 100 records were considered potentially relevant to the keywords for both the exposure and the outcome (i.e. Fifty-three records reporting intervention studies; and forty-seven all other records, namely seven records about observational studies and forty reviews). An additional seven records were identified by checking the full text and the reference list of the forty-seven relevant records. These records (n 47) were subsequently discarded. A total of sixty records or articles, published between 1990 and 2017, reported on intervention studies; fifty-three records were identified through database searching and seven records were identified by checking full texts and reference lists.
During the eligibility assessment, seven articles were excluded because the studies they described did not have a randomised design. The remaining fifty-three articles described studies with a randomised design. However, thirty-nine articles were excluded because they did not include criteria for prediabetes; subjects were solely recruited on the basis of being apparently healthy (nine articles), fulfilling the criteria for overweight, obesity and/or dyslipidaemia (eleven articles), having the metabolic syndrome (fourteen articles) or being at high risk of developing CVD (five articles), defined as having T2D or at least three CVD risk factors (i.e. current smoking, hypertension, hypercholesterolaemia, decreased HDL-cholesterol levels, overweight/obesity, or a family history of premature CHD).
Out of the remaining fourteen articles, five articles reported on subjects at high risk of developing T2D( Reference Mori, Considine and Mattes 25 – Reference Njike, Yarandi and Petraro 29 ) and nine articles reported on subjects with prediabetes( Reference Rhee and Brunt 30 – Reference Wien, Bleich and Raghuwanshi 38 ). Six articles were excluded for the following reasons; one article( Reference Tan and Mattes 27 ) did not include criteria for defining prediabetes; one article( Reference Njike, Yarandi and Petraro 29 ) assessed only diet quality and food group consumption; in one article( Reference Reis, Ribeiro and Costa 26 ) which defined prediabetes as 2-h PG during OGTT of 140–199 mg/dl (7·8–11·0 mmol/l), the mean baseline value of 2-h PG was lower than the bottom cut-off point for defining IGT-prediabetes; one article( Reference Mori, Considine and Mattes 25 ) aimed to investigate the first-meal (0–240 min) and second-meal (240–490 min) effects of nut consumption; one article( Reference Hjorth, Ritz and Blaak 37 ) was excluded because it did not report intervention studies with nuts or seeds, but rather tested ad libitum diets with different glycaemic load or a high-fat/low-carbohydrate hypoenergetic diet; finally, one article( Reference Rhee and Brunt 30 ) was excluded because it did not meet the eligibility criteria with respect to the control; ground flaxseed was tested against equal amount of ground wheat bran. In other words, that study was aimed to determine the effects of flaxseed over the effects of wheat bran, instead of a flaxseed-free diet in general.
All in all eight articles were included in the qualitative synthesis( Reference Njike, Ayettey and Petraro 28 , Reference Hutchins, Brown and Cunnane 31 – Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 , Reference Wien, Bleich and Raghuwanshi 38 ). Seven articles( Reference Hutchins, Brown and Cunnane 31 – Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 , Reference Wien, Bleich and Raghuwanshi 38 ) reported only on subjects with prediabetes and one article( Reference Njike, Ayettey and Petraro 28 ) reported on subjects at high risk of developing T2D. This article( Reference Njike, Ayettey and Petraro 28 ) included criteria for defining prediabetes (i.e. FPG of 100–125 mg/dl or HbA1c of 5·7–6·4 %). At baseline, the mean value of FPG was lower than 100 mg/dl, but the mean value of HbA1c was at or slightly above the lower cut-off point for defining prediabetes. This article was, therefore, included in the present review.
