Introduction
The hand–foot–mouth disease (HFMD) is an acute communicable disease, mostly affecting children under 5 years of age [Reference Zhao1]. It is mainly caused by human enterovirus 71 (EV-A71) and coxsackievirus A16 (CV-A16) [Reference Zhang and Xu2]. The main manifestations are fever, maculopapular rashes or blisters on the hands, soles of feet and buttocks, and ulcers in the oral mucosa. HFMD is transmitted by close contact with virus-contaminated hands, toys, open and weeping skin vesicles or exposure to a contaminated environment [Reference Xie3]. HFMD is generally self-limiting, and patients with no secondary cutaneous infection mostly recover in 2 weeks.
In recent years, several widespread outbreaks of HFMD in China [Reference Zhu4], Singapore [Reference Ang5] and other Western Pacific Regions [Reference Ryu6, Reference Van Tu7], involving millions of children, have become a big threat to public health and a substantial economic burden. In March 2008, the largest pandemic in Asia occurred in China, causing more than 20 000 cases and dozens of deaths. Then, HFMD was defined as a C-class notifiable disease in Mainland China on 2 May 2008. The reported mortality rate of HFMD was 0.03/100 000 during 2008–2017 in China [Reference Zhang8]. Moreover, some patients with neurologic and cardiorespiratory complications, delayed manifestations and poor sanitary conditions were more likely to die [Reference Xu9]. Numerous studies reported that EV-A71, coma and seizures were responsible for HFMD deaths [Reference Zhao10, Reference Xing11]. However, the risk factors for HFMD deaths have still not been completely identified. Thus, this study aimed to quantitatively assess all these qualified studies and identify the risk factors for HFMD deaths.
Materials and methods
Search strategy
A systematic search of the databases PubMed, Medline, Embase, the Cochrane Library, China National Knowledge Infrastructure and Wanfang (Chinese) were performed for relevant studies published before May 2019 using the key words ‘hand foot mouth disease OR HFMD’, ‘death OR fatal OR fatality OR mortality’ and ‘risk factors’. The reference lists of all retrieved studies were screened and checked for potential additional studies. No language restrictions were applied.
Selection criteria and exclusion criteria
The inclusion criteria were as follows: (1) All patients included had HFMD according to the Guidelines on the Diagnosis and Treatment of HFMD [Reference MoHotPsRo12]; (2) the study had a case–control design including death and survival groups; (3) the studies investigated the association between risk factors and death of HFMD; and (4) the survival data were extracted from the primary studies. Reviews, case reports and original articles without fatal outcomes or with repeated samples were excluded.
Data extraction and quality assessment
Data were obtained from each eligible study independently by two reviewers. Disagreements were discussed between the reviewers until consensus was reached. The main characteristics recorded for the selected study included the first author, publication year, country, study period, sample size, age and sex. The proportion of dead patients who had developed HFMD in the presence and absence of each given risk factor was recorded.
Two reviewers independently used the Newcastle–Ottawa quality assessment scale (NOS) to assess the quality of the selected studies [Reference Stang13, Reference Welch, Losos and Tugwell14]. A third reviewer was consulted if a disagreement arose. The NOS was used to score the studies on three criteria: selection, comparability and outcome. The total score ranged from 0 to 9. High-quality studies had an overall score of ⩾5. The details of the quality assessment are presented in Table S1.
