INTRODUCTION
Hepatitis E virus (HEV) is a RNA virus belonging to the genus Orthohepevirus A, which includes two recognised genotypes infecting only humans (HEV-1and HEV-2) and two genotypes infecting either humans or different animal species (HEV-3 and HEV-4) [Reference Hazards EPanel1]. In recent years, HEV has emerged as a threat to public health in developed countries. While the human-restricted HEV-1 and HEV-2 are often associated with outbreaks in developing countries where direct transmission via the faecal–oral route is prominent, HEV-3 and HEV-4 have a zoonotic potential, as they are found in both humans and animals [Reference Wilhelm2]. In Europe, most (sporadic) human HEV infections affect older men and are caused by HEV-3, which is widespread in swine herds [Reference Di Bartolo3, Reference Di Bartolo4], while HEV-4 is more prevalent in Asia [Reference Wilhelm2]. Yet, autochthonous HEV infections caused by HEV-4 in humans and pigs are being reported in several European countries [Reference Colson5–Reference Hakze-van der Honing7], including Italy [Reference Garbuglia8, Reference Monne9].
Although domestic pigs are the main reservoirs of HEV, viral RNA has also been detected in other animals, particularly wild boar and deer [Reference Ivanova10, Reference Di Bartolo11]. Accordingly, consumption of (undercooked/raw) meat and offal from these animals has been associated with human HEV infection [Reference Colson12–Reference Tei14], although the public health importance of this transmission route remains unclear [Reference Purcell and Emerson15, Reference Yugo and Meng16]. Several studies have highlighted that occupational exposure to animals, particularly swine, may play a role in HEV transmission in developed countries [Reference Galiana17–Reference De Schryver19]. Indeed, HEV infection in pigs is mostly asymptomatic and self-limiting, causing mild liver dysfunction with no macroscopic lesions [Reference Casas20]. Moreover, HEV may persist in manure, posing those in direct contact with infected animals or their living environments at risk of infection [Reference Yugo and Meng16].
While HEV is a growing public health concern in Europe, epidemiological data in swine and humans in Italy are scattered and heterogeneous with regard to populations, sample types, diagnostic methods and locations [Reference Di Bartolo3, Reference Monne9, Reference Costanzo21–Reference Masia23], making the magnitude of HEV infection difficult to determine. The aim of this study was to determine the seroprevalence of HEV in the domestic swine population of Northern Italy (where over 62% of Italy's swine population is located) and in the corresponding human population, seeking also to detect the circulating HEV strains. Additionally, we aimed to assess differences in the risk of HEV infection associated with occupational exposure to pigs, foreign travel, medical history, hunting activities and eating habits.
METHODS
Swine sampling
A three-stage sampling design was applied. The first stage determined the HEV seroprevalence in the commercial pig population of the Northern Italian regions of Veneto, Lombardy and Friuli-Venetia-Giulia (Fig. 1). The second stage determined the HEV detection rate in pig faeces at HEV-seropositive herds. The third stage determined the HEV detection rate in tissue samples from slaughtered pigs reared in herds where HEV was detected in faeces. For logistical reasons, these two last stages involved only the herds located in Veneto and Friuli-Venetia-Giulia. All sampling activities were performed during November 2011–April 2014.