The results of search and study selection are presented in Fig. 1. The updated literature search identified one new record, but assessed only dietary nutrient intake and was therefore excluded.
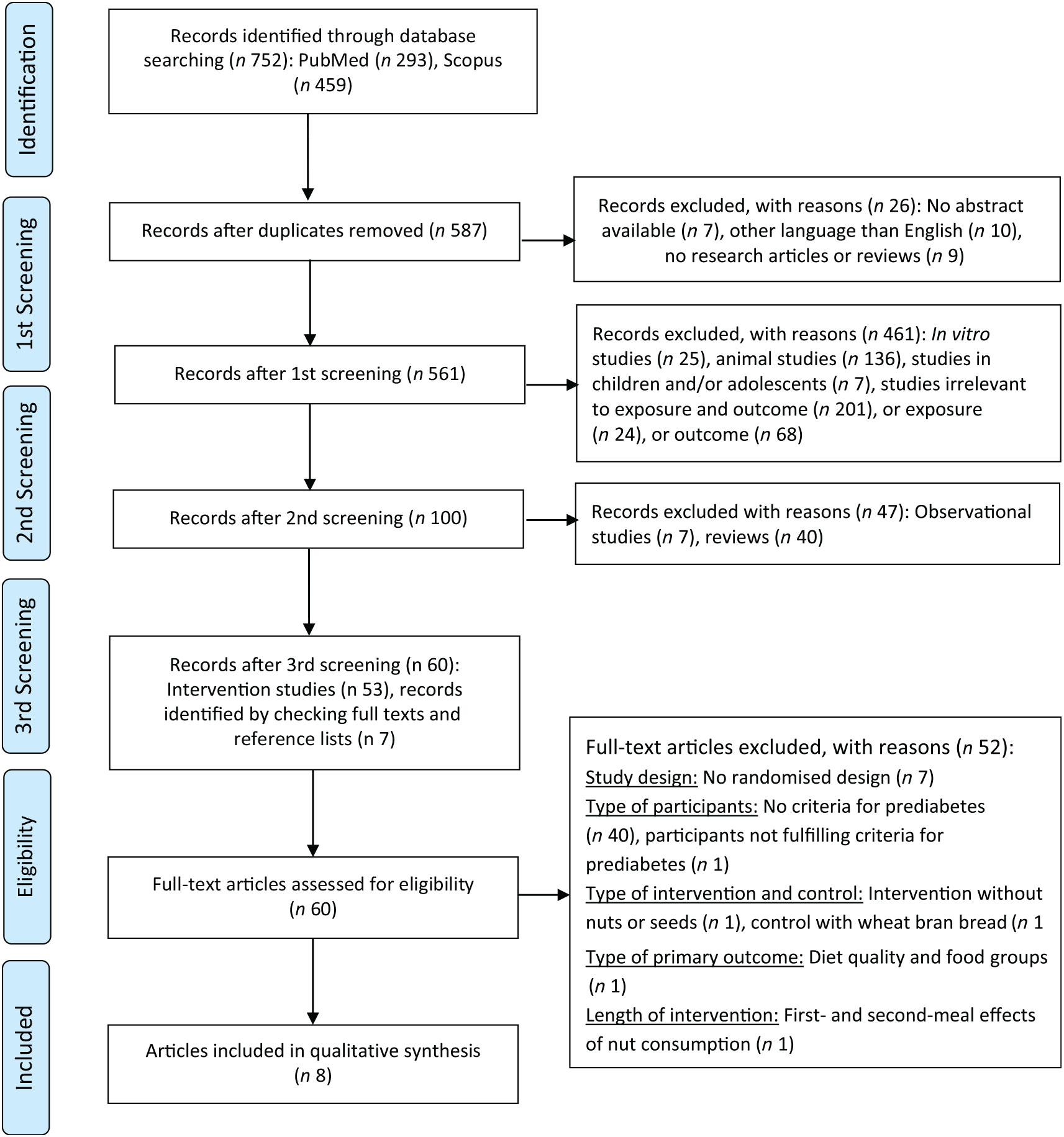
Fig. 1. Preferred Reporting Items for Systematic reviews and Meta-Analyses 2009 flow diagram.
Data extraction
The articles included in the present review were published between 2010 and 2017. All studies are registered. Four articles( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ) were found to be reports of one study. Funding source was disclosed in all articles and conflict of interest was disclosed in all except for one article( Reference Hernández-Alonso, Cañueto and Giardina 35 ).
One study assessed the effects of pistachios on markers of glucose metabolism, lipid profile, inflammation- and glucose-related markers, anthropometric measures and blood pressure( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ), lipoprotein profile( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 ), circulating microRNA related to glucose metabolism( Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ) and urine metabolites( Reference Hernández-Alonso, Cañueto and Giardina 35 ) in overweight or obese adults with prediabetes. A second study( Reference Njike, Ayettey and Petraro 28 ) assessed the effects of walnuts, with or without dietary counselling to adjust energy intake, on markers of glucose metabolism, lipid profile, anthropometric measures, blood pressure and endothelial function in adults at risk of T2D( Reference Njike, Ayettey and Petraro 28 ). A third study( Reference Wien, Bleich and Raghuwanshi 38 ) assessed the effects of almonds on markers of glucose metabolism, lipid profile, anthropometric measures and blood pressure in adults with prediabetes. Finally, two independent studies( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) assessed the effects of two ground flaxseed doses on markers of glucose metabolism, as well as on inflammation markers( Reference Hutchins, Brown and Cunnane 31 ), and blood pressure( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) in overweight or obese adults with prediabetes.
The target population was male and female adult subjects with prediabetes in all( Reference Hutchins, Brown and Cunnane 31 – Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 , Reference Wien, Bleich and Raghuwanshi 38 ) but one study( Reference Njike, Ayettey and Petraro 28 ) where the target population was male and female adult subjects at risk of diabetes. Prediabetes was defined as IFG (FPG: 100–125 mg/dl or 5·6–6·9 mmol/l)( Reference Hutchins, Brown and Cunnane 31 – Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 , Reference Wien, Bleich and Raghuwanshi 38 ) or random blood glucose levels of 140–199 mg/dl( Reference Wien, Bleich and Raghuwanshi 38 ). Risk of diabetes was defined as meeting at least one of the following criteria: overweight with increased waist circumference; prediabetes, defined as FPG >100 and <126 mg/dl or HbA1c 5·7–6·4 %; presence of the metabolic syndrome( Reference Njike, Ayettey and Petraro 28 ). Participants were recruited from the community at primary care centres( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), or through flyers and newspaper advertisements( Reference Njike, Ayettey and Petraro 28 ), or via phone call in order to be screened for prediabetes as part of a research project( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ), or at the workplace, endocrine and diabetes clinics and community-based health fairs( Reference Wien, Bleich and Raghuwanshi 38 ). The total sample size was 371 adults with prediabetes or at risk of diabetes.