Statistical analysis
Odds ratio (OR) with 95% confidence intervals (CI) were used to pool the outcome data. The I 2 test was used to test for statistical heterogeneity. For outcomes with low heterogeneity (I 2 < 50% and P > 0.1), a fixed-effects model (the Mantel–Haenszel method) was used for secondary analysis; otherwise (I 2 ⩾ 50% or P ⩽ 0.1), a random-effects model (the DerSimonian and Laird method) was used [Reference DerSimonian and Laird15]. A sensitivity analysis was further conducted in which one study was removed and the rest were analysed to evaluate whether the results were affected statistically significantly. Thus, a sensitivity analysis was performed to assess the EV-A71 infection, vomiting and convulsion and to evaluate potential sources of heterogeneity in the analyses. Publication bias was evaluated via the visual analysis of funnel plots and conducting Egger's and Begg's tests [Reference Begg and Mazumdar16, Reference Macaskill, Walter and Irwig17]. All tests were considered statistically significant for P values <0.05. Statistical analyses were performed using Review Manager 5.3 (Cochrane Collaboration, UK) and Stata version 14.0 (Stata Corporation LP, TX, USA).
Results
Study selection and description
A total of 889 studies were obtained through database searches, of which 81 were excluded due to duplication. Another 878 studies were excluded after the title and abstract information was reviewed. Four studies were excluded after assessing the full text; two studies included repeated samples and two studies lacked control groups. The final meta-analysis included seven studies [Reference Long18–Reference Deng24]. Six studies were from China and one was from Singapore. A flowchart depicting the study selection process is shown in Figure 1. A total of 1641 patients with HFMD were enrolled in the included trials; of these, 634 died and 1007 survived. All studies were case–control studies. The main characteristics of the included trials are summarised in Table 1.
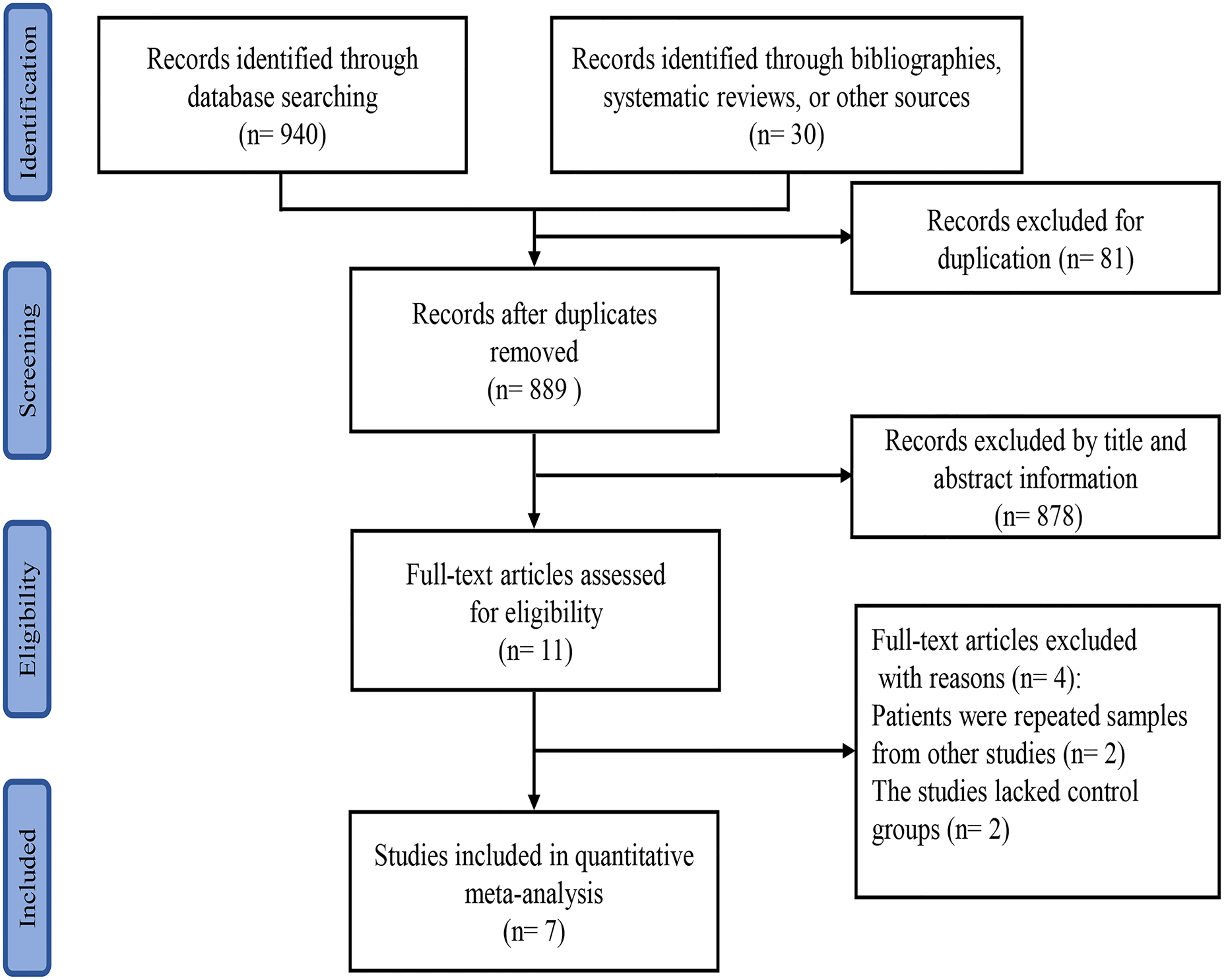
Fig. 1. Flowchart of the study selection process for inclusion in the meta-analysis.
Table 1. Key characteristics of the included studies

RSV, respiratory syncytial virus; CMV, cytomegalovirus; CV-A4, coxsackie A4; NR, non-reported.
Risk factors for HFMD deaths
EV-A71
Five studies [Reference Long18–Reference Song20, Reference Chong23, Reference Deng24] reported the association between EV-A71 infection and the risk of HFMD deaths, involving 433 deaths and 674 survivals. EV-A71 infection was not associated with HFMD death (OR = 2.12; 95% CI 0.87–5.18; I 2 = 81%; P = 0.10) (Fig. 2).
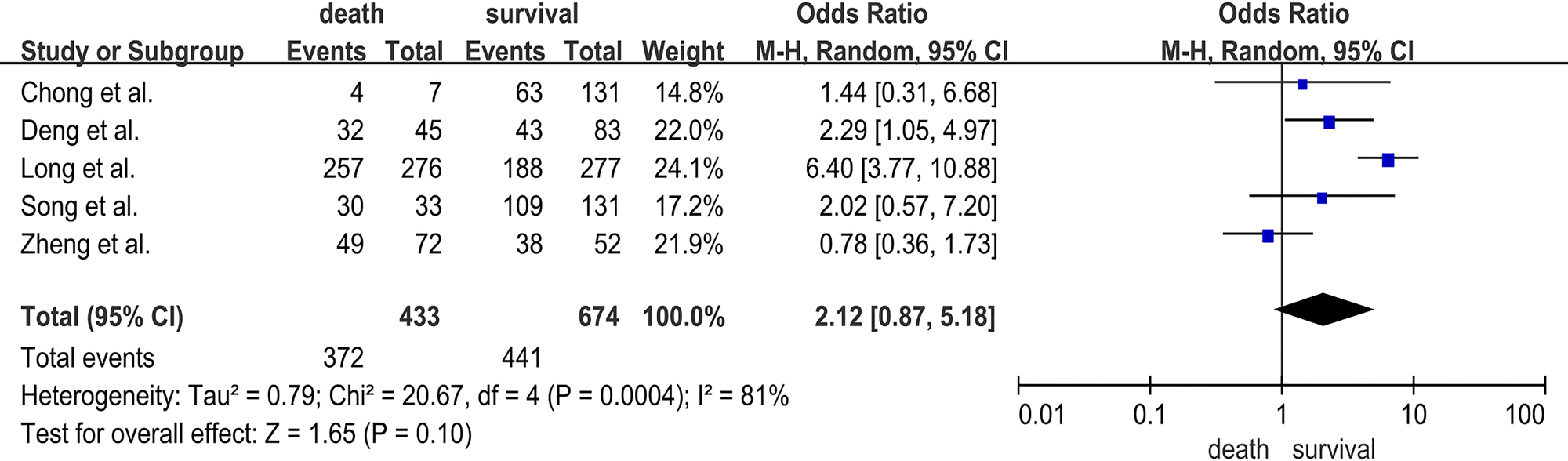
Fig. 2. Forest plots showing the results of the meta-analysis regarding EV-A71 infection.