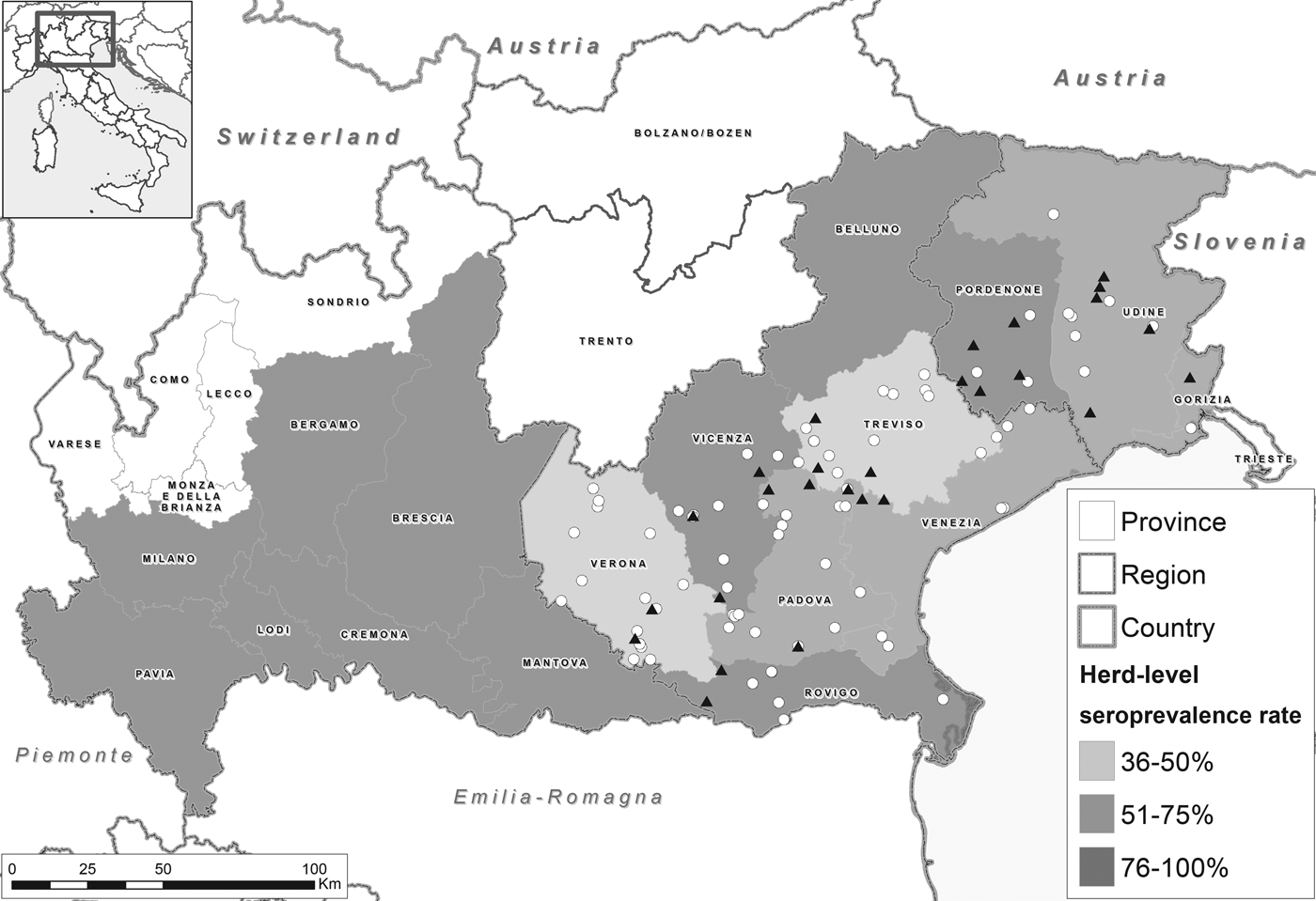
Fig. 1. Map of the study area showing the herd-level seroprevalence rate (anti-HEV IgG antibodies) in pigs per province. Dots indicate farms in which HEV RNA presence was investigated (triangle=positive farms, circles=negative farms).
