No CrossRef data available.
Published online by Cambridge University Press: 16 May 2023
A new ternary intermetallic compound Al3GaCu9 was synthesized experimentally. A high-quality powder diffraction pattern of the compound was collected by an X-ray diffractometer, and its crystal structure was determined using the Rietveld refinement method. Results show that the compound has a cubic cell with the Al4Cu9 structure type (space group 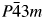 $P\bar{4}3m$ and Pearson symbol cP52). The lattice parameter a = 8.7132(3) Å, unit-cell volume V = 661.52 Å3, calculated density Dcalc = 7.26 g/cm3, and Z = 4. The residual factors converge to Rp = 2.96%, Rwp = 4.06%, and Rexp = 2.57%. The experimentally obtained reference intensity ratio value is 7.04.
$P\bar{4}3m$ and Pearson symbol cP52). The lattice parameter a = 8.7132(3) Å, unit-cell volume V = 661.52 Å3, calculated density Dcalc = 7.26 g/cm3, and Z = 4. The residual factors converge to Rp = 2.96%, Rwp = 4.06%, and Rexp = 2.57%. The experimentally obtained reference intensity ratio value is 7.04.