The voluntary addition of micronutrients to foods, or voluntary food fortification, represents a strategy to alleviate the prevalence of low micronutrient intakes and suboptimal status observed throughout Europe( Reference Cashman, Muldowney and McNulty 1 – Reference Whitton, Nicholson and Roberts 7 ) and also internationally( 8 , Reference Rhodes, Clemens and Goldman 9 ). Since July 2007, the addition of nutrients and other substances to foods has been regulated at a European Union (EU) level through Regulation (EC) No. 1925/2006( 10 ). The discretionary nature of such additions by food manufacturers, in addition to a highly variable and changing food market, presents a challenge with regard to evaluating the impact of fortification on micronutrient intakes. The adoption of 1925/2006/EC has provided for the setting of safe levels of addition of micronutrients and other substances to foods, and several models have been proposed( Reference Flynn, Moreiras and Stehle 11 – Reference Rasmussen, Andersen and Dragsted 13 ); however, these levels have not yet been agreed upon or implemented. Therefore, it is important to monitor fortification practices and consumption of fortified foods in order to assess the efficacy and safety of such additions on an ongoing basis at a EU level.
There are a limited number of studies that track the consumption of fortified foods and the impact of food fortification over time in European adults( Reference Geurts, Westenbrink and Verkaik-Kloosterman 14 , Reference Van Rossum, Verkaik-Kloosterman and Beukers 15 ) and children( Reference Sichert-Hellert, Kersting and Alexy 16 – Reference Sichert-Hellert, Kersting and Schoch 18 ). Data from the Dutch National Food Consumption Surveys of young adults (2003, 2007–10) have shown an increase in the supply of fortified foods and in the consumption of these foods( Reference Geurts, Westenbrink and Verkaik-Kloosterman 14 ), in addition to an increased contribution of fortified foods to micronutrient intakes during the last decade in The Netherlands( Reference Van Rossum, Verkaik-Kloosterman and Beukers 15 ). The Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) study has tracked the consumption of fortified foods by 1- to 18-year-old Germans since 1985( Reference Sichert-Hellert, Kersting and Alexy 16 – Reference Sichert-Hellert, Kersting and Schoch 18 ). Consumption of fortified foods by German children and adolescents increased significantly between 1985 and 1996( Reference Sichert-Hellert, Kersting and Schoch 18 ). Similarly, the contribution of fortified foods to nutrient intakes increased significantly over time for most nutrients( Reference Sichert-Hellert, Kersting and Alexy 16 ).
In response to the issues experienced by some EU Member States in compiling data for the European Commission report on evaluation of 1925/2006/EC, the International Life Sciences Institute (ILSI, Europe) Addition of Nutrients Expert Group has proposed an ‘ideal scenario’ for monitoring changes in micronutrient intake from foods in the context of 1925/2006/EC( Reference Casala, Matthys and Péter 19 ). These criteria include data from detailed nationally representative food consumption surveys, pre- and post-2007, detailed brand information and regularly updated food composition databases. The food consumption data of nationally representative samples of adults in Ireland collected at two time points by the Irish Universities Nutrition Alliance (IUNA; www.iuna.net), along with detailed fortified food composition data, fulfil these criteria and provide an excellent opportunity to analyse the trends in the consumption of fortified foods and its impact on nutrient intakes in a country that has experienced liberal fortification practices. The aims of the present study were to characterise the changes in the supply of fortified foods in Ireland between 1997–9 and 2008–10, to investigate the trends in the consumption of fortified foods by Irish adults, and to assess the impact of the changes in voluntary fortification practices on intakes of macronutrients and on intakes, adequacy and risk of excessive intake of micronutrients.
Experimental methods
The impact of voluntary fortification practices on nutrient intakes in Irish adults between 1997 and 1999 has been described previously by Hannon et al. ( Reference Hannon, Kiely and Flynn 20 ). In order to describe the trends in the impact on nutrient intakes, reanalysis of the raw data collected from both surveys was carried out to obtain uniformity with respect to cut-off points for dietary reference values and identification of under-reporters.