All studies were RCT with a cross-over design ( Reference Hutchins, Brown and Cunnane 31 – Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), a parallel-treatment arms design( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 , Reference Wien, Bleich and Raghuwanshi 38 ) or a combined design (i.e. a parallel-treatment arms design and a cross-over design within each arm)( Reference Njike, Ayettey and Petraro 28 ). Participants in the intervention arm(s) followed a diet that provided 57 g of pistachios( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), or 56 g of walnuts( Reference Njike, Ayettey and Petraro 28 ), or 20 % of total energy intake (mean intake 60 g) from almonds( Reference Wien, Bleich and Raghuwanshi 38 ), a low-dose of flaxseed (i.e. 13 g( Reference Hutchins, Brown and Cunnane 31 ) or 20 g( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 )) or a high dose of flaxseed (i.e. 26 g( Reference Hutchins, Brown and Cunnane 31 ) or 40 g( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 )) per d. Participants were provided with whole half roasted and half roasted and salted pistachios( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), whole unshelled walnuts( Reference Njike, Ayettey and Petraro 28 ), whole raw or dry roasted almonds( Reference Wien, Bleich and Raghuwanshi 38 ) or ground, pre-weighed and pre-packaged doses of flaxseed( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ). In contrast, participants in the control arm were asked to follow an isoenergetic nut-free diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), or received instructions to consume the habitual diet without walnuts and specific walnut-containing products( Reference Njike, Ayettey and Petraro 28 ), or followed an American Diabetes Association diet without tree nuts and peanuts( Reference Wien, Bleich and Raghuwanshi 38 ), or a flaxseed-free diet( Reference Hutchins, Brown and Cunnane 31 ) or their habitual diets without flaxseed( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ). Adherence to the intervention treatment was assessed by counting the empty packages returned to the investigators( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), checking the content of the returned packages( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ), measuring biochemical indices in the plasma, for example, lutein-zeaxanthin and γ-tocopherol levels for pistachio consumption( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), a-tocopherol levels for almond consumption( Reference Wien, Bleich and Raghuwanshi 38 ) and α-linolenic acid levels for flaxseed consumption( Reference Hutchins, Brown and Cunnane 31 ), or by completing 24-h recalls( Reference Njike, Ayettey and Petraro 28 ) or 3-d food/activity records( Reference Wien, Bleich and Raghuwanshi 38 ) at regular intervals. Participants were also instructed to maintain their physical activity during the study, in an attempt to reduce the effect of an altered physical activity pattern on the outcomes evaluated( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 – Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ).
The characteristics of the studies included in the present systematic review are presented in Table 1.
Table 1. Characteristics of the studies included in the review
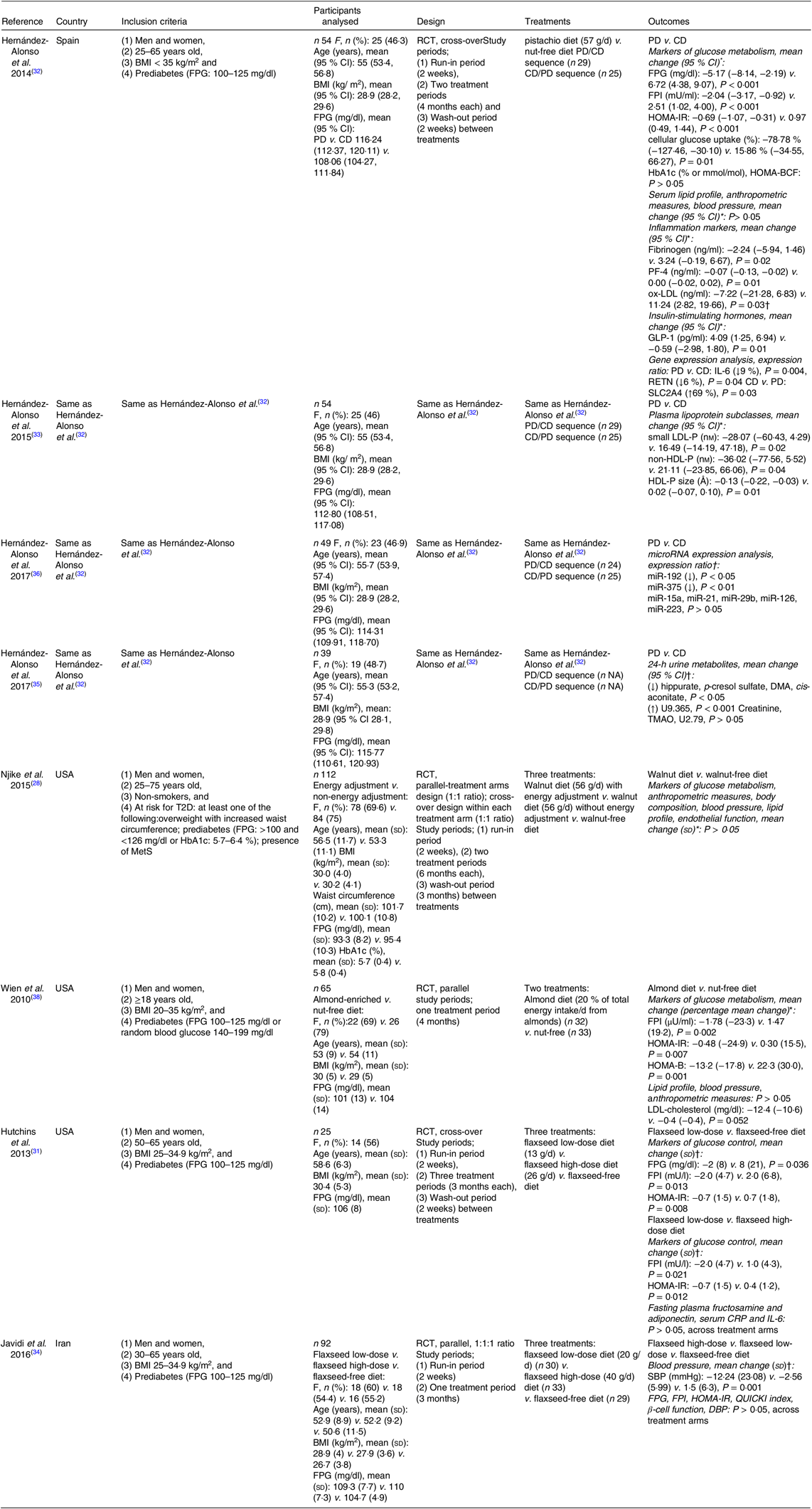
RCT, randomised controlled trial; PD, pistachio diet; CD, control diet; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HOMA-IR, homeostasis model assessment of insulin resistance index; HbA1c, glycated Hb; HOMA-BCF, HOMA of β-cell function; PF-4, platelet factor 4; ox-LDL, oxidised-LDL; GLP-1, glucagon-like peptide-1; RETN, resistin; SLC2A4, solute carrier family 2, facilitated glucose transporter member 4; F, female; small LDL-P, small LDL-particle; non-HDL-P; non-HDL-particle; DMA, dimethylamine; TMAO, trimethylamine N-oxide; T2D, type 2 diabetes mellitus; MetS, metabolic syndrome; HOMA-B, HOMA of β-cell function; CRP, C-reactive protein; SBP, systolic blood pressure; QUICKI index, quantitative insulin sensitivity check index; DBP, diastolic blood pressure.
