Introduction
Obsessive–compulsive disorder (OCD) is a neuropsychiatric disorder with core clinical features of obsessions and/or compulsions. Repetitive behaviors or mental acts are performed to prevent or reduce distress or potentially dreaded outcomes.Reference Adam, Meinlschmidt, Gloster and Lieb 1 The lifetime and 12-month prevalences of OCD are 2.3% and 1.2%, respectively.Reference Ruscio, Stein, Chiu and Kessler 2 Studies show that the gender ratio of OCD is relatively even, but with slight female preponderance in adults and slight male preponderance in childhood. Other potential risk factors, such as the postpartum period, premorbid neurocognitive functioning, and streptococcal infections, have also been reported.Reference Fontenelle and Hasler 3 Research on the pathogenesis of OCD has provided growing evidence for the role of the dysfunctional immune system.Reference Arnold and Richter 4 The term “pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections” (PANDAS) was firstly proposed in 1998 to describe a subset of children with abrupt onset or exacerbations of OCD or tics, or both, following streptococcal infections.Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar 5 Research has found significantly more CSF autoantibodies directed against the basal ganglia and thalamus in patients with OCD than in control patients.Reference Gerentes, Pelissolo, Rajagopal, Tamouza and Hamdani 6 In addition, alexithymia presents in 20% to 40% of patients with OCDReference De Berardis, Campanella and Gambi 7 and is associated with higher suicide ideation.Reference De Berardis, Serroni and Marini 8 , Reference De Berardis, Serroni and Campanella 9 Some studies suggest that alexithymia is associated with impaired immune response because increased serum levels of similar type 2 cytokine and tumor necrosis factor alpha are found in both alexithymia and autoimmune disease.Reference Guilbaud, Corcos, Hjalmarsson, Loas and Jeammet 10 , Reference Bruni, Serino and Galluzzo 11 An epidemiological study has demonstrated a higher incidence of subsequent OCD among patients with systemic autoimmune diseases. Specifically, the risk of OCD was significantly increased in patients with systemic lupus erythematosus, dermatomyositis, and Sjögren’s syndrome.Reference Wang, Chen, Chiang, Hsu and Shen 12 A systemic review has also showed a positive association between some autoimmune skin diseases (ASDs) and OCD or tic disorders.Reference Perez-Vigil, Fernandez de, Brander, Isomura, Gromark and Mataix-Cols 13
ASDs encompass a variety of autoimmune diseases with cutaneous manifestations, including psoriasis, alopecia areata (AA), lichen planus, morphea, vitiligo, hidradenitis suppurativa, and autoimmune bullous dermatoses. These skin diseases not only cause clinical symptoms and cosmetic problems, but also significantly impact patients’ quality of life, and, in some cases, the conditions can be fatal.Reference Karimkhani, Dellavalle and Coffeng 14 - Reference Dai, Tai, Chang, Chen and Chen 18 The principal explanatory mechanism for their susceptibility and onset may be related to interactions between genetic and environmental factors.Reference Dai, Tai, Chang, Chen and Chen 19 - Reference Sushama, Dixit, Gautam, Arora, Khurana and Anubhuti 24 Since the relationship between OCD and systemic autoimmune disease has been demonstrated, it is reasonable to hypothesize that there may be a positive relationship between OCD and ASD. This population-based cohort study aimed to investigate the risk of developing ASD in patients with OCD.
Methods
Data source
The Taiwan National Health Insurance (NHI) program was established in 1995 and covered approximately 99.6% of all Taiwanese residents by the end of 2010. The NHI Research Database (NHIRD) contains comprehensive information about the insured participants, including demographic details (date of birth, sex, and residential location) and details of insurance claims (outpatient and inpatient care, medical diagnoses, prescriptions, and operations). NHIRD data have been widely used in numerous epidemiological studies.Reference Dai, Tai, Chang, Chen and Chen 25 – Reference Dai, Tai, Chang, Chen and Chen 31 To protect individual privacy, a unique identification number is assigned to each beneficiary and enciphered before the data are released for scientific purposes. The diagnostic codes used for this study were based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The institutional review board of Taipei Veterans General Hospital approved this retrospective study and waived the requirement for informed consent from patients (2018-07-016AC).