Analysis of swine sera
The target pig population consisted of 4184 commercial cross-bred pig herds, i.e. breeding herds with ⩾5 animals and fattening herds with ⩾50 animals registered in the 23 provinces within the aforementioned three regions in 2010, when this study was set up. Sample size calculations based on an expected herd-level seroprevalence of 50%, 95% confidence level and 5% precision returned a total of 353 herds to be sampled. However, for logistical reasons, only 300 farms could be sampled; these were randomly selected in proportion to their underlying population by province and type of production (farrow-to-finish, farrow-to-feeder, fattening and weaning herds). Serum samples were collected within the framework of statutory surveillance activities for swine vesicular disease and Aujeszky's disease. From each farm, the sera of nine animals were randomly selected for HEV testing, corresponding to an expected within-farm seroprevalence of 30% [Reference Di Bartolo4], 95% confidence level and 5% precision. In total, 2700 individual serum samples were obtained (Table 1). Sera were tested for the presence of anti-HEV antibodies (IgG) using an in-house non-competitive indirect ELISA (97·5% sensitivity and 87·8% specificity) developed by the Istituto Zooprofilattico Sperimentale della Lombardia ed Emilia Romagna (IZSLER), according to manufacturer's instructions. Samples with S/P values >10 were considered positive, and negative if S/P values <10.
Table 1. Total number of farms and sera tested for HEV IgG antibodies and total number of farms and tissues analysed for HEV RNA presence
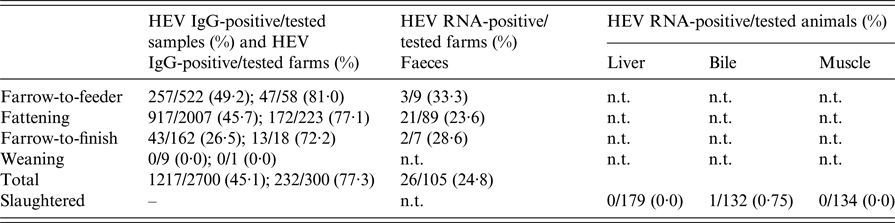
n.t., not tested.
Farms were subdivided for pig production categories.
Analysis of swine faeces
For HEV detection in swine faeces, besides sampling 70 (out of 232) HEV-seropositive herds, two (out of 68) HEV-seronegative herds were sampled, as they were epidemiologically linked to the HEV-seropositive ones. Moreover, faeces from a convenience sample of 33 pig herds whose HEV serological situation was unknown were also tested. From each herd, up to 10 pools of faeces from 10 different pens were collected. As the likelihood of detecting HEV in faeces is higher in pigs of 80–120 days of age [Reference Di Bartolo3], faecal sampling focused on this age group. In total, 959 faecal pools were collected (Table 1) and analysed by real-time reverse transcription polymerase chain reaction (RT-PCR) targeting a 70 bp fragment of the open reading frame 3 (ORF3) region as previously described [Reference Monne9]; positive samples were also confirmed by nested RT-PCR amplifying a 458 bp fragment of the ORF2 encoding the constitutive protein of the capsid.
Analysis of swine tissues
Presence of viral RNA was investigated in diaphragmatic muscle, liver and bile samples collected at slaughterhouse from pigs originating from four herds with HEV-positive faeces. In total, 179 animals were tested on at least one of these three tissues (Table 1); 177 of these animals were slaughtered at 9 months of age, whereas two animals were slaughtered at 5 and 6 months of age for the production of traditional Italian ‘porchetta’ (seasoned and slow-roasted whole pig) to be cooked in smaller pits. All muscle/liver samples were analysed as described previously [Reference Di Bartolo4, Reference Monne9], whereas a pre-treatment step was applied to bile samples before RNA extraction by diluting them 1:10 in sterile phosphate-buffered saline to reduce potential inhibitory activity in RT-PCR. All extracted RNAs were further processed as reported elsewhere [Reference Monne9].
Immunohistochemical testing was also performed on a total of 72 liver samples (from three different farms) fixed in 10% buffered formalin and embedded in paraffin; slide staining was performed using the automated immunostainer Benchmark Ultra (Ventana, Roche). Tissue sections of 3 µm underwent proteolytic antigen retrieval by incubation with Protease 2 (Roche) at 36 °C for 12 min, and then were incubated with a casein solution (Antibody Diluent with Casein, Roche) at 36 °C for 12 min to block non-specific sites. Sections were incubated for 40 min at room temperature with 1:50-diluted anti-HEV polyclonal primary antibody (Abbiotec), which recognises several putative HEV proteins including protein ORF3, the immunogenic protein from the viral capsid and structural proteins. Finally, the sections were incubated with casein solution at 36 °C for 12 min and processed with the chromogenic detection kit ultraView Universal DAB Detection Kit (Ventana, Roche) according to the manufacturer's instructions. Negative control sections were included in each run by replacing the primary antibody with the buffer to exclude the presence of non-specific reactions with the reagents used.
