Introduction
Patagonian toothfish (Dissostichus eleginoides Smith, 1898) is endemic to the Southern Hemisphere. It has a circumpolar distribution and is found around sub-Antarctic islands and seamounts. In the Atlantic Ocean, it extends north onto the Patagonian shelf and Uruguay, while in the Pacific Ocean, it occurs off Chile and Peru (Møller et al., Reference Møller, Nielsen and Fossen2003; Collins et al., Reference Collins, Ross, Belchier and Reid2007; Taki et al., Reference Taki, Kiyota, Ichii and Iwami2011; Aramayo, Reference Aramayo2016). In Ecuador, Patagonian toothfish was recorded in research campaigns during 2008, occurring between 1200 and 1400 m depth, corresponding to the extreme northern area of its distribution (de González et al., Reference de González, Maroto-Castaño and Muñoz-Recio2008).
Patagonian toothfish is a commercial species in the Tropical Pacific Ocean (TPO) region of South America and fished by bottom longline. Patagonian toothfish has been fished since 1980 off Chile, and since 1999 off Peru (Sancho-Andrade et al., Reference Sancho-Andrade, Ortiz -Von Halle and Naranjo-Cuvi2002; Collins et al., Reference Collins, Brickle, Brown and Belchier2010). An experimental fishery was developed in Ecuador between 2017 and 2022 for the assessment of the opening of commercial exploitation of Patagonian toothfish.
All the countries have reported bycatch in the Patagonian toothfish fishery. Bycatch includes non-target species that are landed and sold, species that are retained for consumption onboard, as well as those non-target species that are discarded. Discards are the portion of the catch returned to the sea (Alverson et al., Reference Alverson, Freeberg, Murawski and Pope1994). Common species reported as bycatch in longline fisheries for Patagonian toothfish fishery include grenadiers (Macrouridae), morid cods (Moridae), and skates (Rajiformes) (Collins et al., Reference Collins, Brickle, Brown and Belchier2010).
In Peruvian waters, reported bycatch has included chimaera (Hydrolagus sp.), sharks (Somniosus sp.), skates (Bathyraja sp.), teleosts (Nezumia sp. and Trachyrincus helolepsis), octopus (Benthoctopus sp.), and king crabs (Lithodes sp. and Paralomis longipes) (Aramayo, Reference Aramayo2016). Bycatch is also a risk for the conservation of protected, threatened, and endangered species. Sharks and rays are highly vulnerable species in surface and bottom longline fisheries (Chaves, Reference Chaves, Leal-Filho, Azul, Brandli, Lange-Salvia and Wall2021), including in Ecuadorian waters (Sepa et al., Reference Sepa, Coello, Herrera and Zambrano2021). In Chile, an interaction of the Patagonian toothfish fishery with marine mammals (e.g. killer whales and sperm whales) and seabirds (e.g. Diomedea spp. and Macronectes giganteus) has also been reported (Guerrero and Arana, Reference Guerrero and Arana2009; Céspedes et al., Reference Céspedes, Vargas and Adasme2016).
Ecuador has been a cooperating party in The Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) since 2018 but became a member in 2022 (meetings: CCAMLR-XXXVII/14 and CCAMLR-41). Parties to CCAMLR must demonstrate responsibilities in the use and collection of data related to marine Antarctic resources. Therefore, the objective of this study was to provide initial data on the species and size composition, and species diversity, of the bycatch observed over the period 2017–2021. This study could be considered a baseline for determining the effect of Patagonian toothfish fishery on demersal species and benthic.
Materials and methods
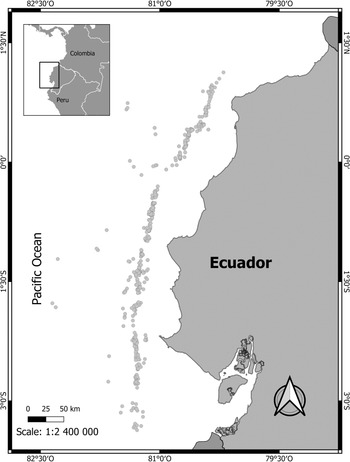
We considered bycatch as non-target species landed and sold as well as those discarded. Bycatch data from an experimental fishery of Patagonian toothfish (June 2017–July 2021) were analysed. The data correspond to fishing trips undertaken by the same fishing vessel (only one vessel had a fishing permission), recorded by observers onboard. Fishing deployments (sets) were conducted at depths of 1000–2700 m (Figure 1).

Figure 1. Study area and locations of observed hauls of the experimental fishery of Patagonian toothfish (D. eleginoides) in Ecuadorian oceanic waters, between June 2017 and July 2021.
Fishing gear was a horizontal bottom longline with a principal line and vertical drop lines rigged every 10 m. The hooks were circular with a crooked tip, size 14/0 (Sepa et al., Reference Sepa, Coello, Herrera and Zambrano2021).
