Introduction
Cobalt is a relatively rare but important metal widely used in production of super alloys, special steel, carbides, diamond tools, magnets, rechargeable batteries and many others (USGS, 2022). It also plays a vital catalytic role in life evolution and biochemical synthesis (He et al., Reference He, Wang, Yang, He, Zhong, Wang, Han and Jin2021; Russell, Reference Russell2022). In 2020, the worldwide mine production of cobalt amounted to 140,000 metric tons, with the biggest producer being the Democratic Republic of Congo (~95,000 tons), followed by Russia, Australia, Philippines, Cuba and Canada (Statista, 2021). In Nature, over 100 Co-bearing minerals have been identified, among which 66 mineral species have been approved by the Commission of New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA–CNMNC) (Hazen et al., Reference Hazen, Hystad, Golden, Hummer, Liu, Downs, Morrison, Ralph and Grew2017). Natural cobalt oxide minerals are very rare (Hey, Reference Hey1962). The spinel-structured Co2+-oxide mineral cochromite, (Co,Ni,Fe)(Cr,Al)2O4, is one such, found in the Bon Accord nickel deposit, South Africa (De Waal, Reference De Waal1978). The Co3+ minerals such as linnaeite (Co2+Co3+2S4) and heterogenite (Co3+O(OH)) also occur in some metal sulfide mines (Hey, Reference Hey1962; Deliens and Goethals, Reference Deliens and Goethals1973). Although synthetic Co3O4 has been studied widely (e.g. Natta and Schmidt, Reference Natta and Schmidt1926; Hendriks and Albrecht, Reference Hendriks and Albrecht1928; Osaki, Reference Osaki2018), and the existence of natural Co3O4 predicted by Hazen et al. (Reference Hazen, Hystad, Golden, Hummer, Liu, Downs, Morrison, Ralph and Grew2017), its occurrence in Nature has not been reported until now.
During a mineralogical investigation on the cobalt ores from the Sicomines copper–cobalt mine, Democratic Republic of Congo, a mineral with the composition of Co3O4 was found, characterised, submitted to the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA–CNMNC) and approved under IMA2017-080 (Lei et al., Reference Lei, Chen, Wang, Zhang, Huang, Lu and Du2017). The mineral name ‘guite’ (symbol Gui) is in honour of Prof. Xiangping Gu (1964–) of Central South University, Changsha, Hunan province, China. Prof. Gu obtained a BS degree in 1983 and a MS degree in 1986, both from the Central South University, and a DSc degree in 2003 from Hiroshima University in Japan. In the past 40 years he has made significant contributions to mineralogical research and teaching in China; in particular to new mineral discoveries, and is the leading author of over 12, and the co-author of over 22 new minerals to date. The type material of guite is deposited in Geological Museum of China in Beijing with the catalogue number M13711 and a cotype sample is deposited at the RRUFF Project (deposition # R180022) (http://rruff.info).
Here, we present detailed descriptions of the morphology, composition, physical property, and crystallography of this mineral using optical microscopy, electron probe microanalysis (EPMA) and X-ray diffraction (XRD).
Occurrence and mineral association
Guite was found in the Sicomines copper-cobalt mine at 10°44'17.4"S, 25°22'50.4"E, ~11 km southwest of Kolwezi City, Democratic Republic of Congo. The Sicomines Cu–Co deposit is located at the northwest end of the world famous Katanga Cu–Co belt extending from Congo (Kinshasa) to Zambia (Fig. 1a). The Cu–Co ores are hosted in the Proterozoic Roan Formation, which is composed of red and grey–green sandstone and mudstone (R1 group), dolostone and dolomitic sandstone (R2 group) and dolomitic siltstone and mudstone (R3 group) (Fig. 1b). The Co-bearing dolomite in the R2 group is considered to be the source of cobalt (Chen et al., Reference Chen, Liu, Yang, Wang, He and Li2012). The samples were taken from the drilling cores in the R2 group (Fig. 1b). Guite is supposed to be a supergene cobalt oxide formed from the precipitation of a cobalt-bearing solution at weakly alkaline and oxidising conditions:

Fig. 1. (a) Simplified regional geological map of the Katanga Cu–Co belt and the location of the Sicomines mine (after Zhao, Reference Zhao2016). Legend: 1. Neozoic cover; 2. Basement strata; 3. Nguba and Kundelungu Formations; 4. Roan Formation; 5. Cu–Co deposits. (b) Plan geological map of the Sicomines Cu–Co deposit (after Chen et al., Reference Chen, Liu, Yang, Wang, He and Li2012). Legend; 1: R3 Group of Roan Formation: dolomitic siltstone; 2: R2 Group of Roan Formation: talc-bearing argillaceous dolostone, silicified dolostone, dolomitic shale and siltstone; 3: R3 Group of Roan Formation: coarse-grained arkoses and conglomerate; 4: faults; 5: sampling locations.