Human sampling
In parallel with swine faecal sampling, a cohort study of HEV in swine workers was performed. Swine workers in the sampled farms were asked to provide a serum sample for HEV serological testing along with a questionnaire covering basic information on demographics, eating habits, hunting activities, previously experienced hepatitis symptoms and travel abroad (Table 2). For comparison purposes, two groups of people non-occupationally exposed to swine were sampled from the general population: (i) people following an omnivorous diet, and (ii) people following a vegetarian/vegan diet. The number of subjects to be recruited in these groups was such to guarantee the identification of a statistically significant difference (α = 0·05) in the risk of being HEV-seropositive with a confidence level of 95% and a power of 80%; a minimum of 100 subjects per group were then to be sampled.
Table 2. Human HEV seropositivity rates and risk ratios for the variables assessed for association with HEV seropositivity in the overall binomial regression analysis
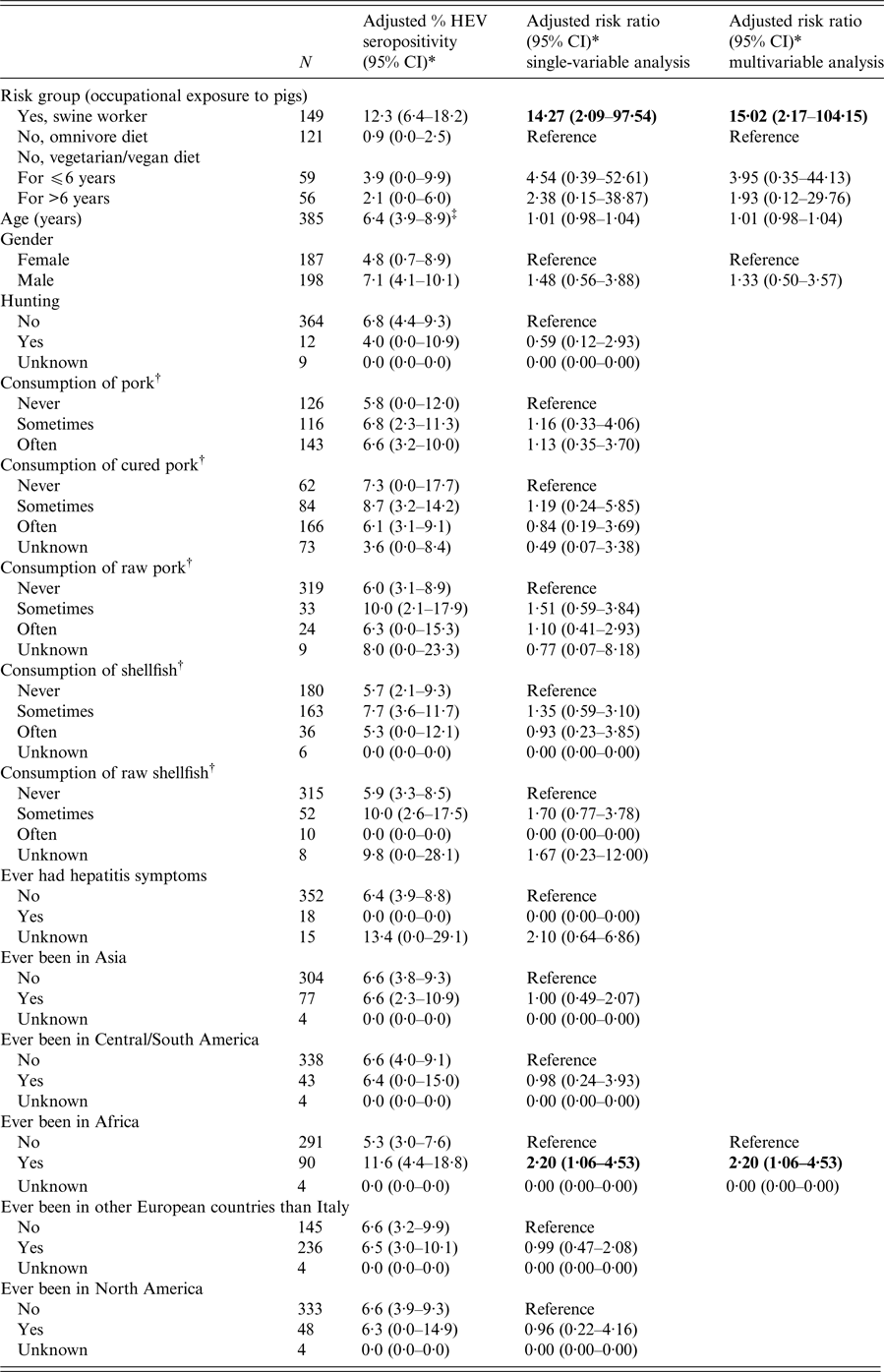
95% CI, 95% confidence interval. Statistically significant risk ratios are highlighted in bold.
* Adjusted for age (continuous variable expressed in years), gender, risk group (occupational exposure to pigs), except for the eponymous variables and clustering of swine workers at the farm level.
† Risk group (occupational exposure to pigs) excluded from the model because of collinearity with this variable.
‡ Estimated at the overall average age of participants (42 years).
The omnivores were recruited from the general population of Veneto region via an online recruitment campaign. The same was done to recruit individuals following a vegetarian/vegan diet, with the online recruitment campaign targeting local vegetarian/vegan blogs and websites. Like the swine workers, these participants provided a serum sample and completed the aforementioned questionnaire. Participants were informed about the objective and the methods of the study, which was approved by the Ethical Committee of the Padua's University Hospital, and were enrolled on a voluntary basis, with no financial incentive being given; informed written consent was obtained from all participants.