* Data analysis based on all randomised participants.
† Data analysis based on participants without missing data and/or not excluded for being outliers; all tests were two-sided, and significance was defined as P ≤ 0·05.
Effects of pistachios on cardiometabolic markers in adults with prediabetes
Markers of glucose metabolism
Significant decreases were found for FPG and fasting plasma insulin (FPI) concentrations, homeostasis model assessment of insulin resistance (HOMA-IR) and cellular glucose uptake in lymphocytes after consumption of pistachios for 4 months compared with treatment with a nut-free diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ) (Table 1). Other markers of glucose metabolism, that is, HbA1c and homeostasis model assessment of β-cell function were not significantly different between the two dietary treatments.
Lipid profile
No significant differences were found between the pistachio diet and the nut-free diet with respect to changes in serum lipid profile (i.e. total, HDL-, LDL- and VLDL-cholesterol concentrations, total-to-HDL-cholesterol ratio, LDL-to-HDL-cholesterol ratio, TAG)( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ) (Table 1). However, evaluation of the lipoprotein profile of participants showed favourable effects for the pistachio diet. Specifically, small LDL particle concentrations, non-HDL particle concentrations (i.e. sum of total VLDL and LDL particles) and HDL particle size were found decreased in the pistachio diet compared with the nut-free diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 ) (Table 1).
Glucose- and inflammation-related markers
Fibrinogen, platelet factor 4 and oxidised LDL concentrations were found decreased, whereas glucagon-like peptide-1 (GLP-1) concentrations were found increased in the pistachio diet compared with the nut-free diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ) (Table 1). Data from gene expression analysis showed a down-regulation in the expression of Toll-like receptor 2, Toll-like receptor 4, solute carrier family 2, facilitated glucose transporter member 3, IL-6 and resistin, and an up-regulation in the expression of solute carrier family 2, facilitated glucose transporter member 4 after the pistachio diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ). The expression of Toll-like receptor 2, Toll-like receptor 4, facilitated glucose transporter member 4, IL-6 and resistin was up-regulated, whereas the expression of facilitated glucose transporter member 3 was down-regulated after the nut-free diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ). Significant differences were found between the pistachio diet and the nut-free diet with respect to gene expression analysis data; in particular, the expression of IL-6 and resistin was decreased in the pistachio diet compared with the nut-free diet, whereas the expression of facilitated glucose transporter member 4 was increased in the nut-free diet compared with the pistachio diet( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ) (Table 1).
Circulating micro-RNA
Data from the microRNA analysis showed up-regulation in the expression of miR-15a, miR-21, miR-29b, miR-126 and miR-223, down-regulation in the expression of miR-375 and same expression for miR-192 after the pistachio diet. In contrast, the expression of all studied microRNA was up-regulated after the nut-free diet( Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ). No significant differences were found between the pistachio diet and the nut-free diet with respect to miR-15a, miR-21, miR-29b, miR-126 and miR-223, whereas the expression rate of both miR-192 and miR-375 was significantly decreased in the pistachio diet compared with the nut-free diet( Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ) (Table 1).
Urine metabolites
Several 24-h urine metabolites were found significantly decreased in the pistachio diet compared with the nut-free diet( Reference Hernández-Alonso, Cañueto and Giardina 35 ); these included metabolites of the gut microbiota-derived metabolism (i.e. hippurate, p-cresol sulfate and dimethylamine) and the tricarboxylic acid cycle (i.e. cis-aconitate). In contrast, U9.365, an unknown metabolite, was found significantly increased in the pistachio diet compared with the nut-free diet( Reference Hernández-Alonso, Cañueto and Giardina 35 ) (Table 1). No significant differences were found with respect to the changes in creatinine, trimethylamine N-oxide and U2.79 (unknown metabolite) between the pistachio diet and the nut-free diet( Reference Hernández-Alonso, Cañueto and Giardina 35 ) (Table 1).
Anthropometric measures and blood pressure
No significant differences were found between the pistachio diet and the nut-free diet with respect to changes in anthropometric measures (i.e. weight, BMI, waist circumference) and blood pressure( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ) (Table 1).
Effects of walnuts on cardiometabolic markers in adults with prediabetes
Markers of glucose metabolism
No significant differences were found between the walnut diet and the walnut-free diet, in the energy-adjusted or the ad libitum diet arms, with respect to changes in measured markers of glucose metabolism (i.e. fasting blood glucose and HbA1c concentrations)( Reference Njike, Ayettey and Petraro 28 ) (Table 1). No significant changes from baseline were found for fasting blood glucose after 6 months in the walnut diet or the walnut-free diet, whereas HbA1c was found significantly increased both in the walnut diet with energy adjustment (0·05 %), the ad libitum walnut diet (0·10 %), and the walnut-free diet (0·06 %)( Reference Njike, Ayettey and Petraro 28 ).
Lipid profile
No significant differences were found between the walnut diet and the walnut-free diet, in the energy-adjusted or the ad libitum diet arms, with respect to changes in total, HDL-, LDL-cholesterol, total-to-HDL-cholesterol and TAG( Reference Njike, Ayettey and Petraro 28 ) (Table 1). Total and LDL-cholesterol concentrations were found to improve after 6 months on either the walnut diet or the walnut-free diet( Reference Njike, Ayettey and Petraro 28 ).