Appearance and physical properties
Guite occurs as granular aggregates up to 500 μm in size associated closely with heterogenite in quartz, in which anhedral to subhedral single crystals of guite are estimated to be several to tens of μm (Fig. 2). Guite has a dark grey colour with metallic opaque lustre and black streak. The Mohs hardness is estimated to be 6–6.5. The mineral is brittle with uneven fracture. The calculated density is of 6.003 g/cm3 according to the empirical formula and unit cell volume from powder X-ray diffraction. Guite is non-magnetic as tested by a magnet needle.
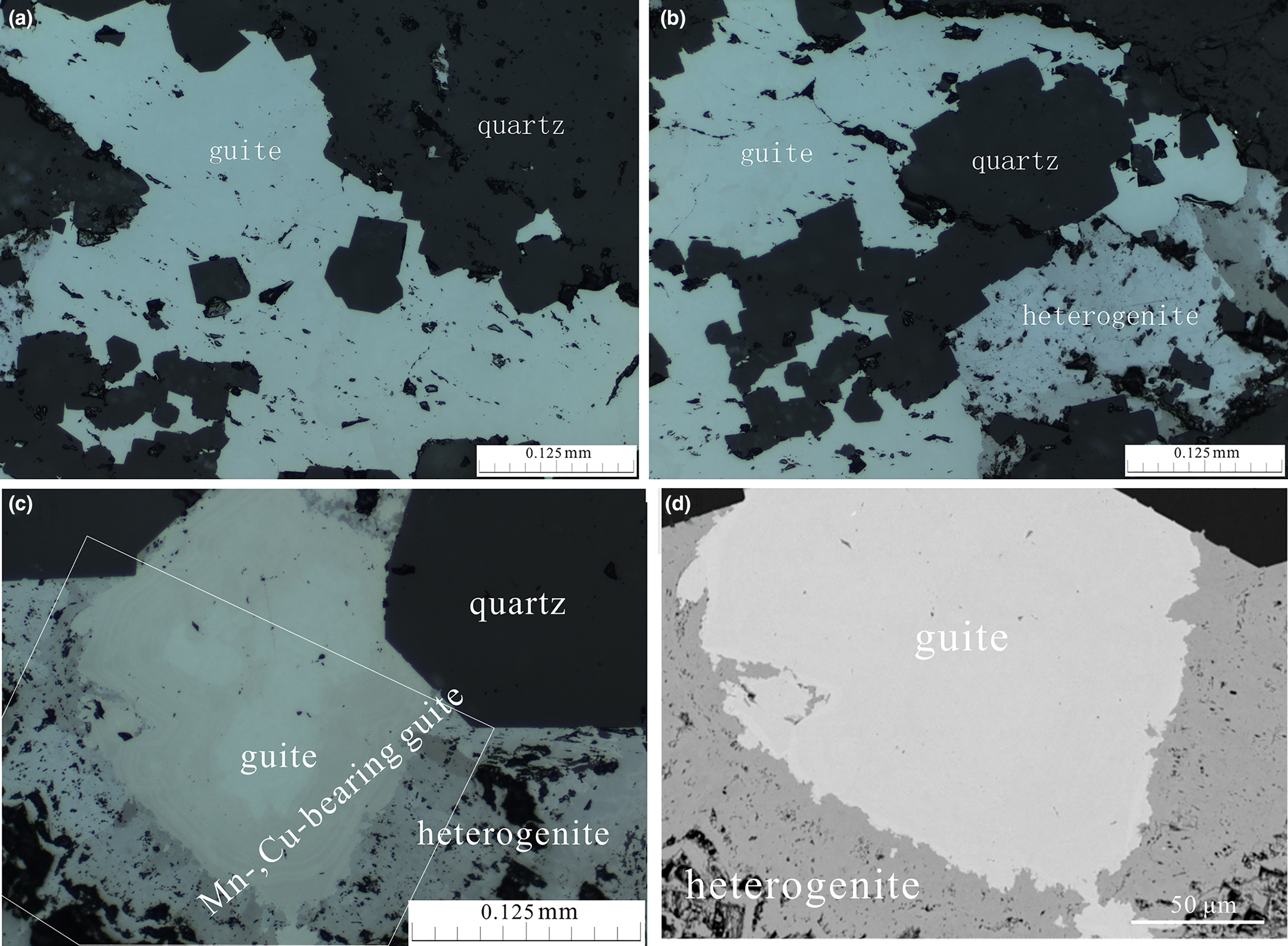
Fig. 2. Photomicrographs in reflected light (a–c) and back-scattered electron image (d) of guite. (a) Guite filling the interstitial space between quartz; (b) guite replaced by heterogenite in the interstitial space of quartz; (c) compositional zonation of guite – the core of almost pure Co3O4 surrounded by Mn-, Cu-bearing Co3O4, further replaced by heterogenite; and (d) back-scattered electron image of the area framed in (c).
In reflected light microscopy, guite has a white colour with no internal reflections. Zonar variation of reflectance and colour, from bright white (Fig. 2a,b) to brownish grey (Fig. 2c), is observed due to compositional zonation. The reflectance values (R, %) at different wavelength (λ, nm) measured using TIDAS MSP400 attached to LEICA DM2500p with SiC standard are shown in Table 1. The colour index is calculated relative to an equienergy lamp source. The data indicate that the reflectance of guite is ~25.4% for pure Co3O4 and apparently decreases to 22.9% when Cu, Mn and Si are incorporated (Table 1).