The three groups were mutually exclusive. In total, 149 subjects were enrolled in the group of swine workers (median age 43 years, range 16–74; 85% males), 121 in the group of omnivores (median age 43 years, range 20–85; 38% males) and 115 in the group of vegetarians/vegans (median age 39 years, range 19–73 years; 23% males). Serum samples were taken at the Outpatient Service of Microbiology and Virology of Padua's University Hospital or directly on farm upon visit of a specialised nurse. After collection, serum samples were refrigerated at 4 °C until arrival at the laboratory and stored in aliquots at −20 °C until testing for anti-HEV IgG antibody detection using the commercial Wantai HEV-IgG ELISA kit (Beijing Wantai Biological Pharmacy Enterprise, China), according to the manufacturer's recommendations.
Data analysis
A ‘design-based’ analysis was performed to account for the multilevel serosurvey design for pigs, including the province and type of production as strata, the herds as clusters (principal sampling units) and weighting adjustment for the corresponding population from which the sample was drawn.
For humans, seropositivity rates were calculated for the three groups of participants under study, and their differences were tested for significance using binomial regression including cluster-robust standard errors to account for clustering of swine workers at the farm level; estimates were always adjusted for age and gender. This approach was also used to assess factors associated with HEV seropositivity over the three groups of participants, as well as in each group of participants. Variables were first assessed univariately and those showing a P < 0·20 for the association with the outcome were included in a multivariate model built in backward stepwise fashion. Non-significant (P > 0·05) variables were dropped one-by-one from the multivariate models after having evaluated the significance of each partial effect. Associations were expressed as adjusted risk ratios (RR) providing 95% confidence intervals (95% CI). Statistical analysis was performed using STATA 13 (StataCorp, College Station, Texas, USA).