Food consumption data
The North/South Ireland Food Consumption Survey (NSIFCS) (1997–9)( 21 ) investigated the habitual intakes of foods and nutrients in Irish adults aged between 18 and 64 years (n 1379: 662 men and 717 women) using a 7 d estimated food diary. During the NSIFCS, a fieldworker made four visits (on average 30 min per visit) to the participants, at their home or place of work, during the survey period: a training session, where the participants were instructed how to complete the food and beverage diary (to include nutritional supplements); a visit on day 2 and day 4 or 5, where the fieldworker reviewed the diary and clarified any details where necessary; a final visit on day 8 (at the end of the survey period). A detailed description of the survey design and methodology for the NSIFCS is available elsewhere( Reference Harrington, Robson and Kiely 22 , Reference Kiely, Flynn and Harrington 23 ). The National Adult Nutrition Survey (NANS) (2008–10)( 2 ), also carried out by the IUNA, collected detailed dietary intake data and health and lifestyle characteristics of a representative sample of Irish adults aged >18 years (n 1500: 740 men and 760 women). A 4 d semi-weighed food diary (including at least one weekend day) was used to collect food and beverage intake data. Participants received a training in which they were provided with a digital food scale (Tanita) and a food diary, and were instructed how to weigh and record all the food and beverages consumed, including nutritional supplements, using the participants’ own food and beverage intake from the previous day as an example. The researcher carried out two detailed reviews of the diary to ensure completeness and to clarify, where necessary, food and beverage descriptors. Participants were also asked to keep any food packaging from the food and beverages consumed during the survey period. A detailed description of the survey methodology for the NANS is available elsewhere( 2 ). For both surveys, a hierarchical approach was used for the quantification of foods consumed: (1) weighing (NSIFCS: 36 %; NANS: 46 %); (2) photographic food atlas (NSIFCS: 10 %; NANS: 16 %); (3) manufacturer's product information (NSIFCS: 11 %; NANS: 10 %); (4) IUNA food weights (NSIFCS: 9 %; NANS: 4 %); (5) food portion sizes( Reference Mills and Patel 24 , 25 ) (NSIFCS: 20 %; NANS: 11 %); (6) household measures (NSIFCS: 2 %; NANS: 11 %); (7) estimation (NSIFCS: 11 %; NANS: 2 %).
In the present study, adults aged 18–64 years (n 1274: 634 men and 640 women) were selected from the NANS in order to allow comparison with those from the NSIFCS. Analysis of the demographic features of both survey samples has shown them to be a representative sample of Irish adults at each time point with respect to age, sex, social class and geographical location( 26 , 27 ). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals, University College Cork and the Human Ethics Research Committee of University College Dublin. Written informed consent was obtained from all the subjects. A concise overview of the methods pertinent to the present study is outlined below.
Food composition data
Nutrient intake data collected from both surveys were estimated using WISP©, which included data from the UK food composition tables, McCance and Widdowson's The Composition of Foods 6th Edition(28) (NANS only) and 5th Edition( Reference Holland, Welch and Unwin 29 ) plus supplemental volumes( Reference Chan, Brown and Buss 30 – Reference Holland, Widdowson and Unwin 38 ), and the Irish Food Composition Database( Reference Black, Ireland and Møller 39 ). The Irish Food Composition Database has been consistently updated during each Irish national nutrition survey to reflect the most recent composition data for fortified foods, nutritional supplements, composite dishes and Irish brands consumed that were not adequately characterised by the UK food composition tables. In both surveys, the accuracy of food composition, as well as consumption, was aided by asking the participants to retain food packaging during the survey period.
Identification of fortified foods and fortified food consumers
For both surveys, fortified foods were identified by the presence of vitamins and/or minerals in the ingredient list on the food label. ‘Fortification’ refers to the voluntary addition of micronutrients by food manufacturers and excludes (semi) mandatory addition of vitamins A and D to fat spreads and skimmed milk to ensure ‘nutritional equivalence’ and addition of micronutrients to flour for the purposes of ‘restoration’. These additions are considered ‘indigenous’ for the purpose of the present study. Fat spreads and skimmed milk, which were fortified with nutrients other than vitamins A and D, were included as fortified foods; however, the vitamin A and D content of such foods was not included as fortified sources of vitamins A and D.
Pre-fortification levels of micronutrients in fortified foods were obtained from the manufacturers or by using compositional data for unfortified equivalent of a food. Participants of the NSIFCS and NANS were classified as fortified food consumers if they consumed a fortified food at least once during either survey period.