Anthropometric measures and blood pressure
No significant differences were found between the walnut diet and the walnut-free diet, in either the energy-adjusted or the ad libitum diet arms, with respect to changes in anthropometric measures (i.e. BMI, waist circumference, percent body fat, visceral fat) or blood pressure( Reference Njike, Ayettey and Petraro 28 ) (Table 1). Both the walnut diet with energy adjustment and the walnut-free diet were found to reduce waist circumference after 6 months of treatment. The ad libitum walnut diet was found to increase body fat percentage and visceral fat compared with baseline( Reference Njike, Ayettey and Petraro 28 ).
Endothelial function
No significant differences were found between the walnut diet and the walnut-free diet, in the energy-adjusted or the ad libitum diet arms, with respect to changes in the flow-mediated dilation( Reference Njike, Ayettey and Petraro 28 ) (Table 1). Endothelial function was found to improve after 6 months on either the walnut diet or the walnut-free diet( Reference Njike, Ayettey and Petraro 28 ).
Effects of almonds on cardiometabolic markers in adults with prediabetes
Markers of glucose metabolism
Significant differences were found for FPI concentrations, HOMA-IR and homeostasis model assessment of β-cell function between the almond diet and the nut-free diet, whereas no significant differences were found between the two treatments with respect to FPG and HbA1c( Reference Wien, Bleich and Raghuwanshi 38 ) (Table 1). Significant decreases were found for measured and estimated markers of glucose metabolism (i.e. FPG and FPI concentrations, HbA1c, HOMA-IR and homeostasis model assessment of β-cell function) after consumption of almonds for 4 months. Similar results were obtained for the nut-free diet, with the exception of FPI concentrations, HOMA-IR and homeostasis model assessment of β-cell function which were increased( Reference Wien, Bleich and Raghuwanshi 38 ).
Lipid profile
No significant differences were found between the almond diet and the nut-free diet with respect to lipid profile( Reference Wien, Bleich and Raghuwanshi 38 ) (Table 1). Total cholesterol, LDL-cholesterol and TAG concentrations were found decreased, whereas HDL-cholesterol concentration increased after 4 months of almond consumption. Similar results were obtained for the nut-free diet, with the exception of total cholesterol, TAG which were found increased( Reference Wien, Bleich and Raghuwanshi 38 ).
Anthropometric measures and blood pressure
No significant differences were found between the almond diet and the nut-free diet with respect to changes in anthropometric measures (i.e. body weight, BMI and waist circumference) or blood pressure( Reference Wien, Bleich and Raghuwanshi 38 ) (Table 1).
Effects of flaxseeds on cardiometabolic markers in adults with prediabetes
Markers of glucose metabolism
In the study by Hutchins et al.( Reference Hutchins, Brown and Cunnane 31 ), FPG concentrations were significantly decreased in the flaxseed low-dose treatment (13 g/d) compared with the flaxseed-free treatment. FPI concentrations and HOMA-IR were significantly decreased in the flaxseed low-dose treatment (13 g/d) compared with both the flaxseed high-dose (26 g/d) and flaxseed-free treatments. No significant differences were found for plasma fructosamine across treatments( Reference Hutchins, Brown and Cunnane 31 ) (Table 1). After 3 months in the flaxseed low-dose treatment, FPG and FPI concentrations, as well as HOMA-IR, were decreased. Treatment with a flaxseed dose twice as high as the low dose did not improve markers of glucose metabolism( Reference Hutchins, Brown and Cunnane 31 ). In the study by Javidi et al.( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ), no significant differences were found for markers of glucose control across treatments (Table 1). FPG concentrations were improved after 3 months in the flaxseed low-dose (20 g/d), flaxseed high-dose (40 g/d) or flaxseed-free treatment, whereas FPI concentrations did not significantly change in any of the treatments. Both insulin resistance and insulin sensitivity indices were improved in the flaxseed low-dose treatment. β-Cell function was significantly increased in both the flaxseed high-dose and the flaxseed-free treatments( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ).
Inflammation markers
Treatment with either a low-dose or a high-dose flaxseed dietary supplement for 3 months did not significantly affect markers of inflammation (i.e. serum concentrations of IL-6 and C-reactive protein) or adiposity (i.e. plasma concentrations of adiponectin)( Reference Hutchins, Brown and Cunnane 31 ) (Table 1).
Blood pressure
Significant differences were found across the three treatment arms with respect to systolic blood pressure; this was decreased in the flaxseed high-dose (40 g/d) and the flaxseed low-dose (20 g/d) treatments, whereas systolic blood pressure was increased in the flaxseed-free treatment. No significant differences were found for diastolic blood pressure across treatments( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) (Table 1).
Risk of bias assessment
Results about risk of bias assessment are provided in Table 2.
Table 2. Risk of bias assessment of the studies/reports included in the review
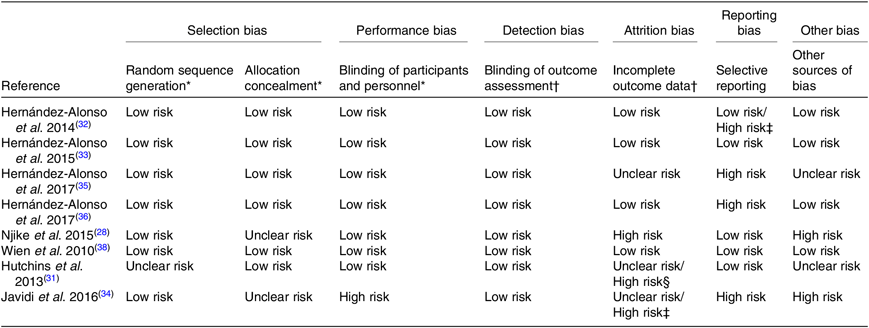
* Evaluation per study.