RESULTS
HEV seroprevalence in pigs
In total, 232/300 (77·7%) farms had at least one HEV-positive serum sample (Table 1). Adjusting for the serosurvey design resulted in a farm-level seroprevalence of 75·6% (95% CI 70·3–80·2%) (Fig. 1). This was highest in farrow-to-feeder farms (81·6%, 95% CI 69·1–89·8%, n = 58 farms), followed by fattening (75·5%, 95% CI 69·5–80·6%, n = 223), farrow-to-finish (68·0%, 95% CI 41·0–86·7%, n = 18) and weaning farms (0·0%, 95% CI 0·0–97·5%, n = 1). Excluding the one weaning farm sampled, farm-level seroprevalence did not differ significantly among the types of farms (χ 2-test, P = 0·4806).
With a total of 1217/2700 (45·1%) HEV-positive serum samples, the adjusted pig-level seroprevalence was estimated at 43·1% (95% CI 39·3–47·0%). Seroprevalence was highest in farrow-to-feeder farms (47·7%, 95% CI 39·6–56·0%, n = 522 sera), followed by fattening (44·0%, 95% CI 39·5–48·6%, n = 2007), farrow-to-finish (23·1%, 95% CI 13·8–36·1%, n = 162) and weaning farms (0·0%, 95% CI 0·0–33·6%, n = 9). Excluding the sera from the weaning farm, the pig-level seroprevalence differed significantly among the types of farms (P = 0·0109). Specifically, seroprevalence in pigs of farrow-to-feeder farms differed from that of pigs in fattening (P = 0·0032) and farrow-to-finish farms (P = 0·0028), but the seroprevalence of the pigs housed in these two latter types of farms did not differ significantly with one another (P = 0·4405). Undersampling of farms as mentioned in the methods had no consequences given the higher observed than expected prevalence.
HEV detection in pig faeces and tissues
In total, 26/105 (24·8%) farms had at least one faecal sample positive for HEV (Table 1), of these 25/26 belonged to HEV-3 and one to HEV-4, as reported previously [Reference Monne9]. The latter genotype was detected in a farm in which HEV-3 was detected as well. All liver (n = 179) and diaphragmatic muscle (n = 134) samples tested negative, only 1/132 bile sample tested positive. This sample was taken from a 5-month-old animal whose muscle and liver sample tested negative for HEV. All immunohistochemical analyses tested negative.
HEV seropositivity in humans
Anti-HEV IgG antibodies were detected in 14·1% (21/149) of swine workers, 0·8% (1/121) of omnivores and 2·6% (3/115) of vegetarians/vegans. Seropositivity rates adjusted for age and gender were as follows: swine workers 12·3% (95% CI 6·4–18·2%), omnivores 0·9% (0·0–2·5%) and vegetarians/vegans 3·0% (0·0–6·6%). While adjusting for age and gender, seropositivity in swine workers was significantly higher than that of the omnivores (P = 0·007) and vegetarians/vegans (P = 0·041), but these two groups were not significantly different from each other (P = 0·291).
In the overall risk factor analysis (Table 2), the only factors significantly associated with HEV seropositivity was occupational exposure to pigs (swine workers vs. omnivorous population: RR 15·02, 95% CI 2·17–104·15, P = 0·006) and having travelled to Africa (been in Africa once or more times vs. never been in Africa: RR 2·20, 95% CI 1·06–4·53, P = 0·033). Given the limited number of HEV positivities in the groups of omnivores (#1) and vegetarians/vegans (#3), the group-specific risk factor analysis was performed only for the swine workers. In this group, only age (continuous variable expressed in years) was significantly associated with HEV seropositivity (for every 1-year increase in age: RR 1·03, 95% CI 1·01–1·06, P = 0·007).