Table 1. Reflectance data for guite.

Colour index:
[1] x = 0.3298, y = 0.3325, Y = 25.4, λd = 480 nm, Pe = 1.44%
[2] x = 0.3320, y = 0.3335, Y = 22.9, λd = 481 nm, Pe = 0.55%
Chemical composition
The chemical composition of guite was determined on a Shimadzu EPMA-1720 microprobe by wavelength dispersive spectroscopy with operating conditions of accelerating voltage = 15 kV, beam current = 10 nA and beam size = 1 μm. Detectable elements include Co, Cu, Mn, Si and O; the contents of Fe, Mg, V and Ni are below the detection limits (~200 ppm) according to qualitative scans at specific wavelengths. Quantitative analysis was carried out using the following standards (and lines): pure Co (CoKα); pure Cu (CuKα); pure Mn (MnKα); pure Si (SiKα), and pure Fe3O4 (OKα). The ZAF4 correction program supplied with the instrument was used for the correction calculation. Compositional zoning is often observed (e.g. Fig 2c), mainly due to the variation of the contents of Cu (up to 2.50 wt.%) and Mn (up to 1.78 wt.%). The average of 20 electron-microprobe analyses is Co 71.53, Cu 0.58, Mn 0.67, Si 0.25, O 26.78, total 99.82 wt.% (Table 2). The empirical formula calculated on the basis of 4 oxygen atoms per formula unit and with partitioning of Co2+ and Co3+ to achieve charge balance is: (Co2+0.92Cu2+0.02Si4+0.02)Σ0.96(Co3+1.98Mn3+0.03)Σ2.01O4.00. The simplified formula is (Co2+,Cu2+,Si4+)(Co3+,Mn3+)2O4 and the ideal formula is Co2+Co3+2O4.