Adequacy of micronutrient intakes
In consumers of fortified foods, the adequacy of a number of micronutrient intakes, both including and excluding the added nutrient component from voluntary fortification, was assessed using the estimated average requirement or EAR cut-point method, proposed by Beaton( Reference Beaton, Shils, Olson and Shike 40 ) and described by the Institute of Medicine( 41 ), in which the population prevalence of inadequate intakes is computed as the proportion of the group with intakes below the median requirement (EAR). The present study used the UK Department of Health EAR( 42 , 43 ) to assess the population prevalence of inadequate intakes. In the case of vitamin D, the Institute of Medicine EAR( 44 ) was applied. Intakes of vitamin D < 5 μg/d were also assessed. As misreporting of food consumption is known to affect the estimate of micronutrient adequacy( Reference Becker and Welten 45 ), under-reporters of energy intake, identified as having an energy intake:BMR( Reference Henry 46 ) ratio of < 1·1( Reference Goldberg, Black and Jebb 47 ), were excluded from the analysis (NSIFCS: 19·1 %; NANS: 28·2 %). As women of childbearing age (18–50 years) have been identified as a subgroup of particular importance due to their high requirement and risk of suboptimal intake of Fe and folate in particular( 2 ), the impact of fortification on the adequacy of these nutrient intakes was examined separately for this subgroup. In addition, the potential impact of increased folic acid intake from fortified foods on the reduction in the risk of neural tube defects (NTD) in infants was estimated with reference to the data of Daly et al. ( Reference Daly, Kirke and Molloy 48 ), in which the erythrocyte folate level of women during early pregnancy was associated with the risk of NTD-affected pregnancy in a continuous dose–response relationship. These data were incorporated into the randomised controlled trial by Daly et al. ( Reference Daly, Mills and Molloy 49 ) who investigated the amount of folic acid a food fortification programme would have to deliver to produce concentrations of erythrocyte folate that is known to protect against NTD. Based on their findings that daily intakes of 100, 200 and 400 μg folic acid would confer a 22, 41 and 47 % reduction in the risk of NTD-affected pregnancy( Reference Daly, Mills and Molloy 49 ), we estimated a reduction in the risk of NTD-affected pregnancy conferred by current fortification practices by linear extrapolation.
Risk of excessive micronutrient intakes
The impact of the changes in voluntary fortification practices on the risk of excessive micronutrient intakes between 1997–9 and 2008–10 was assessed by (1) expressing the 95th percentile (P95) of intake as a percentage of the tolerable upper intake level (UL), and (2) estimating the percentage of the population with intakes exceeding the UL, both including and excluding the added nutrient component from voluntary fortification. The UL is defined as the maximum level of total chronic daily intake of a nutrient (from all sources) that is judged to be unlikely to pose a risk of adverse health effects to humans( 50 ). The present study used UL derived by the European Food Safety Authority to assess the risk of excessive intakes including and excluding the added nutrient component from fortified foods for retinol, vitamin D, vitamin E, preformed niacin, vitamin B6, folic acid, Ca, Zn and Cu( 51 – 59 ). In the absence of a UL set by the European Food Safety Authority for Fe, vitamin C and P, the UL established by US Food and Nutrition Board were applied( 60 – 62 ).
Statistical analyses
Statistical analyses were performed using SPSS version 20.0 (SPSS, Inc.). Mean daily energy intake (kJ) and percentage of contribution to total daily energy intake from all fortified foods and from fortified food categories were determined for consumers of fortified foods, stratified by survey. The distribution of the data was formally assessed for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests and also through visual examination of Q–Q plots and histograms. Results for intake of macronutrients and micronutrients are presented as means and standard deviations or as medians, stratified by survey, and significant differences in the percentage of contribution across the surveys were determined by the Mann–Whitney U test, as all data were positively skewed. Percentile intakes of micronutrients (P5, P50 and P95), both including and excluding added nutrients from voluntary fortification, are presented, and significant differences in intake before and after fortification were determined by the Wilcoxon signed-rank test. For both surveys, the relationship between voluntary fortification and subsequent reduction in the prevalence of inadequate micronutrient intakes in men and women was examined by McNemar's test for categorical variables. The P95 intake of micronutrients was expressed as a percentage of the UL, both including and excluding the added nutrient component from voluntary fortification. For all statistical analyses, an α value of 0·05 was considered statistically significant.