A total of 1500 or 2000 hooks were used per set, which were baited with the fish Auxis thazard. All the bycatch species were identified by two observers onboard using identification guides for the region (Fischer et al., Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995a, Reference Fischer, Krupp, Schneider, Sommer, Carpenter and Niem1995b; Robertson and Allen, Reference Robertson and Allen2015; Ebert and Mostarda, Reference Ebert and Mostarda2016; Froese and Pauly, Reference Froese and Pauly2021). Scientific names were validated according to Eschmeyer's Catalog of Fishes (Fricke et al., Reference Fricke, Eschmeyer and Van der Laan2022).
Each bycatch taxon was reported as the total number of individuals per species. The taxa captured during deployment/retrieval of the fishing gear were also reported as the number of individuals. Bycatch representativity was calculated as the difference percentage between the target catch and non-target, in weight. The diversity of bycatch was determined by Shannon (entropy), equitability (Pielou J), and dominance (1-Simpson) indices. The analysis was carried out using software Past ver. 4.10 (Hammer, Reference Hammer2022).
Results
A total of 60 fishing trips were analysed, comprising 100–330 sets per year. The catch of Patagonian toothfish has decreased since 2019 while the bycatch ratio has increased (Table 1). Bycatch was composed of 51 taxa, comprising fish (52%), skates and rays (23%), sharks (15%), crustaceans (6%), and cephalopods (4%). Macrouridae, Arhynchobatidae, and Somniosidae were the families with the most of bycatch species.
Table 1. Number (n) of trips and sets, fishing depth, Patagonian toothfish landed, bycatch representativity, and number of individuals in each bycatch taxa in an experimental fishery for Patagonian toothfish (D. eleginoides) in Ecuadorian oceanic waters

The years 2017 and 2018 presented the fewest number of taxa records. The most frequent bycatch species (by number of individuals) were Hydrolagus melanophasma, followed by Antimora rostrata, Coryphaenoides delsolari, Coryphaenoides armatus, Etmopterus granulosus, and Centroscymnus owstonii. Other taxa presented a record sporadic (Table 1).
A total of 12 pelagic taxa were captured during the deployment/retrieval of the fishing gear. Coryphaena hippurus presented the highest interannual presence, followed by Pteroplatytrygon violacea, which was the only myliobatiform ray recorded. The other species were caught sporadically (Table 2).
Table 2. Number of individuals fished during the deployment/retrieval of the fishing gear, by species, in an experimental fishery for Patagonian toothfish (D. eleginoides) in Ecuadorian oceanic waters

Dominance index showed a decrease towards 2021. Equitability and Shannon indices presented a positive trend. The diversity was between medium and low (0.5–1.8), according to the Shannon index (Figure 2).

Figure 2. Trends in the diversity indices related to the bycatch of the experimental fishery of Patagonian toothfish (D. eleginoides) in Ecuadorian oceanic waters.
Discussion
Bycatch in the Patagonian toothfish fishery in Ecuadorian oceanic waters included teleost, rays, sharks, crustaceans, and cephalopods. While those are characteristic species groups in deep longline fishing in the southern region of the TPO, the proportion in landings may vary by region, depth, and fishing gear (Collins et al., Reference Collins, Brickle, Brown and Belchier2010; Sellanes et al., Reference Sellanes, Pedraza and Zapata Hernandez2012).
In Ecuador, bycatch had more species than the reports from Chile (between 18 and 25 species) and Peru (11 species) (Lamilla et al., Reference Lamilla, Bustamante, Roa, Acuña, Concha, Melendez, López, Aedo, Flores and Vargas2010; Sellanes et al., Reference Sellanes, Pedraza and Zapata Hernandez2012; Aramayo, Reference Aramayo2016). The most representative species were chimaeras (H. melanophasma), grenadiers (A. rostrata, C. delsolari, and C. armatus), and sharks (E. granulosus, and C. owstonii). H. melanophasma is a species associated with the Patagonian toothfish local fishery in Chile and Peru (Aramayo, Reference Aramayo2016; Ñacari et al., Reference Ñacari, Sepulveda, Droguet, Escribano and Oliva2020). Grenadiers are common species in the Patagonian toothfish fishery globally, and A. rostrata is quite common in the southern TPO (Reyes et al., Reference Reyes, Torres and Reyes2009; Collins et al., Reference Collins, Brickle, Brown and Belchier2010).
Bait has the function of attracting target fishes, but it can also be consumed by non-target species (Emiati et al., Reference Emiati and Brown2022). In Ecuador, the bait was the fish A. thazard, which is one of the food items of large pelagic fishes, including sharks (Aguilar-Palomino et al., Reference Aguilar-Palomino, Galván-Magaña, Abitia-Cárdenas, Muhlia-Melo and Rodríguez-Romero1998; Estupiñán-Montaño et al., Reference Estupiñán-Montaño, Cedeño-Figueroa and Galván-Magaña2009, Reference Estupiñán-Montaño, Cedeño-Figueroa, Estupiñán-Ortiz, Galván-Magaña, Sandoval-Londoño, Castañeda-Suarez and Polo-Silva2019; Trujillo-Olvera et al., Reference Trujillo-Olvera, Ortega-García, Tripp-Valdez, Escobar-Sánchez and Acosta-Pachón2018; Calle-Morán et al., Reference Calle-Morán, Hernández-Téllez, Tibán-Vivar, Intriago-Vera, del Valle-Coello, Loor-Jama and Ganchozo-López2022).