Study cohort and controls
This study was representative of the Taiwanese population and included all individuals diagnosed with OCD between January 1, 2001 and December 31, 2009. OCD was identified by the ICD-9-CM code 300.3, verified by board-certified psychiatrists based on diagnostic interviews and clinical judgment.
For each patient with OCD, four matched controls were randomly selected from the NHIRD. The controls were matched for age, sex, monthly premium, and residence. Monthly premiums were classified into 0 to 500, 501 to 800, and ≥801 U.S. dollars. The residence was classified into five levels of urbanization, with level 1 indicating the most urbanized and level 5 indicating the least urbanized area. Monthly premium and urbanization levels were used to represent socioeconomic status. The Charlson Comorbidity Index (CCI) was used to assess clinical prognosis and comorbidity adjustment. The CCI was composed of 19 physical conditions (ie, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic lung disease, connective tissue disease, peptic ulcer, mild liver disease, diabetes without end-organ damage, hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, tumor without metastases, moderate or severe liver disease, leukemia, lymphoma, acquired immunodeficiency syndrome, and malignant tumors).Reference Charlson, Pompei, Ales and MacKenzie 32 The index date for patients in the OCD group was the date when they were diagnosed with OCD for the first time, whereas the index date for the participants in the control group was the OCD diagnosis date of their respective matched patients. For patients who developed ASD, the length of follow-up was from the index date to the date of the first diagnosis of ASD. The censored time for patients who did not develop an ASD was from the index date to December 31, 2011, or the date of withdrawal from the NHI, whichever occurred first.
Outcomes
The primary outcomes assessed were new-onset ASD, which included the following: psoriasis (ICD-9-CM codes 696.0, 696.1, and 696.8), lichen planus (ICD-9-CM code 697), AA (ICD-9-CM code 704.01), morphea (ICD-9-CM code 701.0), autoimmune bullous diseases (ICD-9-CM code 694), hidradenitis suppurativa (ICD-9-CM code 750.83), vitiligo (ICD-9-CM code 709.01), lupus erythematosus (ICD-9-CM codes 710.0 and 695.4), systemic sclerosis (ICD-9-CM code 710.1), Sjogren’s syndrome (ICD-9-CM code 710.2), and dermatomyositis (ICD-9-CM code 710.3). The participants were considered as having developed an ASD only if the diagnosis was established by board-certified dermatologists or rheumatologists, and the condition occurred at ≥3 outpatient visits. Participants with a previous ASD diagnosis, invalid insurance status, unknown sex status, or unknown covariates were excluded from the ASD incidence.
Statistical analysis
For between-group comparisons, the t-test or Wilcoxon rank-sum test was used for continuous variables, and Pearson’s test was used for categorical variables. The Cox proportional hazards model was used to assess the association between OCD and ASD. Adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) were computed after controlling for age, sex, monthly premium, residence, CCI scores, and annual outpatient visits. Backward variable selection analyses with an entry criterion of 0.05, as a sensitivity test to determine the variables significantly associated with the risk of developing ASDs. Two-sided P < .05 was considered statistically significant. Data management and analyses were performed using Statistical Analysis System software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Characteristics of patients with OCD and controls
A total of 44 324 patients with OCD and 177 296 controls were included in this cohort study. The median (interquartile range [IQR]) age at diagnosis of OCD was 31.8 [22.4-44.1] years, and among the participants, 51.5% were men (Table 1). Age, sex, monthly premium, and residence were matched, and between-group differences among patients with OCD and controls were not observed. Patients with OCD had higher psychological comorbidities, CCI scores, and annual outpatient visits than the controls (P < .001).