† Evaluation with respect to the outcomes of interest to the systematic review measured (objective outcomes) of each report.
‡ For gene expression analysis only.
§ For blood outcome measures only.
The risk of selection bias was generally low except for three articles( Reference Njike, Ayettey and Petraro 28 , Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) which had an unclear risk of bias; one article( Reference Hutchins, Brown and Cunnane 31 ) did not specify any random component in the sequence generation process and two articles( Reference Njike, Ayettey and Petraro 28 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) did not describe any method of allocation concealment. The risk of performance bias was low in all studies except for one( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ); there was no blinding, according to the trial registry, and the study did not treat participants in the intervention and control arms with a similar amount of attention( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ). Blinding of outcome assessment could be ensured in all studies. Thus, all studies had a low risk of detection bias.
Risk of attrition bias was low in four reports( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 , Reference Wien, Bleich and Raghuwanshi 38 ). One report( Reference Hernández-Alonso, Cañueto and Giardina 35 ) had an unclear risk of attrition bias because the rate of exclusion of participants from the analyses was not reported for each treatment sequence( Reference Hernández-Alonso, Cañueto and Giardina 35 ). One report( Reference Njike, Ayettey and Petraro 28 ) had a high risk of attrition bias for the following reasons; the dropout rate was higher among participants in the walnut diet arm compared with the walnut-free diet; there was an imbalance in the reasons for dropping out across treatments; and handling of missing data was not explicitly described( Reference Njike, Ayettey and Petraro 28 ). Two studies( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) had a high risk of bias with respect to blood outcome measures only. Otherwise, the risk of attrition bias was unclear due to incomplete reporting of dropout reasons with respect to the treatment period( Reference Hutchins, Brown and Cunnane 31 ), and because data analysis was restricted to those participants who completed treatment periods( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ).
With respect to reporting bias, four reports( Reference Njike, Ayettey and Petraro 28 , Reference Hutchins, Brown and Cunnane 31 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Wien, Bleich and Raghuwanshi 38 ) had low risk, whereas three reports( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ) had a high risk of reporting bias. In the study by Javidi et al.( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ), blood pressure was not pre-specified in the protocol, despite being one of the primary outcomes of the study. Even though a low and a high dose of flaxseed treatment were tested, no pairwise comparisons were conducted in order to determine which groups differed significantly( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ). In the study by Hernández-Alonso et al.( Reference Hernández-Alonso, Cañueto and Giardina 35 ), changes from baseline according to treatment arm were not reported for any of the urine metabolites, and only those metabolites with statistically significant differences between treatment arms were reported( Reference Hernández-Alonso, Cañueto and Giardina 35 ). There was selective reporting of microRNA expression analysis data( Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ); results were graphically presented, and only those being statistically significant were reported in the text( Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ). Finally, one report( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ) had a high risk of reporting bias for gene expression analysis only; percentage of difference between treatment arms was reported instead of changes from baseline according to treatment arm( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ).
Risk of other bias was low for four reports( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 , Reference Wien, Bleich and Raghuwanshi 38 ). Two reports( Reference Hutchins, Brown and Cunnane 31 , Reference Hernández-Alonso, Cañueto and Giardina 35 ) had an unclear risk of other bias; data handling with respect to evaluation of the carry-over effect was not explicitly presented in one report( Reference Hutchins, Brown and Cunnane 31 ), or the results of the data handling method employed to examine the carry-over effect were poorly presented in another report( Reference Hernández-Alonso, Cañueto and Giardina 35 ). Two reports( Reference Njike, Ayettey and Petraro 28 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) had a high risk of other bias. In the study by Njike et al.( Reference Njike, Ayettey and Petraro 28 ), there were two possible sources of bias; a possible conflict of interest and a design-specific risk of bias. With respect to the latter, no evaluation was performed at the beginning of the second treatment period (9 months) after the 3-month wash-out period, which could not enable evaluation of carry-over effect( Reference Njike, Ayettey and Petraro 28 ). All studies except for one( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) used reliable methods for assessing adherence, namely measuring plasma levels of biochemical indices specific for a given type of nut or seed or evaluating dietary records obtained at regular intervals. However, adherence to the intervention with flaxseeds in the study by Javidi et al.( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) was only assessed by checking the content of the returned packages, which is a much less reliable method.
Discussion
The prediabetic state, a characteristic clinical feature of obesity, is a strong risk factor for T2D. The relative risk of annual T2D incidence ranged between 4·7 and 12·1 for subjects with IFG-defined prediabetes and subjects with both IFG and IGT, respectively( Reference Gerstein, Santaguida and Raina 4 ). While T2D is an irreversible clinical entity, the prediabetic state can be regressed by proper lifestyle changes and this regression is clearly associated with a lower risk for T2D( Reference Bodicoat, Khunti and Srinivasan 39 ). Taking into account that among adults with prediabetes, almost half of them (48·2 %) remained prediabetic( Reference Song, Qiu and Zhang 40 ), efficient as well as sustainable dietary strategies should be formulated for either the regression of prediabetes or the improvement of the biochemical and clinical status of the pre-diabetic patients.