Results
Trends in the consumption of fortified foods
The total number of fortified foods reported increased from fifty-four in the NSIFCS (1997–9) to 150 in the NANS (2008–10) (Table 1). An additional three food categories, fat spreads, yogurt and confectionery, contained fortified foods in the NANS. The number of fortified foods within the food categories also increased between the two surveys, e.g. beverages increased from seven to twenty-six, breads from two to thirteen, milk from four to ten, and cereal bars from one to eight. A higher proportion of adults were consumers of fortified foods in the NANS (82 %) than those in the NSIFCS (66 %) (Table 2). Similarly, mean energy intake from fortified foods (406 kJ (NSIFCS) v. 711 kJ (NANS)) as well as the mean proportion of total energy (TE) (4·6 % TE (NSIFCS) v. 8·4 % TE (NANS)) in consumers of fortified foods increased between the two surveys. The P95 intake of energy from fortified foods increased from 1051 kJ (11·8 % TE) in the NSIFCS to 1820 kJ (20·7 % TE) in the NANS. Ready-to-eat breakfast cereals remained the fortified food category most commonly consumed by Irish adults in the NANS (53 %) and contributed 338 kJ (4·0 %) to mean daily energy intake, similar to the contribution observed in the NSIFCS (367 kJ, 4·1 % TE). However, breads (146 kJ), milk (60 kJ), fat spreads (44 kJ) and beverages (41 kJ) made increased contributions to both the absolute and proportion of mean daily energy intake (1·8, 0·7 and 0·5 %, respectively) when compared with the data from the NSIFCS.
Table 1 Number of fortified foods per food group consumed by the participants of the NSIFCS (North/South Ireland Food Consumption Survey, 1997–9) and the NANS (National Adult Nutrition Survey, 2008–10)
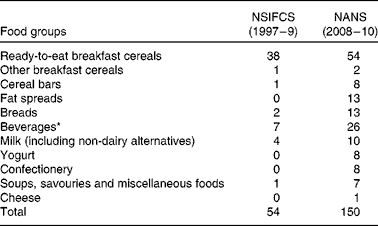
* Beverages included fruit juices, fruit juice drinks and cordials, powdered drinks, and sports and energy drinks.
Table 2 Mean daily energy intake (kJ) and percentage of energy from fortified foods (FF) in Irish adult consumers of FF in the NSIFCS (North/South Ireland Food Consumption Survey) and the NANS (National Adult Nutrition Survey) (Mean values and standard deviations)

* Beverages included fruit juices, fruit juice drinks and cordials, powdered drinks, and sports and energy drinks.
Contribution of fortified foods to nutrient intakes
Relative to the contribution of fortified foods to energy intake (8·4 % TE) in the NANS, fortified foods contributed higher amounts of carbohydrate, starch and dietary fibre (12·8, 15·5 and 10·3 %, respectively), while contributing lower amounts of protein, total fat, saturated fat and monounsaturated fat (5·6, 4·9, 4·2 and 3·9 %, respectively). The contribution of fortified foods to the intake of total sugars (9·1 %), non-milk sugars (9·0 %) and polyunsaturated fat (8·0 %) was in line with their contribution to energy intake. While the contribution of fortified foods to macronutrient intake was significantly greater for all macronutrients (P= 0·000) in the NANS than in the NSIFCS, the greater contribution to mean daily intakes (MDI) of carbohydrate, starch and fibre and the lower contribution to those of protein and fat remained constant (Table 3).
Table 3 Percentage of contribution of fortified foods to mean daily intakes (MDI) of macronutrients in consumers of fortified foods in the NSIFCS (North/South Ireland Food Consumption Survey) and the NANS (National Adult Nutrition Survey)* (Mean values and standard deviations; medians)
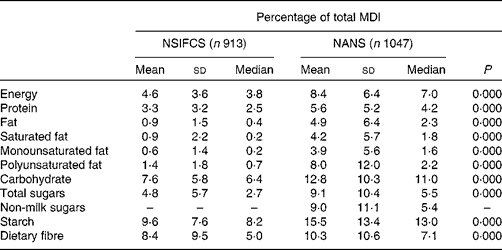
* Significant differences in the percentage of contribution across the surveys were determined by the Mann–Whitney U test.