Table 1. Demographic Data of Patients with Obsessive–Compulsive Disorder and Matched Controls
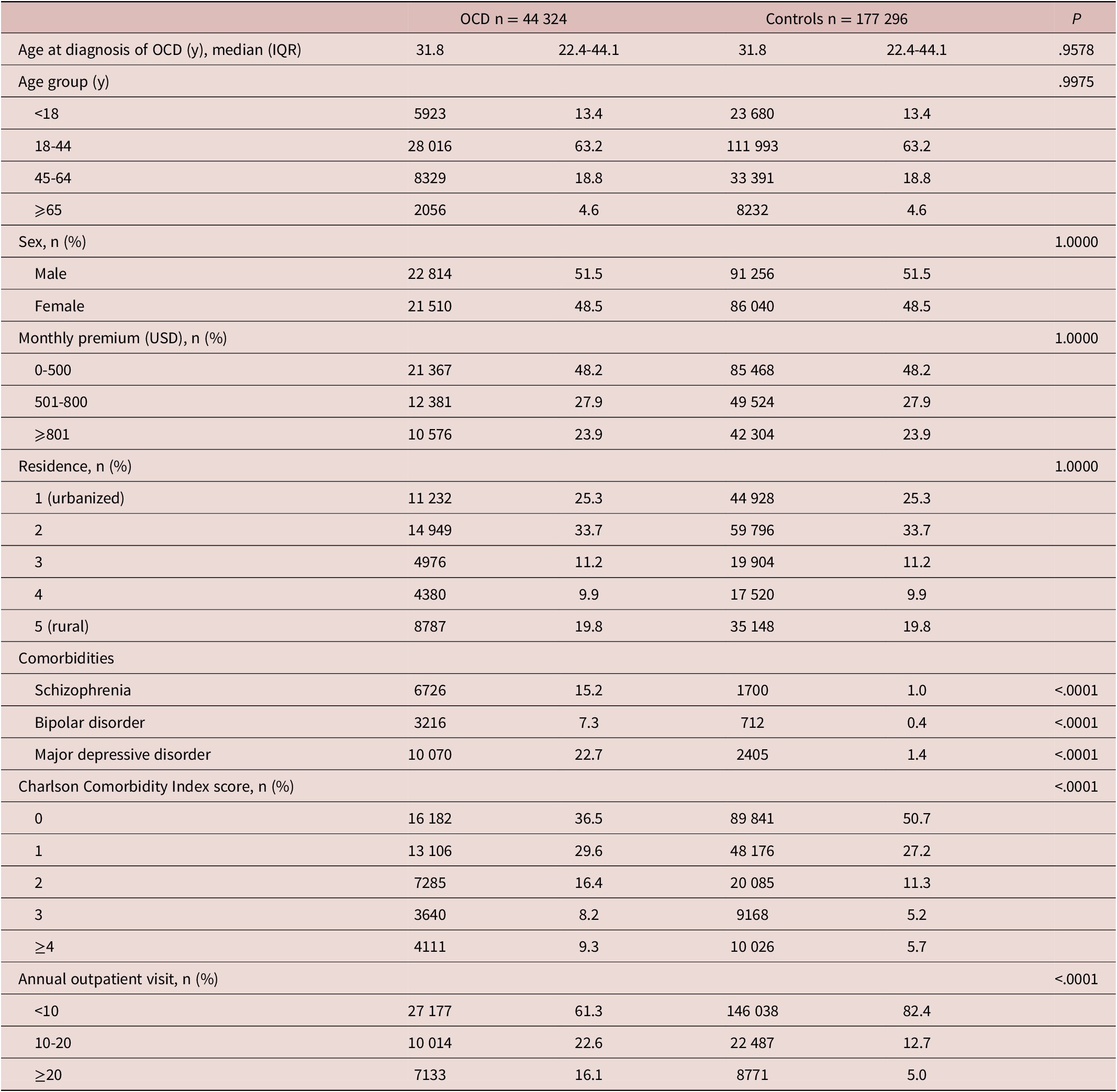
Abbreviations: IQR, interquartile range; OCD, obsessive–compulsive disorder; USD, U.S. dollar.
Risk of ASDs among patients with OCD
During a median (IQR) follow-up of 7.0 [4.6-9.2] years, 646 patients with OCD developed ASD, with an overall rate of 211.28 cases per 100 000 person-years, whereas 304 non-OCD individuals had an ASD, with an overall rate of 24.65/100 000 person-years. After controlling for age, sex, monthly premium, residence, comorbidities, CCI scores, and annual outpatient visits, we observed an increased risk of ASDs among patients with OCD (aHR: 6.36; 95% CI: 5.43-7.45) compared to the controls (Table 2). Younger onset age of ASD (median [IQR]) was observed among the patients with OCD compared to the controls (37.9 [28.1-50.1] vs 42.8 [29.7-54.8] years; P = .0006). The cumulative incidence of ASD among patients with OCD and controls is shown in Figure 1. As shown in Table 3, patients with OCD had a significantly increased risk of developing psoriasis (aHR: 12.52; 95% CI: 8.78-17.85), lichen planus (aHR: 27.22; 95% CI: 13.09-56.60), AA (aHR: 13.69; 95% CI: 9.38-19.98), autoimmune bullous diseases (aHR: 4.30; 95% CI: 2.03-9.11), hidradenitis suppurativa (aHR: 29.95; 95% CI: 3.35-267.62), vitiligo (aHR: 9.35; 95% CI: 5.35-16.62), and lupus erythematosus (aHR: 2.10; 95% CI: 1.52-2.91).
