INTRODUCTION
Asbestos-exposed workers have shown increased all-cause mortality, and mortality from malignant neoplasms, especially from cancers of the stomach, lung, peritoneum and pleura, from mesothelioma as well as from cerebrovascular disease and asbestosis [Reference Harding1]. It is well known that asbestos exposure causes lung and pleural fibrosis, and lungs that are debilitated due to occupational dust may be vulnerable to infections. Death from asbestosis or lung cancer in asbestos-exposed workers may be compensated when resulting from occupational exposure; asbestos-exposed workers might also die of pneumonia, yet it is unknown whether these workers are at increased risk of death from pneumonia because of their previous occupational exposure.
The incidence of pneumonia and its mortality increases with age in adults [Reference Ewig2]. Other factors associated with risk for pneumonia mortality include body mass index, smoking, alcohol consumption, comorbidities and physical exercise [Reference Inoue3]. Pulmonary comorbidities other than chronic obstructive pulmonary disease (COPD) were associated with a high mortality of 24% for hospitalized pneumonia in a large nationwide German study [Reference Ewig2], although we found no data on pneumonia mortality in asbestos-exposed subjects.
We conducted a follow-up study on asbestos-exposed workers previously screened for lung cancer and thereafter classified for fibrotic pleuropulmonary signs using high-resolution computed tomography (HRCT) to examine whether the risk for death from pneumonia is dependent on lung fibrosis.
METHODS
Study population
Data from an earlier study on asbestos-exposed construction workers previously screened for lung cancer by HRCT in 1996–1997 [Reference Tiitola4] were used for this follow-up study. These screened subjects were originally chosen utilizing previous studies and trade union registers. Invitations for screening were submitted to asbestos-exposed workers living in the Helsinki area (n=642), and 602 individuals agreed to participate. Of these there were 85 subjects with asbestosis diagnoses and 601 with bilateral pleural plaques. Current or previous smoking (⩾10 pack-years) was an inclusion criterion for patients without an asbestosis diagnosis, because a high lung cancer risk was pursued at the primary study. As well as a HRCT examination, subjects underwent a health check and answered a questionnaire.
In this follow-up study, sufficient data were available for 590 (580 men, 10 women) of those screened. The mean age of subjects at the time of the HRCT study was 63 years (range 38–81 years), the mean duration of asbestos exposure was 26 years (range 2–48 years) and the latency time from the first exposure to HRCT imaging was 41 years (range 8–61 years). The cohort consisted of 18 never smokers, 407 ex-smokers, 163 current smokers, two persons with no smoking category, and three with no pack-years available. Those with data available had smoked on average 24 pack-years (range 0–88). Table 1 shows the descriptive statistics of the cohort.
Table 1. Descriptive statistics of the cohort at the baseline situation
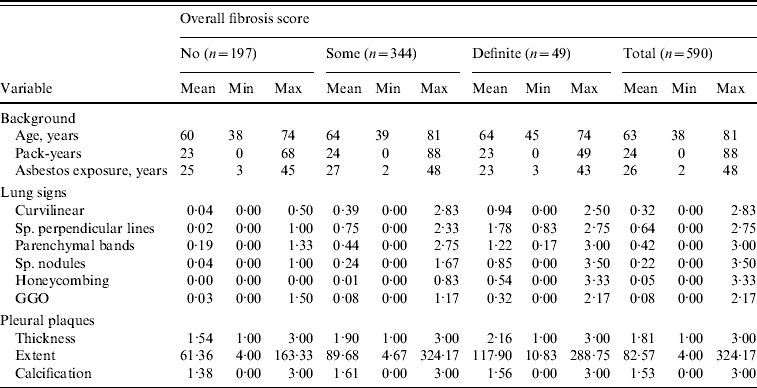
Sp., Sub-pleural; GGO, ground glass opacity.
All patients gave their written informed consent at the time of the primary study and this study plan was accepted by the Ethical Committee of The Finnish Institute of Occupational Health.