Demographic characteristics
Demographic characteristics of fatal HFMD were also analysed in this study. In seven studies [Reference Long18–Reference Deng24], with 634 deaths and 1007 survivals, the pooled OR for male was 0.89 (95% CI 0.71–1.11; I 2 = 37%; P = 0.29) (Fig. 3). Three studies [Reference Song20, Reference Xu22, Reference Deng24] investigated the association between age ⩽3 years and the risk of fatal HFMD, involving 183 deaths and 424 survivals. The pooled OR for age ⩽3 years was 1.55 (95% CI 0.86–2.82; I 2 = 28%; P = 0.15) (Fig. S1). Male sex and age ⩽3 years were not significantly related to HFMD deaths.
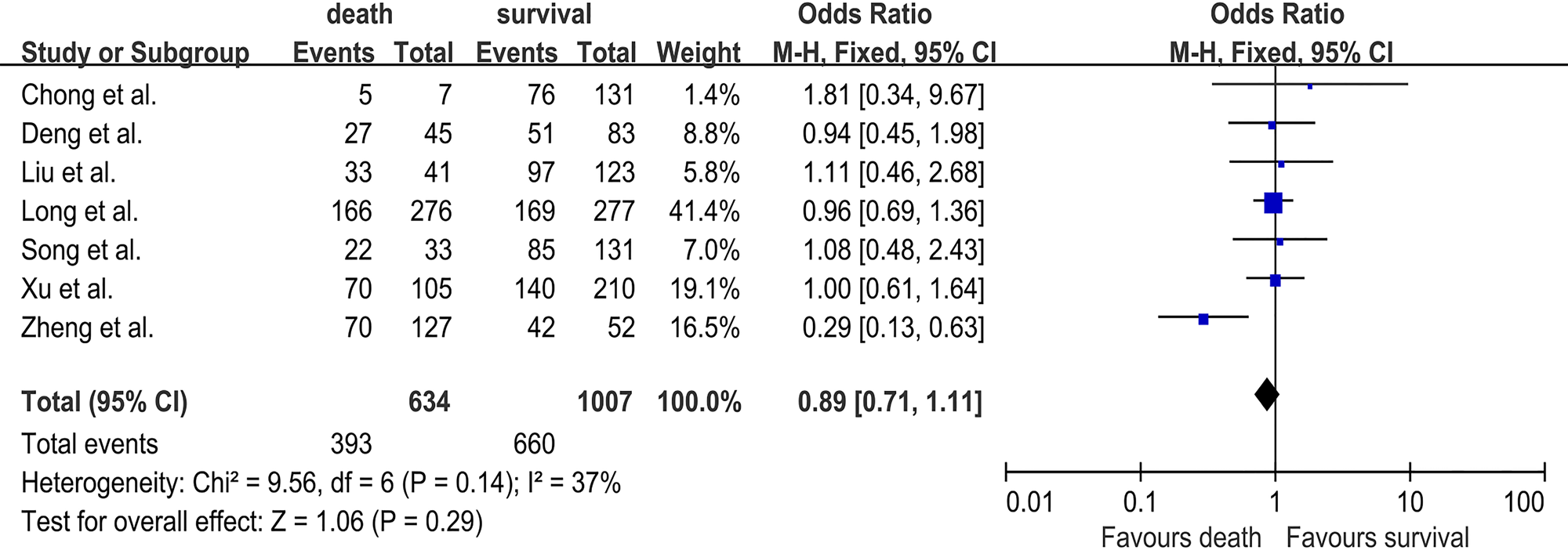
Fig. 3. Forest plots showing the results of the meta-analysis regarding male sex.