Table 2. Hazard Ratios of Autoimmune Skin Diseases Among Patients with Obsessive–Compulsive Disorder
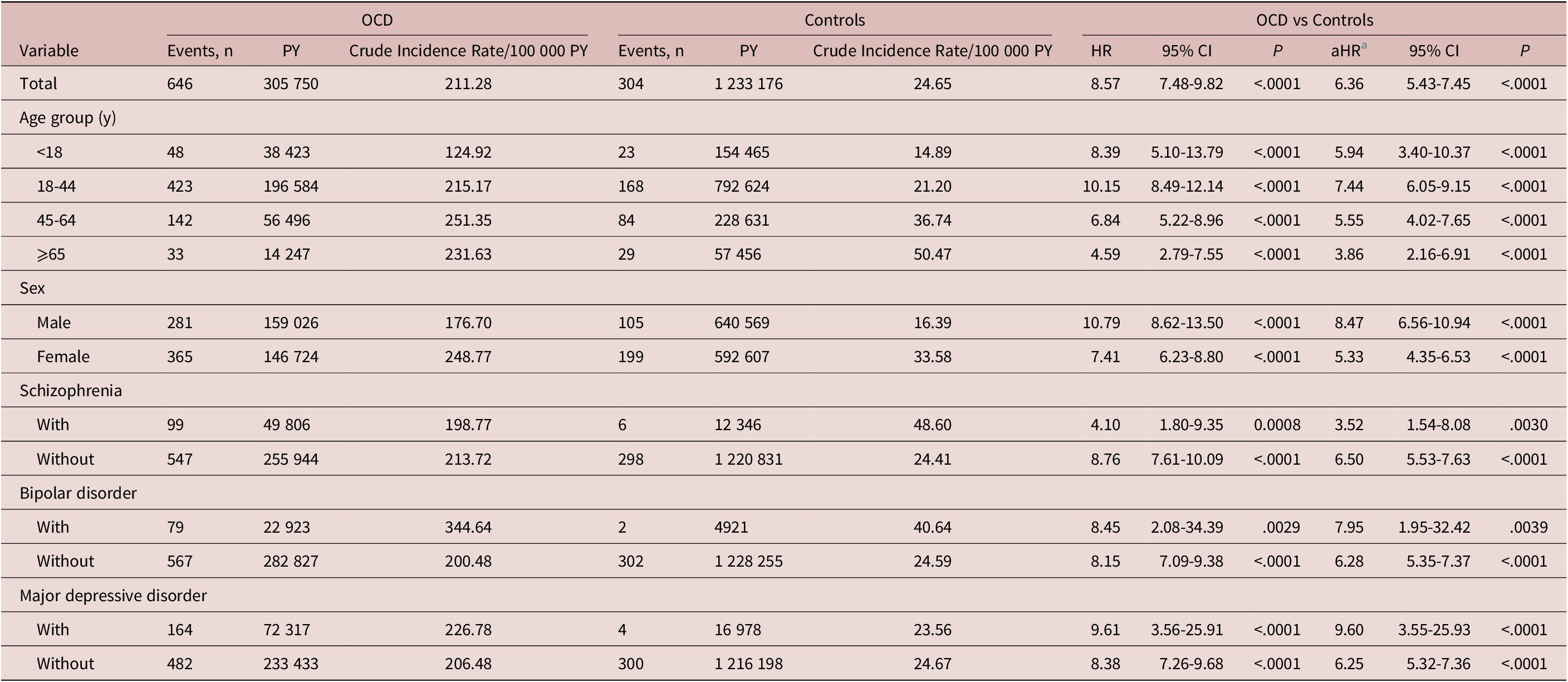
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; OCD, obsessive–compulsive disorder; PY, person-years.
a Adjusted for age, sex, monthly premium, residence, comorbidities, Charlson Comorbidity Index scores, and annual outpatient visits.