Imaging and image interpretation
All patients underwent a chest spiral CT/HRCT (Picker PQ 2000 scanner, Picker International, USA) in supine position with full inspiration. All patients were first imaged supine by single-slice spiral mode at full inspiration. Thereafter, the first subjects (n ~20) were imaged prone with four thin-section cuts (1·5 mm slices, 130 kV, 200 mA, algorithm: sharp) equally spaced from the tracheal bifurcation to the lung bases. The rest of the subjects were imaged with seven such cuts from the aortic arch to the lung bases. All images were printed as hard copies and inspected on light-view boxes. Fibrotic lung signs were assessed independently and bilaterally by three experienced radiologists (0=normal, 5=most advanced finding) observing closely for specific HRCT signs [Reference Huuskonen5]:
-
• subpleural curvilinear opacities (score 0–5),
-
• subpleural septal lines (score 0–5),
-
• parenchymal bands (score 0–5),
-
• subpleural nodules (score 0–5),
-
• honeycombing (score 0–5),
-
• ground glass opacities (score 0–5).
By considering these signs they assessed the overall fibrosis score: 0=normal finding, 1=subclinical mild fibrotic changes, 2=mild fibrosis, 3=moderate fibrosis, 4=severe fibrosis and 5=extreme fibrosis. The average scores of the radiologists were used for statistical analysis and were also trichotomized as ‘no fibrosis’ (average score <0·5, n=197), ‘some fibrosis’ (average score 0·5–1·99, n=344) and ‘definite fibrosis’ (average score ⩾2, n=49). In Finland a fibrosis score ⩾2 is considered sufficient for an asbestosis diagnosis in cases with adequate work history. ‘Some fibrosis’ therefore includes everyone with recognizable fibrosis but without changes sufficient for a diagnosis of asbestosis.
Pleura were similarly scored [Reference Tiitola6]:
-
• slice-by-slice estimated total plaque extent (cm2),
-
• maximum thickness of plaques (1, <5 mm; 2, 5–10 mm; 3, >10 mm),
-
• calcification score: no, slight, considerable, abundant (0–3).
Follow-up
Permission for the follow-up study was given by the Ethics Board of The Finnish Institute of Occupational Health and by Statistics Finland. We drew up the patient register report required by national legislation. No additional ethics committee permission was required at this stage. Mortality dates and the causes of death according to the International Classification of Diseases (ICD-10 by the World Health Organization) were checked in the National Register of Causes of Death (Statistics Finland). This register receives data from death certificates, is nationwide and covers the whole population (quality description: http://www.stat.fi/til/ksyyt/laa_en.html). We paid close attention for codes J10–J18 (influenza and pneumonia), i.e. pneumonias from bacterial or viral aetiology. Patients were linked to register data by using their unique identification codes. The follow-up comprehensively covered deaths between the baseline examination and the end of 2007, about 94% of deaths in 2008, and less comprehensively covered deaths up to 13 April 2009. The lack of complete follow-up in deaths is due to the time lag between the reporting and recording of deaths in the statistical database. The mean follow-up time was 10·44 years (range 0·25–12·98 years), with a total of 6158 person-years of follow-up.
Statistical methods
Cox regression was used to study the association between interstitial lung fibrosis and deaths from pneumonia. Results were adjusted for age and pack-years of smoking. Missing data on pack-years (n=3) were replaced with the group mean values. Both the overall (trichotomized) fibrosis score and the separate lung and pleural signs were studied as risk factors for pneumonia mortality, each in its own model. Cox regression, adjusted for age and pack-years smoked, was used. P values <0·05 were considered significant. We used SPSS 17.0 software (SPSS Inc., USA).
RESULTS
During the follow-up period, 191 deaths occurred in the 590 study subjects. A total of 43/590 subjects (7·3%) died of pneumonia (Fig. 1), which thus contributed to 22·5% (43/191) of all deaths. Pneu-monia was classified as the underlying cause of death in two cases and as the immediate cause of death in the other 41 cases, with some other disease as the underlying cause of death: seven cardiovascular; 16 cancer, of which nine were lung cancers; eight respiratory, of which five were asbestoses, and three were COPD; and 10 deaths for other reasons. There were 7/197 (3·6%) deaths from pneumonia in those with no fibrosis, 30/344 (8·7%) in those with some fibrosis and 6/49 (12·2%) in those with definite fibrosis.