Manifestations
Six studies [Reference Long18–Reference Liu21, Reference Chong23, Reference Deng24] investigated the association between vomiting and the risk of HFMD death and included a total of 529 deaths and 797 survivals. The pooled OR for vomiting was 2.22 (95% CI 0.99–4.99; I 2 = 85%; P = 0.05) (Fig. S2). Three studies [Reference Long18–Reference Song20] investigated the association between cyanosis and the risk of fatal HFMD involving 433 deaths and 460 survivals. The pooled OR for cyanosis was 3.65 (95% CI 1.00–13.33; I 2 = 91%; P = 0.05) (Fig. S3). Convulsion was analysed in four studies [Reference Long18, Reference Zheng19, Reference Liu21, Reference Deng24], and 489 deaths and 535 survivals were enrolled. The pooled OR for convulsion was 0.92 (95% CI 0.31–2.74; I 2 = 88%; P = 0.88) (Fig. S4). Two studies [Reference Long18, Reference Song20] investigated the association between duration of fever ⩾3 days and the risk of HFMD death and included a total of 309 deaths and 408 survivals. The pooled OR for duration of fever ⩾3 days was 0.81 (95% CI 0.20–3.24; I 2 = 87%; P = 0.76) (Fig. S5). Four studies investigated the association between atypical rashes and the risk of fatal HFMD involving 443 deaths and 591 survivals [Reference Long18–Reference Song20, Reference Chong23]. The pooled OR for atypical rashes was 1.07 (95% CI 0.80–1.44; I 2 = 28%; P = 0.65) (Fig. S6). Moreover, abdominal distention was analysed in two studies [Reference Long18, Reference Song20], and 309 deaths and 408 survivals were enrolled. The pooled OR for abdominal distention was 1.68 (95% CI 0.74–3.85; I 2 = 0%; P = 0.22) (Fig. S7). Thus, vomiting, cyanosis, convulsion, duration of fever ⩾3 days, atypical rashes and abdominal distention were not significantly related to HFMD deaths (Table 2).
Table 2. Meta-analysis of risk factors of HFMD death in seven separate studies
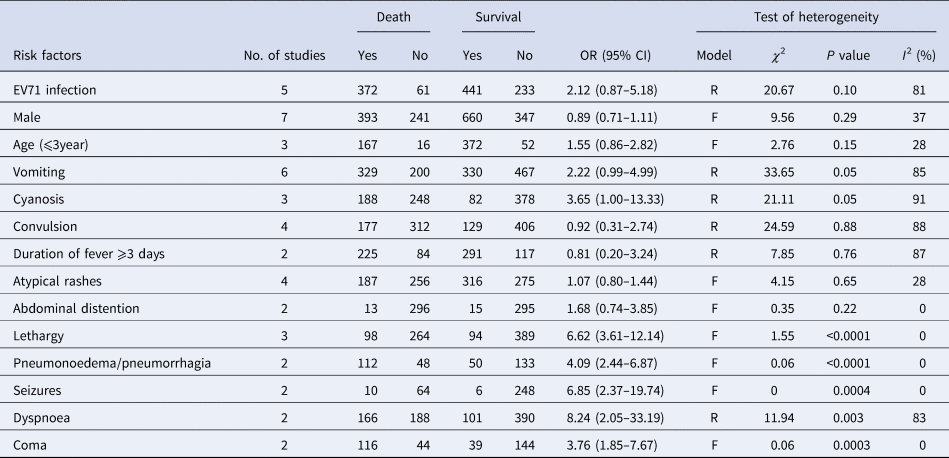
Model: R, random; F, fixed.