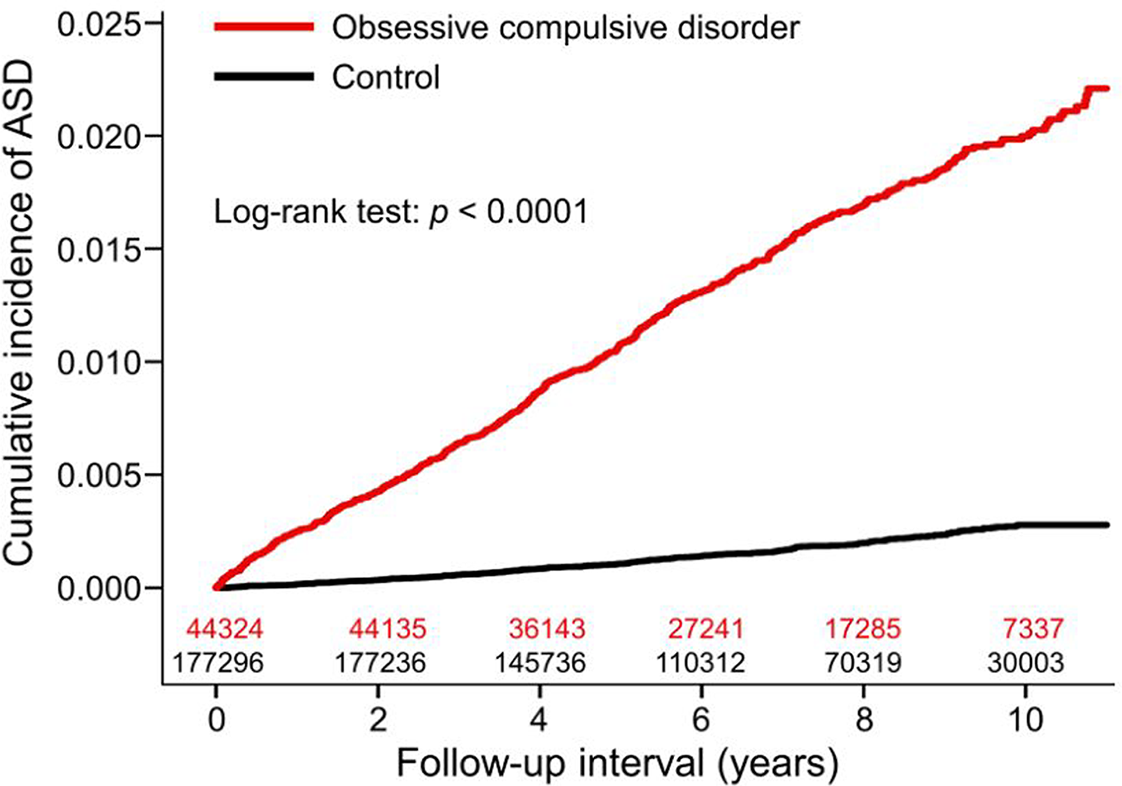
Figure 1. Cumulative incidence of autoimmune skin diseases among patients with obsessive–compulsive disorder and matched controls.