Several dietary factors have been studied in relation to prediabetes and T2D incidence. Increased consumption of energy dense foods deprived of nutrients, total energy and carbohydrate intake, in particular, were linked with the increase in both body weight, the presence of prediabetes and T2D incidence( Reference Weisman, Fazli and Johns 41 , Reference Bagheri, Siassi and Koohdani 42 ). In contrast, healthy dietary patterns, characterised by high intakes of whole grains, vegetables, fruits, legumes, low-fat dairy products and nuts among other food groups, were inversely associated with the presence of prediabetes( Reference Bagheri, Siassi and Koohdani 42 ). Consumption of sugar-sweetened beverages, unprocessed red meat, processed meat and a high- v. a low-glycaemic load diet were directly associated with the risk of developing T2D, whereas consumption of yogurt, whole grains, nuts/seeds and dietary fibre intake was inversely associated with T2D risk( Reference Micha, Shulkin and Peñalvo 43 , Reference Schwingshackl, Hoffmann and Lampousi 44 ).
Consumption of nuts and seeds, together with dietary fibre intake, was found to be the least frequently studied dietary factors in relation to T2D( Reference Micha, Shulkin and Peñalvo 43 ). However, nuts and seeds could serve as attractive snacks for prediabetics for several reasons; they are tasty, relatively cheap and easily available in many forms and types. From a nutritional point of view, they contain slowly digestible carbohydrates and dietary fibres (3·3–12·5 g for nuts and 6·5–27·3 g for seeds)( 15 ) and they are a good source of unsaturated fatty acids and vegetable protein, making them a valuable means for lowering the glycaemic index of the diet( Reference Augustin, Kendall and Jenkins 45 ) thus exerting favourable effects on glycaemic control. Nuts, including peanuts, contain a high amount of good quality fat, 43·9–75·8 %, mostly MUFA and PUFA. Flaxseeds, pumpkin and sunflower seed kernels also contain high amount of fat, 42·2–51·5 %, mostly MUFA and PUFA. The SFA content ranges between 3·8 % and 16·1 % in nuts and 3·7 % and 8·5 % in seeds. Carbohydrates constitute about 12–30 % of the macronutrient content of nuts and 15–29 % of the macronutrient content of seeds( 15 ). Nuts and seeds have, therefore, a high total and unsaturated fat content compared with carbohydrate and SFA content, and could, thus exert favourable effects on glucose control taking into account a recent meta-analysis of randomised controlled feeding trials showing that substitution of carbohydrates with unsaturated fat improved markers of glucose control( Reference Imamura, Micha and Wu 46 ). Evidence also suggests that dietary fat quality is more important than dietary fat quantity in relation to T2D prevention, and a diet that emphasises fat from plant sources over animal sources is considered favourable for the prevention of diabetes( Reference Ley, Hamdy and Mohan 47 , Reference Salas-Salvadó, Martinez-González and Bulló 48 ). In addition, energy intake from SFA was positively associated with measures of IFG and IGT( Reference Guess, Perreault and Kerege 49 ), whereas a moderately high dietary intake of MUFA (10–15 % of total daily energy) and PUFA (4–5 % of total daily energy) was associated with a reduced risk of incident IFG and IGT( Reference Krishnan, Steffen and Paton 50 ). Finally, nut and seed consumption has been associated with increased dietary intake of several vitamins (e.g. vitamin E), macrominerals (e.g. Ca, Mg, K) and trace elements (e.g. Cu, Fe, Zn)( 15 , Reference O’Neil, Keast and Fulgoni 51 – Reference Arya, Salve and Chauhan 53 ). α-Tocopherol, total Mg and Zn intake, from both dietary and non-dietary (i.e. supplements) sources, are all inversely associated with metabolic impairments, defined as IFG, IGT, insulin resistance, or hyperinsulinaemia, as well as with incident T2D( Reference Savolainen, Lind and Bergström 54 – Reference Ranasinghe, Wathurapatha and Galappatthy 58 ).
Few intervention studies with nuts or seeds have been conducted among subjects with prediabetes even though evidence suggests beneficial effects of their nutrient content on glycaemic indices. One possible explanation for this inconsistency is that nuts and seeds are energy-dense foods, primarily due to their high fat content, and could thus induce weight gain when added to the habitual diet. However, evidence from several RCT does not support this cause and effect relationship( Reference Tan and Mattes 27 , Reference Vadivel, Kunyanga and Biesalski 59 – Reference Hull, Re and Chambers 63 ). In the present systematic review, no significant effect of nut or seed consumption on anthropometric indices could have been found since intervention, and controlled treatments were appropriately matched with respect to dietary energy intake. In the studies by Hernández-Alonso et al.( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ) and Wien et al.( Reference Wien, Bleich and Raghuwanshi 38 ), the pistachio diet and the almond diet were compared to an isoenergetic nut-free diet. The energy intake from other fatty foods, mostly olive oil substituted for the energy from pistachios during the nut-free diet period( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Cañueto and Giardina 35 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ), and the energy intake from foods in the meat and fat exchange lists substituted for the 20 % of total energy intake from almonds in the nut-free diet( Reference Wien, Bleich and Raghuwanshi 38 ). In the study by Njike et al.( Reference Njike, Ayettey and Petraro 28 ), consumption of whole walnuts with adjustment for habitual energy intake, or as part of an ad libitum diet, was compared with the habitual diet without walnuts. Participants were asked to reduce portion sizes and received advice to eliminate foods based on their baseline dietary assessment in order to maintain the habitual energy intake. No significant changes were observed among treatments with respect to anthropometric indices. Both RCT which investigated the effects of flaxseed consumption among adults with prediabetes provided participants with pre-weighed doses of ground flaxseed( Reference Hutchins, Brown and Cunnane 31 , Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ). Even though daily total energy and macronutrient intakes were higher during the flaxseed intervention periods compared with the flaxseed-free control period, there were not statistically significant differences across treatment periods( Reference Hutchins, Brown and Cunnane 31 ). Neither dietary intake nor anthropometric indices were evaluated in the study by Javidi et al.( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ) in order to calculate changes across treatment groups; however, participants in the flaxseed-free diet were asked to maintain their habitual diets and substitute each dose of ground flaxseed for the same amount of carbohydrate and fat in their habitual diet. Feeding trials in humans have also shown that nut consumption reduces postprandial feeling of hunger and desire to eat, whereas it increases satiety, which could also explain the lack of weight gain after nut/seed consumption despite their high energy content( Reference Mori, Considine and Mattes 25 , Reference Tan, Dhillon and Mattes 64 , Reference McArthur, Considine and Mattes 65 ). Apart from the potential regulation of postprandial appetite sensations, evidence suggests that reduced fat absorption is another reason for the lack of a weight-promoting effect following nut consumption. The physical properties of nuts, including structure and high fibre content, modify the bioaccessibility and bioavailability of the nutrients they contain( Reference Tan, Dhillon and Mattes 64 ). Thus, nuts may have a high fat content; however, the efficiency of fat absorption is reduced( Reference Carughi, Feeney and Kris-Etherton 66 , Reference Gonçalves de Oliveira, Rodrigues Ferreira Cruz and Mayumi Nakajima 67 ).