Fortified foods in the NANS contributed substantially to the MDI of most micronutrients, relative to their contribution to energy intake, particularly for folate (105 μg, 26·5 %), Fe (3·1 mg, 20·6 %), vitamin B6 (0·8 mg, 21·3 %), riboflavin (0·4 mg, 18·6 %), thiamin (0·4 mg, 18·3 %), preformed niacin (5·0 mg, 16·8 %), vitamin E (1·8 mg, 14·9 %), vitamin D (0·6 μg, 12·5 %) and Ca (111 mg, 11·2 %). A greater contribution to micronutrient intakes, relative to energy intake, was also observed in the NSIFCS (Table 4). When compared with the NSIFCS, fortified foods in the NANS made significantly greater contributions to the intakes of most micronutrients examined, particularly for Ca (NSIFCS: 28 mg; NANS: 111 mg), vitamin E (NSIFCS: 0·3 mg; NANS: 1·8 mg), retinol (NSIFCS: 10 μg; NANS: 39 μg) and vitamin D (NSIFCS: 0·3 μg; NANS: 0·6 μg). The percentage of contribution of fortified foods to the intakes of riboflavin, preformed niacin and Fe was similar across the two surveys. The intake of Na from fortified foods in the NANS (215 mg) was similar to that observed in the NSIFCS (205 mg). The contribution of fortified foods to Na intake in the NANS (8·7 %) was similar to the contribution to energy intake (8·4 %).
Table 4 Micronutrient intakes (total and from fortified foods (FF)) and percentage of contribution of FF to mean daily intake in consumers of FF in the NSIFCS (North/South Ireland Food Consumption Survey) and the NANS (National Adult Nutrition Survey)*

* Significant differences in the percentage of contribution across the surveys were determined by the Mann–Whitney U test.
Table 5 presents the effect of voluntary fortification on the P5, P50 and P95 of micronutrient intakes in men and women consumers of fortified foods in the NSIFCS and the NANS. The addition of nutrients to foods in the NANS (2008–10) contributed an additional 80 μg folate, 0·6 μg vitamin D, 0·6 mg vitamin B6, 0·3 mg thiamin and riboflavin, and 2·2 mg Fe to median intakes in consumers of fortified foods, increasing median intakes significantly (P< 0·001) by 32, 25, 30, 18, 21 and 22 %, respectively. For high intake (as defined by the P95 of intake), the addition of nutrients to foods increased folate by 168 μg (29 %), vitamin B6 by 2·8 mg (45 %), Fe by 4·8 μg (21 %), vitamin D by 1·4 μg (13 %), riboflavin by 0·7 mg (14 %) and preformed niacin by 8·2 mg (16 %). The impact of fortification on median and P95 intakes of these micronutrients in the NANS was more marked than that observed in the NSIFCS (1997–9).
Table 5 Effect of added nutrients on intakes of micronutrients from all sources at the 5th (P5), 50th (P50) and 95th (P95) percentiles in consumers of fortified foods in the NSIFCS (North/South Ireland Food Consumption Survey) and the NANS (National Adult Nutrition Survey)*

* Significant increases in intake after fortification were determined by the Wilcoxon signed-rank test.
Impact of fortification on the adequacy of micronutrient intakes and on the risk of excessive intakes
Table 6 presents the effect of added nutrients on the proportion of consumers of fortified foods with inadequate micronutrient intakes. In both the NANS and the NSIFCS, voluntary food fortification had a modest effect on the adequacy of micronutrient intakes in men. However, in women, the addition of nutrients to foods had a more marked impact on the reduction in the proportion of consumers of fortified foods with inadequate intakes of micronutrients, particularly Fe, folate and vitamin D. The addition of nutrients to foods in the NSIFCS reduced the proportion of women consumers of fortified foods with inadequate intakes of folate from 10 to 2 %, Fe from 56 to 39 % and intakes of vitamin D < 5μg/d from 77 to 72 % (P= 0·000, respectively). Similarly, in the NANS, fortification reduced the proportion of women consumers with intakes of vitamin D < 5 μg/d from 82 to 75 %, and inadequate intake of folate from 10 to 4 % and Fe from 55 to 36 % (P= 0·000, respectively). In women of childbearing age (18–50 years) in the NANS, the addition of nutrients to foods reduced the proportion of consumers with inadequate intakes of vitamin D ( < 5 μg) from 87 to 79 %, folate from 11 to 5 % and Fe from 70 to 47 % (data not shown). While the impact of voluntary fortification on the reduction of inadequate folate intake in women in the NANS appeared modest, the addition of folic acid to foods contributed an additional 72 μg/d to median intake in women of childbearing age (data not shown), which may represent a 16 % reduction in the risk of NTD-affected pregnancies, with reference to the data of Daly et al. ( Reference Daly, Mills and Molloy 49 ).