Table 2. Chemical data for guite (wt.%, N = 20).

S.D. – standard deviation
Raman spectroscopy
Raman spectra of randomly oriented crystals of guite were measured at two laser wavelengths (532.1 and 632.8 nm) with a Horiba LabRam ARAMIS instrument (laser power = 5 mW, resolution = 2 cm–1 and scan time = 10 min). All the five theoretical Raman-active modes, calculated from the factor-group analysis on synthetic Co3O4 (Hadjiev et al., Reference Hadjiev, Ilievl and Vergilov1988), are observed respectively at 197 (F2g), 487 (Eg), 530 (F2g), 625–630 (F2g) and 693–697 (A1g) cm–1 (Fig. 3). The Raman shifts may be attributed to the Co–O stretching vibration modes (500–700 cm–1) and the O–Co–O bending vibration modes (100–500 cm–1) in CoO4 tetrahedra and CoO6 octahedra. The relative intensities of peaks vary for different laser wavelengths. The spectrum of 532.1 nm has the strongest peak at 693–697 (A1g) cm–1 and the spectrum of 632.8 nm has the strongest peak at 197 (F2g) cm–1.

Fig. 3. Raman spectra of guite activated at two laser wavelengths.
Crystallography and crystal structure
Powder X-Kray diffraction data were obtained respectively using a Rigaku D/Max Rapid IIR diffractometer (CuKα; 40 kV; 250 mA; 0.1 mm beam diameter; and exposure time of 1 hour) and a Rigaku Synergy single-crystal diffractometer in Gandolfi powder mode (MoKα; 50 kV; 1 mA; 0.2 mm beam diameter; and exposure time of 20 min). The reflection data are given in Table 3. The eight strongest lines [d in Å (I/I0) (hkl)] are: 4.6714 (16.7) (111), 2.8620 (18.4) (220), 2.4399 (100) (311), 2.3348 (10.4) (222), 2.0230 (24.8) (400), 1.5556 (26.3) (511, 333), 1.4296 (37.7) (440) and 1.0524 (10.1) (731, 553). The unit cell parameters refined from the powder X-ray diffraction data are: a =8.0848(1) Å, V = 528.45(2) Å3 and Z = 8. The calculated density of guite is 6.003 g/cm3 according to the empirical formula.
Table 3. X-ray powder diffraction data (I in %, d in Å) for guite.*

* The strongest lines are given in bold.
The single-crystal X-ray diffraction data for a guite crystal, ~10 μm in size, were collected on Rigaku XtaLAB Synergy-DS diffractometer with microfocus sealed Mo anode tube at 50 kV, 1 mA and 25 s of frame exposure time. The diffraction data were processed with the Rigaku program CrysAlisPro. The crystal structure was determined and refined using SHELX (Sheldrick, Reference Sheldrick2015a,Reference Sheldrickb) included in the software Olex2 (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2009). The crystallographic data and refinement statistics are given in Table 4. The structure was solved in space group Fd $\bar{3}$![]() m and refined with anisotropic displacement for all sites. The occupancies of Co, Cu, Mn and Si at the Co1 and Co2 sites were fixed manually according to empirical formula from the chemical compositions. The final anisotropic full-matrix least-squares refinement on F 2 for 7 parameters was completed with R 1 = 1.32% and wR 2 = 3.52% for all 2007 (69 unique) reflections. The atomic coordinates and displacement parameters are listed in Table 5, and selected bond lengths and angles in Table 6. The bond-valence sums of atoms, calculated according to the actual composition using the parameters given by Brese and O'Keeffe (Reference Brese and O'Keeffe1991), are presented in Table 7. The structure is illustrated in Fig. 4. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
m and refined with anisotropic displacement for all sites. The occupancies of Co, Cu, Mn and Si at the Co1 and Co2 sites were fixed manually according to empirical formula from the chemical compositions. The final anisotropic full-matrix least-squares refinement on F 2 for 7 parameters was completed with R 1 = 1.32% and wR 2 = 3.52% for all 2007 (69 unique) reflections. The atomic coordinates and displacement parameters are listed in Table 5, and selected bond lengths and angles in Table 6. The bond-valence sums of atoms, calculated according to the actual composition using the parameters given by Brese and O'Keeffe (Reference Brese and O'Keeffe1991), are presented in Table 7. The structure is illustrated in Fig. 4. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).