In the present systematic review, the effects of nut and seed consumption on cardiometabolic risk factors among adults with prediabetes could not be directly compared across the RCT included due to the small number of eligible studies, the heterogeneity in terms of design and intervention treatment, as well as the fact that not all studies evaluated the same outcomes. Given the primary aim of the present systematic review only the effects of nut and seed consumption on markers of glucose metabolism were consistently evaluated in all RCT included. In contrast, other cardiometabolic risk factors such as lipidaemic profile and blood pressure were evaluated based on the focus of each RCT. A promising improvement of glucose homeostasis (HOMA-IR, FPG and/or FPI) was shown for pistachios( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 ), almonds( Reference Wien, Bleich and Raghuwanshi 38 ) and flaxseeds( Reference Hutchins, Brown and Cunnane 31 ). The only walnut intervention study among subjects with prediabetes did not show any effect on a wide panel of cardiometabolic risk factors evaluated( Reference Njike, Ayettey and Petraro 28 ). The lipidaemic profile seems to be unaffected by the interventions described in the present review. A modest reduction of LDL-cholesterol was found only after the almond intervention( Reference Wien, Bleich and Raghuwanshi 38 ). Similar improvements in the lipidaemic profile have been previously found in T2D patients after the incorporation of almonds in their diet( Reference Lovejoy, Most and Lefevre 68 , Reference Li, Liu and Liu 69 ). Systolic blood pressure was improved after flaxseed consumption in the study by Javidi etal.( Reference Javidi, Mozaffari-Khosravi and Nadjarzadeh 34 ). Previously published results derived from dietary intervention studies with pistachios in subjects with the metabolic syndrome or T2D showed either no improvement or a modest improvement in the glycaemic and lipidaemic profile, as well as blood pressure( Reference Wang, Li and Liu 62 , Reference Sauder, McCrea and Ulbrecht 70 – Reference Sauder, McCrea and Ulbrecht 72 ). However, the study by Hernandez-Alonso et al. suggested that pistachios may have pleiotropic beneficial effects on subclinical inflammation and lipoprotein quality( Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 32 , Reference Hernández-Alonso, Salas-Salvadó and Baldrich-Mora 33 , Reference Hernández-Alonso, Giardina and Salas-Salvadó 36 ).
All articles were assessed for potential sources of bias with a useful tool that helped us take into consideration possible systematic errors which could interfere with the results reported in each article. In general, the risk of bias was low, thus the results reported herein could be reliable. Nevertheless, further investigation is warranted. Finally, we could not identify any studies about the effects of Brazil nuts, cashews, hazelnuts, macadamias, pecans, pine nuts, peanuts, sunflower seeds and pumpkin seeds on markers of glucose metabolism in adults with prediabetes. We shall, therefore, propose that the effects of these nuts and seeds on markers of glucose metabolism be investigated in adults with prediabetes through well-designed randomised controlled studies.
Strengths and limitations
The present systematic review aimed to integrate all the available information with respect to the effects of nut and seed consumption on biochemical, clinical and anthropometric indices among a specified target population group. This is the first known review which examined the effects of both nuts and seeds on markers of glucose metabolism in adults with prediabetes. We included only RCT, which are considered to be the ‘gold-standard’ method for investigating cause–effect relationships. The review was based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement, and the Cochrane Risk of Bias tool was used to assess the risk of bias. However, a limited number of studies pertained to the aims of the review, and the studies included in the review were not sufficiently homogeneous in terms of design and exposure, for example, different type of nuts and seeds consumed.
Conclusions
In the present systematic review of RCT evaluating the effects of nuts or seeds in adults with prediabetes, pistachios, almonds and flaxseeds improved markers of glucose metabolism. Anthropometric measures, blood pressure and blood lipid profile were not significantly affected by nut consumption, whereas systolic blood pressure was improved by flaxseed consumption. Pistachios may exert pleiotropic metabolic and immunological effects in adults with prediabetes. Taking into account the promising results of the few studies being conducted so far, as well as the need for simple, efficient and sustainable dietary strategies for the prevention of T2D, more and well-powered RCT are needed to ascertain whether nuts and seeds could be a healthy snack for adults with prediabetes.
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
A. N. and T. N. formulated the research question, designed the search strategy and conducted the research. A. N. performed the preliminary screening of identified records. A. N. and T. N. performed subsequent screening of the study selection and the eligibility assessment. A. N. carried out the data extraction and the risk of bias assessment and S. A. and T. N. verified the process. Disagreements at the stages of study selection and risk of bias assessment were resolved through discussion with a third reviewer, S. A. The paper was written by A. N., S. A. critically revised it and T. N. had primary responsibility for the final content. All authors read and approved the final version of the paper.
The authors declare that there are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519001338






