The issue
A considerable number of studies have reported that use of an internal teat sealant introduced into each teat of the dairy cow at the end of lactation, sometimes in combination with a dry cow antimicrobial infusion, successfully prevents clinical mastitis in early lactation. Adoption of this technology on dairy farms has proven highly successful in controlling clinical mastitis (CM) in early lactation and in reducing the use of antimicrobial agents. Several of the studies report the effect may last up to 150 d after calving, but none have proposed a hypothesis as to how this protection can happen long after any introduced ingredient should have disappeared.
Background
Neave et al. (Reference Neave, Dodd and Henriques1950) showed that 50% of intramammary infections (IMI) present at calving of dairy cows persisted for at least 14 d after calving with half developing to CM. Most CM was from infections newly arisen in the dry period, mostly caused by streptococci. Further, cows with a history of infection were those most likely to suffer a new IMI or another IMI in another quarter. However, nearly 30% of IMI detected at calving resolved within 14 d. Later, Smith et al. (Reference Smith, Neave, Dodd and Brander1966, Reference Smith, Neave, Dodd, Jones and Gore1967b) reported that more cows were infected at calving than were infected at drying off with ~30% of new IMI in a lactation occurring in the dry period. This recognition of the importance of the dry period (DP) in the risk of new IMI led to various attempts to control infection in this period. Neave et al. (Reference Neave, Lee and Dodd1951) tried the recently available penicillin, as did Pearson (Reference Pearson1951) to manage pyogenic CM (summer mastitis) in the DP. However, useful success with antimicrobial treatment in the DP required the development of long-acting formulations, the first being cloxacillin (Smith et al., Reference Smith, Neave, Dodd and Brander1966, Reference Smith, Neave, Dodd, Jones and Gore1967b).
Adoption of whole herd treatment at drying off, comprising dry cow antimicrobial infusion (DCAI) into each quarter of each cow, became one of the pillars of the five-point mastitis control plan (Hillerton and Booth, Reference Hillerton and Booth2018). The ability to enhance protection of the mammary gland from infection and disease around the DP has been enhanced by the development of an infused internal teat sealant (ITS, usually containing bismuth subnitrate; Meaney, Reference Meaney1976, Reference Meaney1977). Successful deployment often uses ITS in addition to DCAI, a combination approach (Combo), first described by Woolford et al. (Reference Woolford, Williamson, Day and Copeman1998). The DCAI is usually applied first, followed by the ITS.
Here we present a retrospective examination of the evidence that DP prophylaxis affects the occurrence of CM relatively late in the subsequent lactation. We have used 21 published studies, 13 as used by Rabie and Lean (Reference Rabie and Lean2013) and a later eight (Table 1).
Table 1. Publications comparing various combinations of a negative control, dry cow antimicrobial infusion, an internal teat sealant and a combination of dry cow antimicrobial infusion followed by an internal teat sealant in field trials
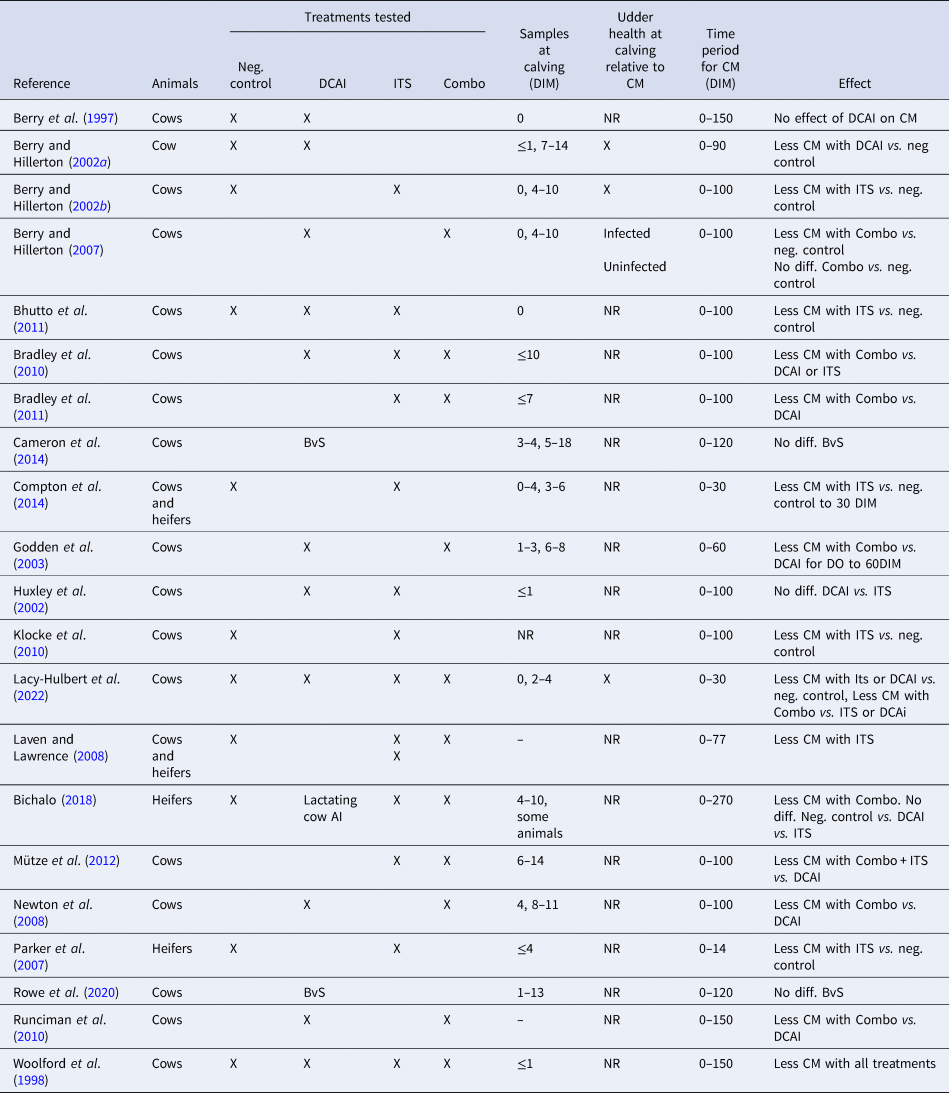
DIM, days in milk; CM, clinical mastitis; Neg. control, untreated control group; DCAI, dry cow antimicrobial infusion; ITS, internal teat sealant; Combo, dry cow antimicrobial infusion followed by internal teat sealant; X, used/reported; NR, not reported; BvS blanket vs. selective DCAI.