Fig. 4. (a) Crystal structure of guite viewed in [0 1 1], showing the chains of CoO6 octahedra (green) along the directions [0 1 1], [1 0 1] and [1 1 0] with isolated CoO4 tetrahedra (blue) occupying the interstitial space of the chains. (b) The shape and edge lengths of CoO6 octahedra (twisted triprism) and CoO4 tetrahedra.
Table 4. Information on crystal and structural refinement for guite.

Table 5. Atomic coordinates and displacement parameters (in Å2) for guite.*

* Occupancies: Co1: Co0.92Si0.02Cu0.02Mn0.01; Co2 Co0.99Mn0.01; O1: O1.00
Table 6. Selected bond distances and angles for guite.

Table 7. Calculated bond-valence sums (in valence units) for atoms in guite.*

* Bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991)
Guite has the spinel-type structure in which the Co1 site is in tetrahedral coordination and occupied by Co2+ with a Co–O distance of 1.942 Å, and the Co2 site is in octahedral coordination and occupied by Co3+ with a Co–O distance of 1.919 Å (Table 6). In compliance with the site symmetry $\bar{3}$![]() m, the six faces of Co3+–O6 octahedra are composed of two equilateral triangles with the O–O distance of 2.550 Å and four isosceles triangles comprising two O–O of 2.689 Å and one O–O of 2.550 Å, which may be described more precisely by a twisted triprism (Fig. 4b). The Co3+–O6 octahedra share the edges at 2.550 Å to form a framework of chains of octahedra in three directions of [0 1 1], [1 1 0] and [1 0 1] with Co2+ occupying the interstitial spaces in tetrahedral coordination among the chains (Fig. 4a). The valence states of Co2+ at the Co1 site and Co3+ at the Co2 site are confirmed by the bond valence sums of the two sites (Table 7), and the different Co–O bonding lengths of the two sites (1.942 vs. 1.919 Å) suggest a high spin electronic structure for Co2+ and a low spin electronic structure for Co3+ according to the ionic radii of Shannon (Reference Shannon1976).
m, the six faces of Co3+–O6 octahedra are composed of two equilateral triangles with the O–O distance of 2.550 Å and four isosceles triangles comprising two O–O of 2.689 Å and one O–O of 2.550 Å, which may be described more precisely by a twisted triprism (Fig. 4b). The Co3+–O6 octahedra share the edges at 2.550 Å to form a framework of chains of octahedra in three directions of [0 1 1], [1 1 0] and [1 0 1] with Co2+ occupying the interstitial spaces in tetrahedral coordination among the chains (Fig. 4a). The valence states of Co2+ at the Co1 site and Co3+ at the Co2 site are confirmed by the bond valence sums of the two sites (Table 7), and the different Co–O bonding lengths of the two sites (1.942 vs. 1.919 Å) suggest a high spin electronic structure for Co2+ and a low spin electronic structure for Co3+ according to the ionic radii of Shannon (Reference Shannon1976).