Three studies [Reference Long18, Reference Liu21, Reference Deng24] were included in the analysis between lethargy and the risk of fatal HFMD involving 362 deaths and 483 survivals. The pooled OR for lethargy was 6.62 (95% CI 3.61–12.14; I 2 = 0%; P < 0.0001) (Fig. 4). Furthermore, pneumonoedema/pneumorrhagia was analysed in two studies [Reference Zheng19, Reference Song20], and 160 deaths and 183 survivals were enrolled. The pooled OR for pneumonoedema/pneumorrhagia was 4.09 (95% CI 2.44–6.87; I 2 = 0%; P < 0.0001) (Fig. S8). Two studies [Reference Song20, Reference Liu21] investigated the association between seizures and the risk of HFMD death and included a total of 74 deaths and 254 survivals. The pooled OR for seizures was 6.85 (95% CI 2.37–19.74; I 2 = 0%; P = 0.0004) (Fig. S9). Three studies [Reference Long18, Reference Song20, Reference Deng24] investigated the association between dyspnoea and the risk of HFMD death, and included a total of 354 deaths and 491 survivals. The pooled OR for dyspnoea was 8.24 (95% CI 2.05–33.19; I 2 = 83%; P = 0.003) (Fig. S10). Coma was analysed in two studies [Reference Zheng19, Reference Song20], and 160 deaths and 183 survivals were enrolled. The pooled OR for coma was 3.76 (95% CI 1.85–7.67; I 2 = 0%; P = 0.0003) (Fig. S11). Therefore, lethargy, pneumonoedema/pneumorrhagia, seizures, dyspnoea and coma were found to be significantly associated with HFMD death (Table 2).
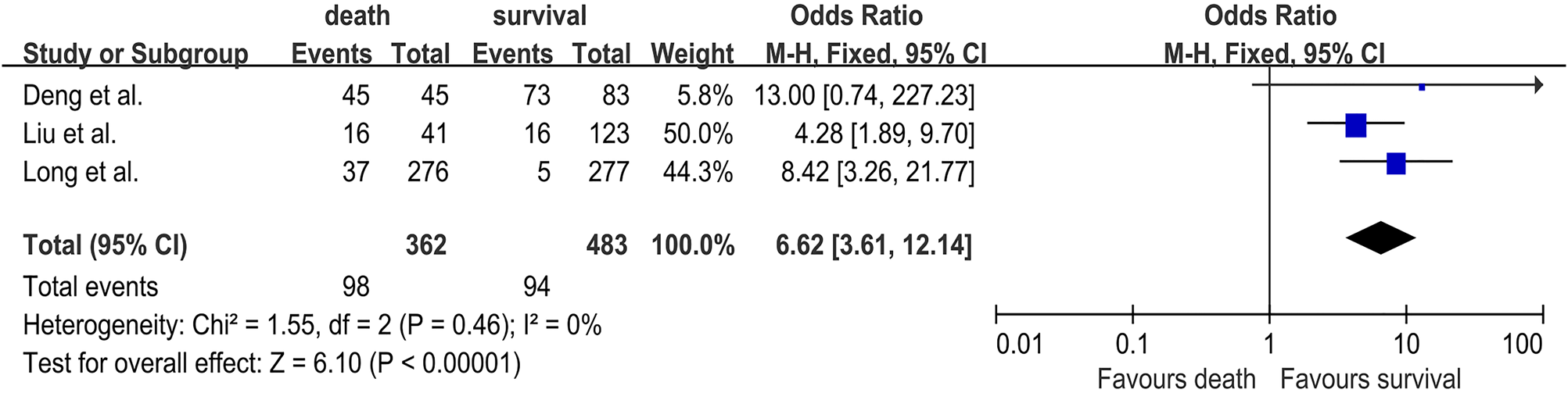
Fig. 4. Forest plots showing the results of the meta-analysis regarding lethargy.