Infections and clinical mastitis
An IMI is defined as the presence of pathogenic bacteria, known to cause an inflammatory response in mammary gland tissue, likely to be sustained in the mammary gland, at least temporarily. A new DP IMI is the identification of pathogenic bacteria in one or more milk samples taken soon after calving from a quarter known to be free of that pathogen at drying off. Thus, a quarter can be defined as infected or uninfected at calving. The time when a sample is taken post calving affects the determination of infected vs. uninfected quarters as an IMI may be eliminated or a new infection arise at any time following calving.
Clinical mastitis is defined as the observation of abnormalities in the appearance (the presence of clots and/or discolouration) of milk or udder tissue (swelling, tenderness and elevated temperature).
Dry period management to control new intramammary infections
Numerous trials have described the efficacy of DCAI or ITS or Combo in preventing new DP IMI, generally showing a trend that ITS is at least equal to DCAI in preventing a new DP IMI in uninfected quarters and that Combo is superior to either DCAI or ITS (see meta-analysis of results from 12 studies, Rabie and Lean, Reference Rabie and Lean2013). Results are based on the bacteriology of quarter samples taken at or soon after calving. Errors are likely in these data, varying between trials, according to when the samples were taken and if confirmatory results were obtained from duplicate samples or subsequent samples. Of the 21 studies considered here, 17 used sampling within a very few days of calving but only eight of those added any confirmatory data from other sampling (Table 1). Without results from both of separate samplings, it cannot be known conclusively when a new IMI occurred and whether it was real and sustained or simply transitory. In some studies, the single ‘calving’ sample was not taken until 10 or more days after calving (days in milk, DIM) so any IMI may have been acquired after calving rather than during the dry period. See Table 1 for details.
Dry period management to control new clinical mastitis
Rabie and Lean (Reference Rabie and Lean2013) suggested, using 21 sub trials, that the overall evidence is that DCAI or ITS or Combo treatment over the DP reduces the incidence of CM, relative to no treatment, in the period 2–100 d post calving. The time varies between reports, and as much as 150 DIM has been claimed in later studies not considered in the 2013 review. However, the relationship of timing of new IMI, whether it occurs in the DP or soon post calving, has not been considered in any of the published studies. This leaves an important question, namely, were the CM cases in quarters infected or uninfected at calving?
When considering quarters identified as infected at calving, Berry and Hillerton (Reference Berry and Hillerton2002a) found fewer CM in DCAI treated cows vs. untreated cows in the first three months post calving, and Berry and Hillerton (Reference Berry and Hillerton2002b) found fewer CM in ITS treated cows vs. untreated cows in the first 100 d post calving. Similarly, Berry and Hillerton (Reference Berry and Hillerton2007) found fewer CM in Combo treated cows vs. DCAI treated quarters in the first 100 d post calving, again in those quarters identified as infected at calving. Berry and Hillerton (Reference Berry and Hillerton2002a and Reference Berry and Hillerton2002b) had both earlier found a slightly higher proportion of coliform CM post calving in ITS treated cows vs. either untreated or DCAI treated cows.
Godden et al. (Reference Godden, Rapnicki, Stewart, Fetrow, Johnson, Bey and Farnsworth2003) reported fewer CM cases up to 60 DIM in Combo treated cows opposed to DCAI treated cows, but no effect on Gram-negative infections. Runciman et al. (Reference Runciman, Malmo and Deighton2010) found less CM in Combo treated cows than in DCAI treated cows, principally for up to 21 DIM but the effect continued for more than 100 DIM, most notably against Gram-negative pathogens.