Discussions and implications
In literature, numerous structural and physico-chemical data of synthetic Co3O4 have been reported (e.g. Natta and Schmidt, Reference Natta and Schmidt1926; Hendriks and Albrecht, Reference Hendriks and Albrecht1928; Liu and Prewitt, Reference Liu and Prewitt1990; Douin et al., Reference Douin, Guerlou Demourgues, Menetrier, Bekaert, Goubault, Bernard and Delmas2009; Osaki, Reference Osaki2018). All of them have the spinel structure with highly ordered occupation of Co2+ in the tetrahedral site and Co3+ in the octahedral site at room temperature and ambient atmosphere, with the unit cell a ranging from 8.065 Å to 8.086 Å, the Co–Otet bonding lengths in CoO4 tetrahedra from 1.908 Å to 1.988 Å and the Co–Ooct bonding lengths in CoO6 octahedra from 1.893 Å to 1.933 Å. A linear regression between Co–Otet and Co–Ooct yields:
The negative correlation between Co–Otet and Co–Ooct seems to indicate a variation of Co2+–Co3+ distribution between the tetrahedral site and the octahedral site. On the other hand, the high-temperature structural determinations of synthetic Co3O4 indicated a tendency of disorder of Co2+–Co3+ distribution between the tetrahedral site and the octahedral site at high temperatures (e.g. Liu and Prewitt, Reference Liu and Prewitt1990; Hazen and Yang, Reference Hazen and Yang1999; Douin et al., Reference Douin, Guerlou Demourgues, Menetrier, Bekaert, Goubault, Bernard and Delmas2009).
Guite is a member of the oxyspinel group in the spinel supergroup (Bosi et al., Reference Bosi, Biagioni and Pasero2019), which may be classified into Al-, V-, Cr-, Fe-, Co-subgroups according to the elements in the octahedral site, with various end-members of Mg, Si, V, Mn, Fe, Co, Cu, Zn, Ge and Sb occupying the tetrahedral site. The spinel structure is of special significance for probing the material states in the deep mantle (e.g. Hazen and Yang, Reference Hazen and Yang1999). As summarised and compared in Table 8, guite is outstanding in the group as it has the smallest unit cell, the biggest density and the highest reflectance. The presence of Si (up to 0.02 apfu), Mn (up to 0.08 apfu) and Cu (up to 0.09 apfu) in guite may predict the natural existence of end members SiCo2+2O4, Mn2+Co3+2O4 and CuCo3+2O4, which have been synthesised in the laboratory (e.g. Morimoto et al., Reference Morimoto, Tokonami, Watanabe and Koto1974; Gautier et al., Reference Gautier, Barbato and Brenet1982; Petrov et al., Reference Petrov, Krezhov and Konstantinov1989). It is interesting to note that hausmannite (Mn2+Mn3+2O4) and hetaerolite (ZnMn3+2O4) adopt a tetragonal structure, topologically similar to the spinel-type structure (Yamamoto et al., Reference Yamamoto, Kawano, Achiwa and Higashi1983; Jarosch, Reference Jarosch1987). The high-pressure transformation of guite, such as that of chromite to xieite and chenmingite (Chen et al., Reference Chen, Shu and Mao2008; Ma et al., Reference Ma, Tschauner, Beckett, Liu, Greenberg and Prakapenka2019) and magnesioferrite to maohokite (Chen et al., Reference Chen, Shu, Xie and Tan2018), is still unknown though it has been shown to be stable up to 1201 K and 8.7 GPa (e.g. Liu and Prewitt, Reference Liu and Prewitt1990; Golosova et al., Reference Golosova, Kozlenko, Nicheva and Savenko2020).
Table 8. Unit cell data, calculated density, bonding lengths in tetrahedra and octahedra, and reflectance of the minerals and high pressure phases in the oxyspinel group.