Large pelagic fish were also caught by the gear, presumably during the deployment/retrieval of the fishing gear. These included seven species of shark (Pseudocarcharias kamoharai, Prionace glauca, Sphyrna lewini, Sphyrna zygaena, Alopias pelagicus, Alopias superciliosus, and Isurus oxyrinchus), P. violacea, and four species of teleost (C. hippurus, Xiphias gladius, Ruvettus pretiosus, and Thunnus albacares). Sharks have commercial importance in Ecuador but are largely taken as incidental species (Martínez-Ortiz et al., Reference Martínez-Ortiz, Aires-Da-Silva, Lennert-Cody and Maunder2015; Coello and Herrera, Reference Coello and Herrera2018), while the teleosts observed are all targeted by local artisanal fisheries.
The observed diversity was medium to low, with a negative trend for dominance and a positive for evenness. The results are consistent with the relatively low diversity of the deep seabed, compared to the shallow water, but also noting that these were based on longline-caught fish and so will not be representative for the whole deep-sea fish assemblage in the area. There was a decrease in the diversity of species on the seabed as depth increased, which is reflected in the bycatch of the Patagonian toothfish fishery (Grassle, Reference Grassle1989; Costello and Chaudhary, Reference Costello and Chaudhary2017; Myers et al., Reference Myers, Anderson, Liggins, Harvey, Roberts and Eme2021).
In the period 2008–2010, research fishing was carried out in deep waters off the mainland coast of Ecuador (de González et al., Reference de González, Maroto-Castaño and Muñoz-Recio2008; González-Troncoso, Reference González-Troncoso2009, Reference González-Troncoso2010). In 2008, the trawls were carried out between 500 and 1500 m, and in the same year, the Patagonian toothfish was recorded, between 1200 and 1400 m. The most representative species, according to the weight, were reported to be Merluccius gayi, Rouleina attrita, C. delsolari, Dicrolene filamentosa, Haliporoides diomedeae, Dicrolene nigra, Alepocephalus spp., Nematocarcinus agassizii, Coryphaenoides carminifer, Nezumia spp., Etmopterus spp., Centroscyllium nigrum, Hydrolagus spp., Coryphaenoides anguliceps, and Benthoecetes tanneri (de González et al., Reference de González, Maroto-Castaño and Muñoz-Recio2008).
The mean diversity reported by de González et al. (Reference de González, Maroto-Castaño and Muñoz-Recio2008) was 2.58 (SD ± 0.58), which is higher than our results. This is because trawling provides a view of the wider assemblage structure, while the longline refers to the proportion of the assemblage that is susceptible to being captured by the fishing gear.
Fish, sharks, rays, and other taxa (e.g. sea turtles) could be part of the bycatch in the Patagonian toothfish fishery. However, the low and sporadic presence of those taxa is because bottom longlines have a low impact on non-target species, contrary to surface longlines; despite this, they could affect certain benthic species and change the community structure in the long term (Pham et al., Reference Pham, Diogo, Menezes, Porteiro, Braga-Henriques, Vandeperre and Morato2014; Oliver et al., Reference Oliver, Braccini, Newman and Harvey2015). The removal of vulnerable organisms could decrease their population, leaving it to the most resilient species, inclusively promoting an increase of opportunistic fauna in the ecosystem. In this sense, it is necessary to monitor, assess, and take decisions for improving fishing practices (e.g. fishing gear) and reducing bycatch (Pham et al., Reference Pham, Diogo, Menezes, Porteiro, Braga-Henriques, Vandeperre and Morato2014; Gilman et al., Reference Gilman, Hall, Booth, Gupta, Chaloupka, Fennell, Kaiser, Karnad and Milner-Gulland2022).
The diversity results in 2017 should be considered as minimum estimates, because the identification of species by fishing observers has improved since 2018. That occurred due to the complexity of the identification of deep-sea species present in Ecuadorian seas. However, the possible bias did not affect the trend in diversity indices. Patagonian toothfish fishery presented a decline in the catch trend and an increase in the bycatch for the period 2017–2021. It could show a negative effect on the target species population as well as on the bycatch species.
Data
The data that support the findings of this study are available from the Public Institute of Aquaculture Research and Fishery. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from M. Herrera with the permission of the Public Institute of Aquaculture Research and Fishery.
Acknowledgements
We are grateful to G. Sandoval for elaborating the study area map. This study is a part of the project ‘Distribution, abundance, and biological aspects of Patagonian toothfish (Dissostichus eleginoides) in Ecuadorian oceanic waters’ supported by the Public Institute of Aquaculture Research and Fisheries as well as ‘TRANSMARINA C.A.’.
Author's contribution
René Zambrano: definition, conceptualization, methodology, formal analysis, writing – original draft, writing – review and editing, and visualization. Dialhy Coello: definition, conceptualization, methodology, and writing – original draft. Marco Herrera: definition, conceptualization, project administration, and funding acquisition.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interest
None.