Table 3. Hazard Ratios of Individual Autoimmune Skin Diseases Among Patients with Obsessive–Compulsive Disorder
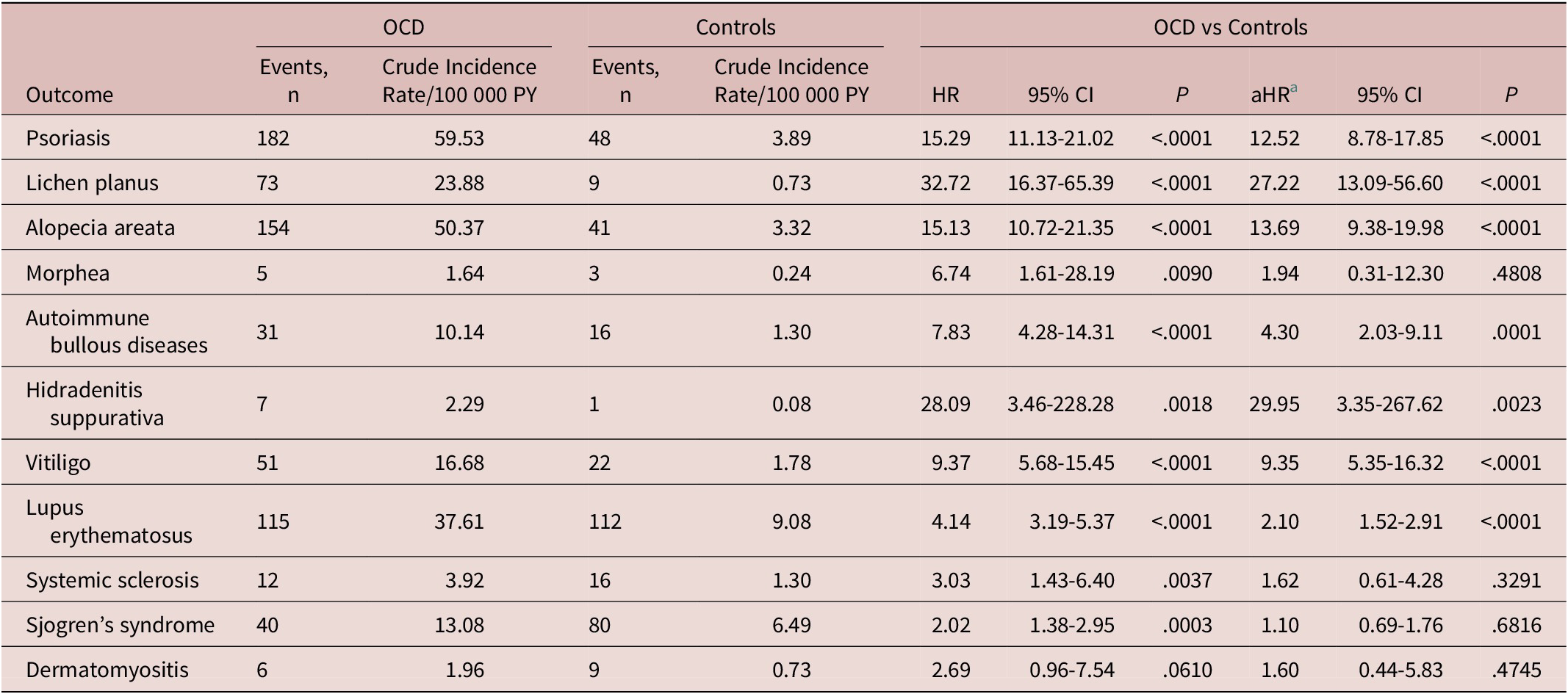
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; OCD, obsessive–compulsive disorder; PY, person-years.
a Adjusted for age, sex, monthly premium, residence, comorbidities, Charlson Comorbidity Index scores, and annual outpatient visits.
Discussion
This cohort study found the incidence of ASD to be approximately six times higher among patients with OCD compared to controls. After stratification by sex and age, the risk of ASDs remained significantly increased for both sexes and all age groups. In this study, elevations of the observed risk among individual ASDs showing heterogeneity (eg, aHR: 12.52; psoriasis: 27.22; lichen planus), which may be due to the differences in the pathogenicity or the degree of autoimmunity between the diverse ASDs.Reference Richard-Miceli and Criswell 33 The wide Cis of the aHRs of individual ASDs indicate that the results should be interpreted with caution, regardless of the statistical significance. Further studies with larger sample sizes and longer observation periods are necessary to confirm our findings.
Previous studies have demonstrated a higher risk of systemic autoimmune diseases among individuals with OCD. A retrospective cohort study of 63 165 Taiwanese patients with systemic autoimmune diseases found an elevated risk of subsequent OCD. Specifically, the significantly increased risk of OCD was observed in systemic lupus erythematosus (1.65, 1.07-2.54), dermatomyositis (3.25, 1.04-10.17), and Sjögren’s syndrome (2.38, 1.53-3.72).Reference Wang, Chen, Chiang, Hsu and Shen 12 A systemic review of 62 studies found an association between OCD and autoimmune diseases, including rheumatic fever, multiple sclerosis, and systemic lupus erythematosus. However, the conclusions were limited by the scarcity of research studies and methodological issues.Reference Perez-Vigil, Fernandez de, Brander, Isomura, Gromark and Mataix-Cols 13 Our study also supports the association between OCD and lupus erythematosus. However, in contrast to the results of previous reports, our study did not observe a significant association between OCD and some systemic autoimmune diseases, including systemic sclerosis, Sjogren’s syndrome, and dermatomyositis. This may be due to the small number of incident cases and limited statistical power. Our results, together with the results of previous studies, suggest that OCD may be significantly associated with both systemic and cutaneous autoimmune diseases.