Table 6 Effect of fortification on the percentage of consumers of fortified foods with inadequate micronutrient intakes, excluding under-reporters in the NSIFCS (North/South Ireland Food Consumption Survey, 1997–9) and the NANS (National Adult Nutrition Survey, 2008–10)*

EAR, estimated average requirement.
* McNemar's test used to determine the significant differences in the prevalence of inadequate intake with and without the inclusion of added nutrients.
† Values in parentheses denote female average requirement.
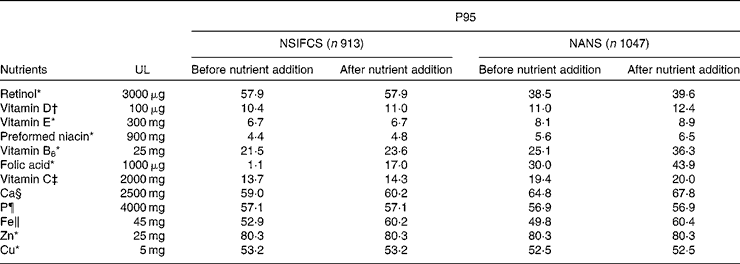
Table 7 presents the effect of food fortification on the P95 of micronutrient intakes as a percentage of the tolerable UL. The P95 as a percentage of the UL did not approach 100 % for any micronutrient, when excluding or including fortification. Although a small proportion ( < 2 %) of men and women consumers of fortified foods in the NSIFCS and the NANS exceeded the UL for some micronutrients (Fe, Cu, Zn, retinol and vitamin B6), the inclusion of added nutrients from fortified foods did not contribute to an increase in the risk of intakes exceeding the UL.
Table 7 95th Percentile (P95) intake as a percentage of the tolerable upper intake level (UL) in consumers of fortified foods in the NSIFCS (North/South Ireland Food Consumption Survey, 1997–9) and the NANS (National Adult Nutrition Survey, 2008–10)
Discussion
The present study provides data on recent trends in the consumption of fortified foods and its impact on nutrient intake in adults over time in a EU country with a history of liberal fortification practices. Our examination of fortified food consumption over 10 years has shown that the supply of fortified foods in Ireland increased between 1997–9 and 2008–10, resulting in a greater proportion of adults consuming fortified food, from 67 % in the NSIFCS to 82 % in the NANS, and a greater contribution of fortified foods to mean daily energy intake (NSIFCS: 4·6 %; NANS: 8·4 %). The fortified foods driving this increase were predominantly from staple food categories such as milk, fat spreads and breads. While ready-to-eat breakfast cereals remained the fortified food category most commonly consumed by Irish adults, the proportion of consumers of this fortified food group decreased somewhat (NSIFCS: 62 %; NANS: 53 %), associated with an increasing proportion of adults consuming porridge (non-fortified) as an alternative to breakfast cereal (NSIFCS: 15 %; NANS: 23 %).
The present study shows that the overall nutrient profile of fortified foods consumed in the two surveys remained predominantly higher in carbohydrate, starch and dietary fibre and lower in protein and fat, relative to energy contribution. Concerns have been raised about the potential for liberal voluntary fortification practices to drive unfavourable consumption patterns of macronutrients( Reference Meltzer, Aro and Andersen 63 ). However, the liberal fortification practices experienced in Ireland do not support this. The data of the NANS show that the contribution of fortified foods to fat intake, in particular saturated fat intake, remained well below their contribution to energy intake. The contribution of fortified foods to intakes of polyunsaturated fat in the NANS increased, a change that is attributable to the inclusion of low-fat spreads as fortified foods, many of which are fortified with vitamin B6 and folic acid. The contribution of fortified foods to intakes of total sugars (9·1 %), non-milk sugars (9·0 %) and Na (8·7 %) remained in line with their contribution to energy intake (8·4 %).