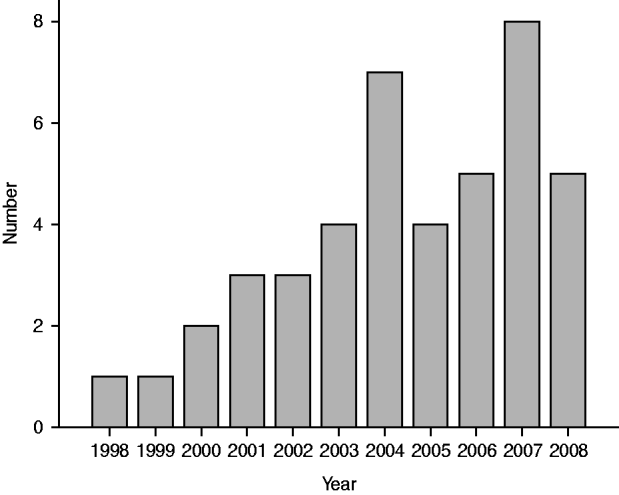
Fig. 1. Number of reported pneumonia deaths by year.
In addition to a positive association with increasing age, the risk of death from pneumonia due to some interstitial fibrosis (HR 2·26, 95% CI 0·98–5·19, P=0·06) and definite interstitial fibrosis (HR 3·70, 95% CI 1·22–11·23, P=0·02) was higher than that of the group with no fibrosis (Table 2). The exposure variable had no significant effect on the outcome.
Table 2. Risk of pneumonia mortality associated with interstitial lung fibrosis (Cox regression). Age and pack-years of smoking forced into the model
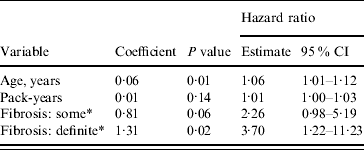
CI, Confidence interval.
* Compared to the ‘Fibrosis: no’ category.
When the effects of separate lung and pleural signs were studied, subpleural perpendicular lines (P=0·01), parenchymal bands (P=0·03), subpleural nodules (P=0·005), honeycombing (P=0·005) and the extent of pleural plaques (P=0·02) emerged as significant predictors for pneumonia mortality (Table 3).
Table 3. Risk of pneumonia mortality associated with separate lung and pleural CT signs. Several fibrotic signs were included as candidates in the forward selection regression model (hazard ratio/classification score)
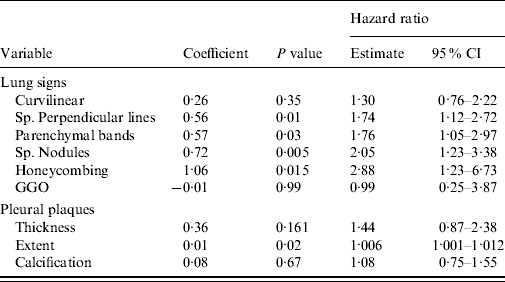
CI, Confidence interval; Sp., sub-pleural; GGO, ground glass opacity.
DISCUSSION
We found a clear association between pleuropulmonary fibrosis and pneumonia mortality. Pre-vious studies on the relation between lung CT signs and overall mortality were mainly restricted to other fibrotic lung diseases. Of the patients with usual interstitial pneumonia or non-specific interstitial pneumonia, a high fibrotic score (the extent of reticulation plus honeycombing) was associated with increased overall death risk [Reference Shin7]. Lung fibrosis at thin-section CT predicted death in patients with interstitial lung fibrosis [Reference Best8–Reference Markowitz11]. The 10-year risk of death due to asbestosis rose sharply with increasing interstitial fibrosis, as identified in the baseline chest X-ray [Reference Markowitz11].