Bradley et al. (Reference Bradley, Breen, Payne, Williams and Green2010) also observed fewer CM in Combo vs. DCAI, this time in high milk cell count (SCC) cows. Overall, they found no difference in low SCC cows. The Combo treated high SCC cows were less likely to incur an enterobacterial CM, whereas the low SCC cows were more likely to suffer an enterobacterial CM. However, they did not differentiate if the CM occurred in the DP or subsequently in the 100 DIM post calving. Bradley et al. (Reference Bradley, Breen, Payne, Williams and Green2011) also reported fewer CM in Combo vs. DCAI treated cows, mostly in the first 21 DIM with the prevalence of coliform IMI in DCAI treated cows twice that in Combo treated cows. Again, they gave no indication when the CM occurred. They notably reported that Combo was equivalent to use of cefquinome (a fourth-generation cephalosporin) DCAI, an antimicrobial with Gram-negative bacteria activity unlike the products more commonly used in DCAI. Cefquinome has been designated a Veterinary Critically Important Antimicrobial, which should not be used as a first line treatment, by the World Organisation for Animal Health (OIE, 2015).
None of the published studies have offered any explanation for this protection against CM many days and weeks after the treatment. Further, many do not consider if the CM was related to an infection detected at calving or arising at any later time in the lactation. Indeed, seventeen of the 21 published studies reviewed (Table 1) either give no timing for the occurrence of CM in the lactation or do not differentiate if the CM occurred in the DP or the subsequent lactation, nor if the quarters that showed CM later in the lactation were infected at calving or not.
If CM is to be used as a proxy for, or another measure of, successful prevention of a new IMI and hence a benefit of any prophylaxis, it is incumbent to identify an IMI at calving. The alternative is to advance a hypothesis of how DCAI or ITS can have an effect that prevents a new IMI in the subsequent lactation, especially by a Gram-negative pathogen, at some time after 4 DIM. At this time, milk is usually considered acceptable for human consumption, meaning that it is free of introduced antimicrobial substances or a teat sealant, or below maximum permitted levels (EMEA, 1998).
The issue: How can dry period prophylaxis prevent new clinical mastitis in the next lactation?
Smith et al. (Reference Smith, Westgarth, Jones, Neave, Dodd and Brander1967a) reported that the activity of dry period antibiotics is limited to three or four weeks after infusion, ie, about half the length of a normal dry period. More extended-life formulations may have a milk withhold of up to 58 d post infusion. Thus, milk at 4 d after calving is free of detectable antimicrobial effects (EMEA, 1998; Hillerton et al., Reference Hillerton, Halley, Neaves and Rose1999). That any long-term effect does not appear to occur for DCAI treated cows suggests the absence of any active ingredient. Importantly, the vast majority of antimicrobials, and those most common in international use, are active against Gram-positive pathogens and rarely or poorly against Gram-negative pathogens.
The various studies of Berry and Hillerton, Bradley et al., and Runciman et al., suggest that a real protection against CM long after any DCAI or ITS activity could be present. What might induce or prevent an IMI developing to CM has not, apparently, been considered. A clue to what may occur is that this is not simply a generic protection against a new IMI and subsequent CM, but that it depends on the timing of infection and the pathogen type involved. Bradley et al. (Reference Bradley, Breen, Payne, Williams and Green2010) said use of pathogen-free status at calving is the most relevant criterion to assess efficacy. Kabera et al. (Reference Kabera, Dufour, Keefe and Roy2018) reported that ITS particles persisted up to an average 4.5 d after calving even though no sealing was evident as the animals were being milked successfully. ITS are composed principally of bismuth salts which have long been known to have some antimicrobial properties, hence their use to treat peptic and gastric ulcers involving Gram-negative Helicobacter spp. in humans (Domenico et al., Reference Domenico, Salo, Novick, Schoch, van Horn and Cunha1997). Notcovich et al. (Reference Notcovich, Williamson, Flint, Yapura, Schukken and Heuer2020) reported some in vitro antimicrobial inhibition by bismuth salts against some strains of mastitis pathogens, including Escherichia coli. Potentially, bismuth salts, the largest ingredient of teat sealants, may not be inactive as initially proposed. That they are active, mostly against Gram-negative bacteria, long after any ITS is absent from the mammary gland is, however, a tenuous claim. The effect of ITS may not simply be as a barrier to entry of pathogens, but no definitive hypothesis has been proposed for an effect when no residue of the supposed active ingredient should still be present.
Research opportunities
Potentially a long-term effect of ITS may occur for CM caused by coliform bacteria, although the field trial results are not unanimous. Indeed, the current state of knowledge is insufficient to substantiate the claims made for protection against CM. The aetiology of IMI and CM in the subsequent lactation requires further study. If the effect is specific to certain pathogen types, then the farm system becomes important. Studies on pastured cows and many loose-housed systems with straw bedding will be less useful as the aetiology is predominantly Gram-positive pathogens, mostly streptococci. A J5 vaccine, applied over the dry period, shows benefits in reduction of Gram-negative mastitis in the subsequent lactation (Hogan et al., Reference Hogan, Smith, Todhunter and Schoenberger1992). No published studies have been found that examine any interaction with ITS treatment, another gap that could be filled.