The observed increase in the supply of fortified foods in Ireland since 1997–9 has resulted in significantly greater contributions to daily intakes of many micronutrients from fortified foods in the NANS than that observed in the NSIFCS, particularly for Fe, folate and other B vitamins, due to the role of fortified breads, cereal bars and fat spreads in the diets of Irish adults. For nutrients such as riboflavin, niacin and Fe, the percentage of contribution of fortified foods to MDI remained largely unchanged between the two surveys, due to the similar intake of ready-to-eat breakfast cereals in the diets of Irish adults in both surveys. While the contribution of fortified foods to the intake of Ca in the NANS (11 %) appeared to have increased since the NSIFCS (3 %), this was not due to added Ca from fortification but was mainly attributable to some fortified foods, such as milk and yogurt, being natural sources of Ca that replace other (non-fortified) natural sources. The median added micronutrient content per average serving of fortified food expressed as a percentage of the EC RDA( 64 ) has not changed substantially between the two surveys( Reference Black, Ireland and Møller 39 , Reference Hennessy 65 ); therefore, these increases in contribution to the total intake of micronutrients are indicative of increased consumption of fortified foods rather than increased levels of addition of micronutrients.
Data from both the NSIFCS and the NANS have shown that women remain the key beneficiaries of voluntary fortification practices in Ireland. The continued voluntary addition of Fe to foods such as ready-to-eat breakfast cereals and cereal bars, observed between 1997–9 and 2008–10, has made a significant contribution to the daily intake of Fe (2·5–3·1 mg/d, accounting for 18–21 % of MDI), and also had a sustained beneficial effect on reducing the prevalence of inadequate Fe intake in women from 56 to 39 % in the NSIFCS and from 55 to 36 % in the NANS, and particularly in women of childbearing age, from 68 to 48 % (NSIFCS) and from 70 to 47 % (NANS).
Although intakes of folate appear to be adequate in women consumers of fortified foods in relation to the average requirement, this requirement does not reflect the separate recommendation of 400 μg supplemental folic acid per d for women of childbearing age( 66 ). In light of low compliance with this recommendation of folic acid supplementation (6 %)( Reference Hennessy 65 ) and the poor folate status observed in this subgroup( Reference Hopkins, McNulty and Walton 67 ), fortified foods in the NANS made a very important contribution to the intakes of folate in women of childbearing age (72 μg/d), estimated to confer a 16 % reduction in the risk of NTD( Reference Daly, Mills and Molloy 49 ). The added folic acid component observed in the NANS was greater than that observed in the NSIFCS (49 μg/d) by Hannon et al. ( Reference Hannon, Kiely and Flynn 20 ), reflecting the role of folic acid-fortified breads, milk and fat spreads in the diets of adults during 2008–10. Our estimate of added folate was comparable to that observed in an intervention that removed folic acid-fortified foods from the diets of Northern Irish women aged 19–40 years( Reference Cuskelly, McNulty and Scott 68 ), which resulted in a decrease of 78 μg/d, leading to a 12 % decrease in erythrocyte folate levels. Furthermore, in a recent analysis of folate status of the total NANS sample (age 18–90 years), Hopkins et al. ( Reference Hopkins, McNulty and Walton 69 ) reported that consumers of folic acid-fortified foods had significantly better folate status than non-consumers.
Previous analysis of the NANS dataset has shown a substantial prevalence of inadequate vitamin D intake( Reference Hennessy 65 ), along with poor vitamin D status (as defined by status that is considered inadequate for bone health) observed in 40 % of Irish adults( Reference Cashman, Muldowney and McNulty 1 ). The voluntary addition of vitamin D to foods during both surveys made a notable contribution to daily intake (increasing from 8 % in the NSIFCS to 13 % in the NANS) and increased the intake of vitamin D in consumers of all fortified foods; however, the increase was not sufficient to markedly reduce the proportion of consumers below the average requirement of 10 μg/d. The addition of vitamin D to foods had a modest impact on reducing the proportion of intakes below 5 μg/d. The mean vitamin D intake increased by 0·6 μg/d between the two surveys, half of which was contributed by voluntary fortification practices. The modest impact of vitamin D fortification on adequacy has also been observed in the USA, where, despite the fortification of milk with vitamin D, intake of vitamin D increased, but not sufficiently to lower the prevalence of intakes below the EAR( Reference Calvo 70 , Reference Fulgoni, Keast and Bailey 71 ). By contrast, the voluntary policy on vitamin D fortification of fluid milk and milk products in Finland( Reference Pietinen, Männistö and Valsta 72 ) has resulted in significant increases in vitamin D intake and decreases in vitamin D insufficiency and deficiency( Reference Laaksi, Ruohola and Ylikomi 73 ). Our recent estimate of median vitamin D intake from an average serving of a vitamin D-fortified food in the NANS (48 % consumers) was 1·2 μg/serving (P25: 0·7 μg/serving; P75: 2·0 μg/serving)( Reference Hennessy 65 ), representing 12 % of the EAR proposed by the US Institute of Medicine( 44 ).