Publication bias
The Egger's and Begg's tests provided no evidence of publication bias for males (P = 0.909 and 0.368, respectively). The funnel plot appeared generally symmetrical (Fig. S12).
Sensitivity analysis
In sensitivity analyses in which one study was excluded at a time from each analysis, the summary estimates were not substantially altered for EV-A71 infection, vomiting and convulsion (Figs S13–S15).
Discussion
Several outbreaks of HFMD have been reported from regions such as Malaysia [Reference Abubakar25, Reference Shimizu26], Singapore [Reference Ahmad27], Mainland China [Reference Ding28], Brunei [Reference AbuBakar29], Western Australia [Reference McMinn30], the USA [Reference Alexander31] and Germany [Reference Kehle32]. Although most HFMD episodes are usually mild and self-limiting, a minority of patients may rapidly progress to severe complications and lose their lives [Reference Xu9]. Between 2008 and 2014, more than 10 million cases, including 3000 deaths from HFMD, were reported to the countrywide disease-reporting system in Mainland China [Reference Liu33]. Factors associated with severe or fatal HFMD include EV-A71, a high fever of over 39 °C for more than 3 days, a raised WBC count >10.8 × 109/L, vomiting, tachycardia, lethargy, hyperglycaemia and leucocytosis [Reference Zhao10, Reference Yang34, Reference Li35]. However, the risk factors for HFMD death are not completely clear. Thus, it is significant to determine the risk factors associated with the occurrence of death for patients with HFMD. This meta-analysis was designed to explore the risk factors for HFMD death and help distinguish patients at risk of developing fatal HFMD at an early stage.
Seven case–control studies were included in this meta-analysis. In this study, the risk factors for fatalities were lethargy, pneumonoedema/pneumorrhagia, seizures, dyspnoea and coma. Moreover, EV-A71, male, age, vomiting, cyanosis, convulsion, duration of fever ⩾3 days, atypical rashes and abdominal distention were not associated with fatal HFMD.
Several clinical studies indicated that EV-A71-related HFMD was always associated with more severe symptoms, such as acute flaccid paralysis, brainstem encephalitis, rapid fatal pulmonary oedema and haemorrhage, whereas CA6 and CA10 caused only mild symptoms [Reference Wu36]. In addition, some researchers held the belief that EV-A71-infected patients faced an increased risk of mortality compared with other viruses. In this study, EV-A71 infection alone was not responsible for HFMD-related deaths. EV-A71 is a neuronophagic virus that mainly affects the brainstem, causing encephalitis, aseptic meningitis and other neurological disorders characterised by vomiting, coma, startle, frequent convulsions and light-reflex insensitivity. This inconsistency may be attributed to missing virology investigation data in the survival group.
Lethargy was reported in lots of studies as a predictor of severe HFMD and confirmed in this study. It was found that lethargy was a useful clinical symptom of the nervous system involved in early disease [Reference Chang37]. Several studies indicated that pneumonoedema/pneumorrhagia was the major cause of death for critical and severe HFMD [Reference Pan38, Reference Zou39]. Fulminant neurogenic pneumonoedema was found in HFMD-related deaths in Malaysia [Reference Chan40, Reference Ooi41], and it may be caused by the damage to certain areas of the brainstem or an increase in pulmonary vascular pressure and pulmonary endothelial permeability [Reference Smith and Matthay42]. This study showed that pneumonoedema/pneumorrhagia was associated with the mortality of patients with HFMD. Chan et al. thought that seizures were the main symptom of neurological involvement and associated with severe disease, consistent with the results [Reference Chan40]. Deng et al. held that dyspnoea was not a significant risk factor for the development of fatal HFMD [Reference Deng24]; however, Long et al. suggested that dyspnoea significantly increased the risk of HFMD death [Reference Long18], which was confirmed in the present study. Coma, a neurological disorder, was a significant symptom of HFMD death, similar to the results of the present study [Reference Zheng19].