DISCUSSION
This study was conducted to determine the seroprevalence and detection rate of HEV in commercial swine herds in Italy's utmost pig-rich area and to assess the risk for humans to be HEV-seropositive as a function of several factors, including occupational exposure to pigs. Previous Italian studies were limited by the convenience sampling of only a few swine herds [Reference Costanzo21, Reference Masia23, Reference Berto24]. The present study overcame this issue using a structured sampling scheme representative of the underlying swine population. Moreover, a complete picture was provided by looking at HEV serological evidence in humans as well.
Results indicated that HEV is widespread in Italian swine herds, supporting previous findings in Italy [Reference Berto24–Reference Ponterio26] and other European countries [Reference Berto24]. For instance, a study in the United Kingdom reports a pig-level seroprevalence of 93% (n = 629) in 6-month-old pigs [Reference Grierson27]. Other studies report a herd-level seroprevalence of 80% in Spain (n = 85) [Reference Jimenez de Oya28] and 65% (n = 186) in France [Reference Rose29], and a pig-level seroprevalence of 62% (n = 380) in Estonia [Reference Ivanova10] and 61% (n = 108) in Scotland [Reference Crossan30]. We also found farrow-to-feeder herds to have the highest seroprevalence, followed by fattening, farrow-to-finish and weaning herds, possibly reflecting the primary productive/age groups represented. For instance, in farrow-to-feeder farms, which are open-cycle herds with sows producing piglets that are sold at 24–28 days for fattening elsewhere, only sows (which usually show the highest HEV seropositivity) were sampled conforming to statutory surveillance activities. In fattening herds, where there are pigs of different ages (usually from 24–28 up to 280 days), some of which would have already seroconverted and some would have not, we found an intermediate seroprevalence. Piglets younger than 60 days are not sampled for swine vesicular disease and more in general they were not included in our study due to maternal immunity. Farrow-to-finish herds, being closed-cycle herds, should introduce new animal less frequently than the others, thereby limiting the introduction of infections; this could explain the lowest HEV seropositivity rates therein. However, a limitation of this study was the lack of information on other factors that may have also played a role in determining the observed seropositivity rates, e.g. type of farm management, infrastructural characteristics of the premises themselves, biosecurity measures implemented, etc. These factors may vary from farm to farm and might not be necessarily associated with the type of farm itself.
Failure to detect HEV in tissues may be due to the age of the pigs slaughtered, as all but two animals were destined to cured ham production and were therefore slaughtered at 9 months of age, and the only positive sample (from bile) was collected from a 5-month-old pigs. This is somewhat reassuring with regard to foodborne transmission of HEV from cured pig products, of which Italy is a big producer and consumer, as also evidenced by other studies [Reference Di Bartolo4].
Genetic analyses confirmed the wide presence of HEV-3 and the co-circulation of HEV-4 among pigs in Italy. For more detailed information on the genetic similarities of the HEV-4 detected here, we refer to the previous publication dedicated to this finding [Reference Monne9]. HEV-4, which is typical of the Asian continent, is believed to have just recently been introduced in Europe [Reference Hakze-van der Honing7, Reference Monne9]. Given the high pathogenicity of this genotype, more focused studies are recommended to better understand how and to which extent this genotype has spread across Europe. We also found that occupational contact with pigs was associated with seropositivity to HEV in humans. HEV-3 and HEV-4 circulating in Europe have a high level of nucleotide identity between swine and human strains [Reference Di Bartolo4], and a recent systematic review and meta-analysis of 12 cross-sectional studies in which HEV seroprevalence (IgG) was compared between people with and without occupational contact with swine has identified a significant association between occupational exposure to swine and seropositivity to HEV [Reference De Schryver19]. However, the high heterogeneity over the studies (due to, e.g. variations in population susceptibility, test performance, etc.) precluded the calculation of a pooled measure of association. Although this heterogeneity makes also the direct comparison of seropositivity rates among studies rather inappropriate, it is worth reporting that our seropositivity rate of 14·1% among swine workers lays within the range of the (significantly higher) seropositivity rates among people occupationally exposed to pigs reported in the literature, i.e. from 11% to 76% [Reference De Schryver19]. Our finding therefore adds to the growing body of evidence that direct contact with pigs is a risk factor for human HEV infection. In the absence of an effective vaccine against HEV, prevention for swine workers, including farmers, butchers and veterinarians, can only rely on the implementation of hygiene and individual protection. Yet, more targeted interventions might be planned in the future once an assessment of the working conditions leading to higher risk of HEV infection among swine workers will be performed. As regard to travel to Africa as a risk factor for HEV positivity, a recent comprehensive review has showed that HEV has spread into the human populations of at least 28 of the 56 African countries, with the continent as a whole being among the most severely affected parts in the world [Reference Kim31].