Although the exact pathophysiology for these findings is unknown, some immuno-inflammatory hypotheses in OCD may explain the relationship between OCD and ASD risk.Reference Gerentes, Pelissolo, Rajagopal, Tamouza and Hamdani 6 First, plasma levels of IL-2, IL-4, IL-6, IL-10, and TNF-α were significantly higher in drug-naïve patients with OCD than in controls.Reference Rao, Venkatasubramanian, Ravi, Kalmady, Cherian and Yc 34 These inflammatory cytokines also appeared to be centrally involved in the pathogenesis of different types of ASD, and similar findings were also noted in ASD.Reference Kunz and Ibrahim 35 - Reference Sushama, Dixit, Gautam, Arora, Khurana and Anubhuti 41 Second, family studies have suggested that elevated rates of autoimmune diseases, including psoriasis vulgaris, are found in mothers of individuals with OCD.Reference Mataix-Cols, Frans and Perez-Vigil 42 Mothers of children fulfilling the criteria of PANDAS were 1.86 times more likely than their counterparts to report having at least one autoimmune disease.Reference Murphy, Storch, Turner, Reid, Tan and Lewin 43 According to the results, a common genetic link may also explain the association between OCD and ASD. Third, regulatory T cells (Tregs) act by secreting anti-inflammatory cytokines and inhibiting pro-inflammatory cellular responses.Reference Sakaguchi, Yamaguchi, Nomura and Ono 44 Recent findings have indicated their unique role in autoimmune diseases. Both insufficient suppression of immune responses and imbalance of Tregs are considered crucial for the initiation and perpetuation of autoimmune diseases.Reference Dejaco, Duftner, Grubeck-Loebenstein and Schirmer 45 Patients with OCD generally exhibit a reduced percentage of Tregs. Furthermore, reduced percentages of Treg cells were also associated with a longer duration of OCD.Reference Rodriguez, Morer and Gonzalez-Navarro 46 This suggests that the imbalance and dysfunction of Tregs are likely involved in the disruption of immune homeostasis in OCD. Overall, abnormalities in cytokine production, imbalance of Tregs, and genetic factors may contribute to the development of ASDs in patients with OCD.
This study had some limitations. First, the NHIRD lacked some important confounding factors, such as disease severity, body mass index, and a history of smoking and alcohol consumption. Second, since we only included those who sought consultation and treatment, the incidence of OCD might have been underestimated. Third, there could be a possibility of misclassification of ASD and OCD diagnoses. However, these diseases were diagnosed by board-certified dermatologists, rheumatologists, and psychiatrists, thus yielding high diagnostic validity and stability. Fourth, patients with OCD showed higher annual outpatient visits, which may also increase the diagnosis rate of ASDs. However, the risk of developing ASDs among patients with OCD was still significantly higher after adjusting for confounding factors. Fifth, there might be other confounding factors that were hard to recognize such as intellectual disability. Finally, almost all our participants were Taiwanese, so the external validity of our findings remains uncertain.
In conclusion, patients with OCD were at an increased risk of developing ASDs compared to matched controls. Clinicians should be aware of this potential association and consider dermatological referrals as needed. Further studies are required to elucidate the underlying biological mechanisms.
Abbreviations
- AA
-
alopecia areata
- aHR
-
adjusted hazard ratio
- ASD
-
autoimmune skin disease
- CCI
-
Charlson Comorbidity Index
- CI
-
confidence interval
- HR
-
hazard ratio
- ICD-9-CM
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- NHIRD
-
National Health Insurance Research Database
- OCD
-
obsessive–compulsive disorder
Funding Statement
This study was funded by the Ministry of Science and Technology, R.O.C. under Grant MOST 107-2314-B-075-032-MY3-2.
Disclosure
The authors do not have anything to disclose.