The male patients were more susceptible to HFMD death than female patients [Reference Wang43]. In this study, the male-to-female ratio of fatal cases was 1.63:1 and no significant association was found between male sex and HFMD death. The results of this study showed that the ratio of children aged >3 years was 91.2%, which was higher than the rate reported in Vietnam [Reference Nguyen44]. Moreover, it was found that age was not a risk factor for HFMD death probably because the immune system of children reaches a stable state at about 5 years of age and low immunity makes no significant difference between fatal and non-fatal cases. The manifestations of digestive system, abdominal distention and vomiting were not the risk factors for HFMD death in this study, as confirmed by some previous studies [Reference Long18]. Long et al. found that children with the symptom of cyanosis were at risk of severe HFMD [Reference Long18]. However, the present study showed that cyanosis was not associated with HFMD death. Furthermore, convulsion was reported to increase the risk of death from severe HFMD; four included studies confirmed the conclusion [Reference Long18, Reference Zheng19, Reference Liu21, Reference Deng24]. In the present meta-analysis, convulsion was not related to the mortality of HFMD. Although boys enjoy outdoor activities, they often pay no attention to hygiene, but it is not the key to increase the mortality of HFMD. Some previous studies regarded duration of fever ⩾3 days as a neurological manifestation and found that it increased the risk of death. However, the results indicated that the duration of fever ⩾3 days was not associated with HFMD death. The occurrence of atypical rashes may be associated with mortality [Reference Chong23], contrary to the results of this study. However, only seven fatal cases were included in this study, reducing the strength of the results.
This study had several inevitable limitations. First, three of seven included studies were published in Chinese and the quality of studies might differ from the ones in English. Second, most of the included studies focused on the patients in China; only one study reported the epidemiology of fatal cases in Singapore. After excluding the study from Singapore, the summary estimates were not substantially altered. Therefore, including the study from Singapore into this meta-analysis was reasonable. Third, several studies might have a selection bias. It was ideal that the patients were randomly enrolled in the survival group, but not all patients had undergone aetiological examinations. Thus, only patients with aetiological examinations were enrolled in the control group. Moreover, several manifestations, such as coma and abdominal distention, were reported in two included studies. Finally, though detailed sensitivity analyses were undertaken, given the heterogeneity in the study protocols, clinically relevant differences could have been missed and might be better assessed in a meta-analysis of individual patient data. There could be additional confounders not accounted for in the analysis. Also, not all of the trials reported each of the outcomes analysed.
This study aimed to identify the manifestations of fatal HFMD, so that early treatment might be started to reduce the mortality. The results of the present study revealed that neurological manifestations, such as lethargy and seizures, increased the mortality of patients with HFMD. Screening patients with HFMD for these abnormal clinical presentations was beneficial in predicting fatal nervous system disorder, allowing timely initiation of appropriate interventions. Due to no effective antiviral therapies up to now, vaccines have become the most effective solution in preventing EV-related HFMD. Moreover, two inactivated EV-A71 vaccines were available in China, showing good efficacy and safety in HFMD prevention [Reference Zhu45–48]. In addition, hand washing, disinfecting common areas and limiting exposure by keeping ill children out of school are also very effective prevention measures for HFMD. Therefore, multivalent EV and coxsackievirus vaccines should be developed and recommended to protect children from HFMD.
In conclusion, the results suggested that lethargy, pneumonoedema/pneumorrhagia, seizures, dyspnoea and coma increased with HFMD deaths. EV-A71 infection, male, vomiting, cyanosis, convulsion, duration of fever ⩾3 days, age, atypical rushes and abdominal distention were not associated with HFMD death. It is vital to screen patients with HFMD for these abnormal clinical presentations, allowing timely initiation of appropriate interventions to reduce the mortality.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268819002279
Acknowledgements
None.
Financial support
None.
Conflict of interest
The authors declare no conflicts of interest.