We found no significant effects of diet on HEV seropositivity, as the rate among the omnivores did not differ significantly from that of vegetarians/vegans, even when accounting for how long the vegetarians/vegans did not eat meat. Moreover, consuming specific ‘risky’ food items like pork or shellfish, either raw or cooked, was not significantly associated with HEV seropositivity in this study. Lack of significant differences in HEV seropositivity between meat consumers and vegetarians have been reported previously in the USA [Reference Ditah32], but in contrast to hepatitis E in developing countries, sporadic cases in developed countries have mainly been associated with pork consumption, particularly raw/undercooked offal [Reference Miyamura33]. However, it has been pointed out that it would not be completely fair to attribute the high seropositivity to HEV in developed countries to pork consumption alone, as despite some indications that this might sometimes be relevant [Reference Wichmann6], raw/undercooked swine offal consumption remains infrequent and cannot explain the increasing HEV seroprevalence in developed countries [Reference Teshale, Hu and Holmberg34]. A recent French study [Reference Mansuy35] involving 10 569 blood donors found an overall IgG prevalence for HEV of 22·4%, with an increased risk of HEV IgG positivity among those eating pork meat, pork liver sausages, game meat, offal and oysters, whereas drinking bottled water was associated with a lower prevalence of anti-HEV IgGs. Yet, these authors concluded that eating habits alone cannot fully explain the exposure to HEV, and that contaminated water may also play a role in HEV transmission [Reference Mansuy35].
Available data on HEV seropositivity in Italy are limited to Southern regions and suggest that 1·3–2·9% of people without hepatitis are HEV-seropositive [Reference Scotto36], although a retrospective follow-up study (1978–1991) on acute nonA–nonB hepatitis cases at a single referral centre in Northern Italy showed autochthonous cases of acute HEV infections since the 1980s [Reference Stroffolini37]. A recent Italian study on seropositivity to HEV among mainly young adults living in the city of Rome who underwent human immunodeficiency virus testing, showed an overall HEV seropositivity of 5·4% and a significant association with male homosexual intercourses, suggesting that besides the oro-faecal and zoonotic transmission, certain sexual practices may also contribute to HEV transmission [Reference Lanini22], as well as blood transfusions and solid organ transplants [Reference Lhomme38].
In conclusion, HEV is widespread in commercial swine herds in Northern Italy, where most of Italy's swine population is located. The circulation of HEV-4, together with the predominant HEV-3, in these swine herds is a cause for concern, as HEV-4 is known to cause more severe illness in humans [Reference Hakze-van der Honing7]. Moreover, occupational exposure to pigs stood out as a significant risk factor for HEV seropositivity in humans. Altogether, these findings support current evidence indicating that swine is the most likely source of HEV infection in Italy.
ACKNOWLEDGEMENTS
The authors are grateful to Giovanni Loris Alborali for his support in data collection. This study was supported by the IZSVe research programme RC20/2010 funded by the Italian Ministry of Health.
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The part of this study involving human subjects received ethical approval from the Ethical Committee of the Padua's University Hospital (Ethical Approval Number 307/AO/14). Participants were enrolled on a voluntary basis, with no financial incentive being given. Informed written consent was obtained from all participants. Swine data were generated from statutory veterinary public health surveillance activities for swine vesicular disease and Aujeszky's disease in Italy and the EU, so ethical approval was not required.