The minimal impact of food fortification on reducing the prevalence of inadequate intakes of vitamin A, Ca, Mg and Zn was attributable to the infrequent addition of these micronutrients to foods.
In the absence of agreed safe levels of addition of micronutrients to foods, the safety of voluntary fortification practices during 1997–9 and 2008–10 in Ireland was assessed in the present study relative to the tolerable UL. The data on the potential for intakes that exceed the UL reported in the present study do not indicate cause for concern. In general, the proportion of fortified food consumers with total intakes exceeding the UL during both surveys was low ( < 2 %). Additionally, high intakes as defined by the P95 of intake were less than the UL when the base diet, fortified food and nutritional supplement use were included in the estimate. These findings are consistent with those reported for adults in a number of European countries( Reference Flynn, Hirvonen and Mensink 74 ). Regarding the safety of voluntary fortification practices over time in Ireland, micronutrient intakes at the P95 in both surveys did not exceed or even approach the UL, with or without the inclusion of added nutrients from voluntary fortification.
When interpreting the findings of any study, it is important to consider the strengths and weaknesses. A key strength of the present study was the consistent methodology and design used by the IUNA when conducting the surveys, both of which were nationally representative of Irish adults aged 18–64 years. Food and beverage intake data were collected by qualified nutritionists to a very high level of detail, involving collection of brand-level data and the retention of food packaging by participants. Furthermore, the identification and classification of voluntarily fortified foods was kept consistent during both surveys. A high proportion of food and beverages consumed during both surveys were weighed, and the prospective nature of the food and beverage diary limits recall bias that may be associated with other methods of dietary assessment. As misreporting of food and energy intake is a known issue in dietary surveys and can result in an over- or underestimate of the prevalence of inadequate nutrient intake, the present study identified and excluded under-reporters of energy intake when conducting analysis to estimate the prevalence of inadequate intake.
In summary, the present study characterising the changes in fortified food consumption in nationally representative samples of Irish adults aged 18–64 years between 1997–9 and 2008–10 has shown that the supply of fortified foods has increased. This has resulted in a greater proportion of adults consuming fortified foods (from 67 to 82 %) and a greater contribution of fortified foods to mean daily energy intake (from 4·6 to 8·4 %). Over this time, the overall nutrient profile of fortified foods consumed, relative to energy contribution, has remained higher in carbohydrate, starch and dietary fibre and lower in protein, fat and saturated fat. The contribution of fortified foods to the intakes of polyunsaturated fat, total sugars, non-milk sugars and Na was in line with their contribution to energy intake. The present study indicates that women, particularly those of childbearing age, have remained key beneficiaries of voluntary fortification practices in Ireland. When compared with the data from 1997 to 1999, we have shown that the continued voluntary fortification of foods in Ireland has increased protection against NTD-affected pregnancy by folic acid and maintained the beneficial impact on the adequacy of Fe intake in women of childbearing age. While the continued voluntary addition of vitamin D to foods has improved intakes, this has had only a modest effect on the high proportions of men and women with inadequate vitamin D intakes. Increased consumption of fortified foods did not contribute to increased risk of intakes exceeding or even approaching the tolerable UL for any micronutrient in adults. The present study in a country with a history of liberal fortification practices provides new data on the nutritional impact of recent changes in voluntary fortification of foods in the EU.
Acknowledgements
The authors thank the participants of both surveys, without whom the study would not have been possible.
The present study was supported by funding from the Irish Department of Agriculture, Food and the Marine (DAFM) under the Food for Health Research Initiative (2007–12). The DAFM had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: Á. H. carried out the data analysis and drafted the manuscript; E. M. H. provided the data from the NSIFCS for the data analysis; J. W. contributed to the design and implementation of the NANS study and provided expert advice on the drafting of the manuscript; A. F. was involved in the conception of the work, was a grant holder and provided expert advice on the drafting of the manuscript. All authors reviewed and approved the final manuscript.
The authors declare that there are no conflicts of interest.