Pneumonia and influenza are ranked eighth as a cause of death in the USA [Reference File and Marrie12]. In Spain, the incidence of community-acquired pneumonia in subjects aged ⩾14 years was 1·62 cases/1000 person-years with a reported overall mortality of 5% [Reference Almirall13]. Of those aged ⩾65 years, the corresponding numbers were 14/1000 person-years with a reported 30-day case-fatality of 12·7% [Reference Vila-Corcoles14]. Pneumonia has been associated with pre-existing lung disease. Pre-existing lung disease was a risk factor for pneumonia in the elderly, occurring in 13·0% of cases vs. 3·8% of controls [Reference Koivula, Sten and Mäkelä15]. The same held true in a German study where chronic pulmonary disease was associated with pneumonia [Reference Schnoor16]. The outcome from pneumococcal pneumonia in tertiary medical care was worse in those with a pre-existing lung disease (relative risk 2·0) [Reference Marfin17].
We are not aware of studies comparing pneumonia rates between asbestos-exposed and non-exposed populations. Increased markers of pulmonary inflammation were found in asbestos-exposed subjects in Finnish studies, indicating a fibrosing inflammatory process in the lungs [Reference Lehtonen18, Reference Lehtimäki19]. The increased risk of death from pneumonia in those with lung fibrosis may be due to an increased risk of acquiring pneumonia, a poorer prognosis when diseased, or both.
The effect of pleural plaques on pneumonia mortality, although statistically significant, was minor in this study. We were unable to find any previous literature reports on such an association or its possible mechanisms. The risk of dying from pneumonia and of developing pleural plaques may share a common mediator. Asbestos-related disease progression may occur in both the pleura and the lungs. Pleural plaques might thus be an indicator of lung fibrosis, which itself is associated with an increased risk of death from pneumonia. This was true to some extent in our study, because adjusting for the overall fibrosis score diluted the effect of this pleural variable.
Fibrotic lung and pleural HRCT changes present in this cohort have been reported as cross-sectional papers [Reference Huuskonen5, Reference Tiitola6], and thus the methodological discussions are not repeated here in detail. Regarding fibrotic signs, intraclass correlations between observers varied between 0·20 and 0·64 and within an observer between 0·23 and 0·80. All used scores were average values of three observers. The fact that about the first 20 patients were imaged with fewer slices might have caused a minor non-differential misclassification, which is not likely to affect the main results of this study. We were able to follow up the whole cohort, but the status (dead/alive) of some single individuals in 2009 remains uncertain. They may number a few at the most and this is unlikely to affect the overall results of this study in any significant manner. No pneumonia deaths were reported that year. We did not use a previous asbestos diagnosis as a grouping criterion in the analyses, because such old clinical diagnoses may lack exact standards. Our subjects were all exposed to asbestos, which may dilute some of the effects found. Therefore, the risks found in this study are likely to be underestimates. We did not try to quantify the exposure any further than adding up the years of exposure, because the workers had several lifetime tasks and exposures, most of which are difficult to evaluate exactly. On the contrary, the study sample was rather homogeneous, consisting of workers with no major difference in social class or ethnicity. Those exposed to asbestos and suffering from lung fibrosis are blue-collar workers with mostly suboptimal healthy behaviour [Reference Pekkanen20]. Smoking itself is an independent risk factor for pneumonia [Reference Huttunen, Heikkinen and Syrjänen21], as is increasing age. In our material smoking did not significantly increase the risk of dying from pneumonia. This is most likely due to a bias in the selection of the material: smoking was an inclusion criterion only for those without a previous asbestosis diagnosis. Smokers' lungs were therefore probably in some respect free of disease. Due to the ban of asbestos use, those exposed are now elderly people, as in this study. These current or former workers are therefore especially vulnerable to pneumonia, and they should be vaccinated against influenza [22] and Pneumococcus [Reference Willems23].
In conclusion, pleuropulmonary fibrotic changes were found to be a risk factor for death from pneumonia in asbestos-exposed workers.
DECLARATION OF INTEREST
None.