*high-pressure phase. 1. This work; 2. Knop et al. (Reference Knop, Reid, Sutarno and Nakagawa1968); 3. Fregola et al. (Reference Fregola, Bosi, Skogby and Halenius2012); 4. Cámara et al. (Reference Cámara, Bindi, Pagano, Pagano, Gain and Griffin2019); 5. Nair et al. (Reference Nair, Fu, Voigt, Su and Brueckel2014); 6. Palache et al. (Reference Palache, Berman and Frondel1944), Hill (Reference Hill1984); 7. Cooley and Reed (Reference Cooley and Reed1972); 8. Verger et al. (Reference Verger, Dargaud, Rousse, Rozsalyi, Juhin, Cabaret, Cotte, Glatzel and Cormier2016); 9. Rao (pers. Comm.); 10. Reznitskii et al. (Reference Reznitskii, Sklyaov and Ushchapovskaya1995); 11. Plumier (Reference Plumier1962); 12. Reuter et al. (Reference Reuter, Riedel, Hug, Arndt, Geisler and Behnke1969); 13. O'Neill et al. (Reference O'Neill and Dollase1994); 14. Hastings and Corliss (Reference Hastings and Corliss1962); 15. Palache et al. (Reference Palache, Berman and Frondel1944), Kyono et al. (Reference Kyono, Gramsch, Yamanaka, Ikuta, Ahart, Mysen, Mao and Hemley2012); 16. Ma et al. (Reference Ma, Tschauner, Beckett, Liu, Greenberg and Prakapenka2019); 17. Ishii et al. (Reference Ishii, Kojitani, Tsukamoto, Fujino, Mori, Inaguma, Tsujino, Yoshino, Yamazaki, Higo, Funakoshi and Akaogi2014); Chen et al. (Reference Chen, Shu and Mao2008); 18. De Waal (Reference De Waal1978), Hirota et al. (Reference Hirota, Inoue, Mochida and Ohtsuka1990); 19. Ueno et al. (Reference Ueno, Sato and Kino1999); 20. Nesterov and Rumyantseva (Reference Nesterov and Rumyantseva1987), Bosi et al. (Reference Bosi, Andreozzi, Halenius and Skogby2011); 21. Palache et al. (Reference Palache, Berman and Frondel1944), Gaudon et al. (Reference Gaudon, Pailhe, Wattiaux and Demourgues2009); 22. Chen et al. (Reference Chen, Shu, Xie and Tan2018); 23. Rashmi et al. (Reference Rashmi, Naik, Jayadevappa, Sudhamani, Patil and Naik2017); 24. Palache et al. (Reference Palache, Berman and Frondel1944); 25. Kiselev et al. (Reference Kiselev, Proskurnina, Voronin and Cherepanov2007); 26. Ahmed et al. (Reference Ahmed, Afify, El Zawawia and Azab2012); 27. Waerenborgh et al. (Reference Waerenborgh, Figueiredo, Cabral and Pereira1994); 28. Sasaki et al. (Reference Sasaki, Prewitt, Sato and Ito1982); 29. Yagi et al. (Reference Yagi, Marumo and Akimoto1974); 30. Forster and Hall (Reference Forster and Hall1965); 31. Ottemann and Nuber (Reference Ottemann and Nuber1972), Welch et al. (Reference Welch, Cooper and Hawthorne2001); 32. Bonazzi et al. (Reference Bonazzi, Chelazzi and Bindi2013), Dunn et al. (Reference Dunn, Peacor, Criddle and Stanley1988); 33. Bosi et al. (Reference Bosi, Hålenius and Skogby2014); 34. Solano et al. (Reference Solano, Frontera, Puig, Obradors, Ricart and Ros2014); Bozi et al. (2019); 35. Collyer et al. (Reference Collyer, Grimes, Vaughan and Longworth1988); Bozi et al. (2019); 36. Jarosch (Reference Jarosch1987); and 37. Yamamoto et al. (Reference Yamamoto, Kawano, Achiwa and Higashi1983).
In the Li-ion batteries industry, synthetic Co3O4 has attracted wide attention as an anode material for its low cost and high theoretical capacity (890 mA h/g) (e.g. Zhang et al., Reference Zhang, Yang, Li, Xie and Shen2017; Xiao et al., Reference Xiao, Zhang, Tang, Fan, Hu, Zhang, Deng and Chen2018). In the Sicomines mine, guite is an important economic mineral and accounts for about one-fifth of the cobalt resource; this discovery of guite originated from the work to increase the recovery rate of cobalt from the ores.
Acknowledgements
The authors thank Prof. Xiande Xie of Guangzhou Institute of Geochemistry, Chinese Academy of Science, for his help in preparing the manuscript, and Prof. Xiangping Gu of Central South University for his assistance in microprobe analyses and structural determination. The manuscript was improved from the comments of Prof. Peter Leverett, Dr. Fernando Camara and an anonymous reviewer.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.27