Introduction
The human immunodeficiency virus (HIV) epidemic of today is characterised by the presence of many severe cases [Reference Belyakov and Rassokhin1], frequently involving the brain [Reference Shelomov2–Reference Bicanic4]. Cerebral toxoplasmosis is a leading cause of the central nervous system disorders in acquired immune deficiency syndrome (AIDS). It is a severe disease, which occupies a third place among fatal diseases [Reference Goncharov, Gubareva and Kobets5]. The causative agent of cerebral toxoplasmosis is Toxoplasma gondii, an opportunistic intracellular parasite that can infect and destroy any nuclear cells.
The prevalence of toxoplasmosis is around 25–30% of the world's human population [Reference Montoya and Liesenfeld6, Reference Pappas, Roussos and Falagas7], significantly varying between regions (from 10% to 80%), within the country and within different ethnic and social groups [Reference Pappas, Roussos and Falagas7–Reference Tenter, Heckeroth and Weiss9]. The highest infection rates are reported in South America (42–72%) and Asia (40%) [Reference Nissapatorn and Sawangiaroen10].
The development of brain T. gondii in the late stages of HIV infection is overwhelmingly associated with the reactivation of latent infection due to strong immunosuppression [Reference Goncharov, Gubareva and Kobets5]. In the later stages, Toxoplasma can disseminate causing damage not only to the brain but also to the lungs, eyes, lymph nodes and to the gastrointestinal tract [Reference Tumash, Kozorez and Zhavoronok11].
The laboratory diagnosis of cerebral toxoplasmosis is fraught with difficulties. First: the production of specific antibodies, especially immunoglobulin M (IgM), is low. Second, the polymerase chain reaction (PCR) assay is not sensitive enough, ranging from 35% to 83% according to different authors [Reference Correia, Melo and Costa12, Reference Saakyan13].
In the absence of immunoglobulin G (IgG), antibodies can be found. High IgG titres (64 and higher in indirect fluorescent antibody test; 6400 and higher in enzyme-linked immunosorbent assay (ELISA)) indicate the phase of reactivation of the latent infection but a low level of IgG does not exclude the development of cerebral toxoplasmosis [Reference Ru and Tang14]. Some authors recommend determining the presence of specific antibodies not only in the blood but also in the cerebrospinal fluid (CSF) [Reference Gubareva15]. The presence of IgG in the CSF is considered a reliable criterion for the reactivation of the infection [Reference Gubareva15]. Other authors argue that a combined seropositivity of plasma and CSF occurs only in 62% of cases [Reference Levine16] and is more likely in the presence of high serum titres [Reference Gubareva15]. Another effective criterion for the reactivation of Toxoplasma infection is the presence of immunoglobulin A T. gondii antibodies in the blood, as their count correlates with the invasion process [Reference Zhao17].
Brain magnetic resonance imaging (MRI) is the main instrumental method for the diagnosis of cerebral toxoplasmosis but the MRI findings that are present in the literature are contradictory. Many authors agree only with the fact that etiotropic therapy causes the reduction of abnormalities on the MRI in patients with cerebral toxoplasmosis within 2 weeks of application [Reference Eggers18–Reference Arama20].
The pathomorphological interpretation of brain lesions in HIV-infected is difficult to accomplish because structural changes that occur in the brain in some opportunistic diseases remain poorly understood [Reference Zinzerling21].
The study aimed to collect clinical, radiological and morphological data on HIV-infected patients with cerebral toxoplasmosis.
Methods
Ethical approval
The study was conducted under the ethical guidelines for retrospective studies and does not disclose data on individual patients.
Participants
The study included 90 HIV-infected patients with cerebral toxoplasmosis. Of these, 73 patients underwent treatment at the Botkin Infectious Disease Hospital and the Center for the Prevention and Control of AIDS and Infectious Diseases in St. Petersburg during 2015–2018. Seventeen patients underwent inpatient treatment at the Novgorod Infectious Diseases Hospital, where they later died within the period between 2016 and 2018. The comparison sample consisted of 225 HIV-infected patients with pathologies other than a brain damage from toxoplasmosis and who underwent inpatient treatment during the same observation period. The study groups were comparable with regard to age and sex.
Diagnosis and testing
In case of positive enzyme immunoassay, HIV infection was confirmed with the immunoblot test. The HIV-1 ribonucleic acid levels were determined using real-time PCR. The flow cytometry was used for counting CD4 cells.
To confirm cerebral toxoplasmosis, laboratory methods and MRI of the brain were used. The laboratory diagnosis was performed by serological detection of T. gondii-specific IgG and IgM antibodies in the blood serum with ELISA; and by molecular detection of T. gondii deoxyribonucleic acid (DNA) in the CSF.
The instrumental methods included brain imaging at 1.5 and 3.0 Tesla. The MRI procedure included T1-weighted (T1-VI) and T2-weighted (T2-VI) sequences, Fluid Attenuation Inversion Recovery (FLAIR), diffusion-weighted imaging (DWI) and T1-weighted spin-echo sequences acquired before and after contrast administration. If necessary, the following procedures were additionally used: diffusion coefficient index (DCI), gradient echo, susceptibility-weighted angiography/susceptibility-weighted imaging (SWI) [Reference Trofimova, Belyakov and Rassokhin22].
Pathomorphological examination included autopsy as well as gross, microscopic and histological examination of internal organs.
The diagnostic criteria for cerebral toxoplasmosis included the presence of cytosis in the CSF (frequency rate, 70%); high amount of lymphocytes and protein in the CSF (frequency rate, 70%); the presence of high and medium titres of Toxoplasma IgG antibodies in the serum sample (frequency rate, 100%); and the presence of T. gondii DNA in the CSF sample (frequency rate, 35.5%). However, the absence of T. gondii DNA did not exclude cerebral toxoplasmosis due to low sensitivity (35%) of molecular method. The final diagnosis was made taking into account the clinical, laboratory, radiological and morphological findings.
Data analysis
Statistical data analysis was performed in Statistica v.9 using parametric and non-parametric methods. The quantitative measures included the arithmetic mean and the standard error of mean (M ± m). All differences were considered significant at P < 0.05.
Results
Cerebral toxoplasmosis was the most frequent opportunistic infection with brain damage diagnosed, being detected in 90 patients. Among them, 75.5% were men and 24.4% women at the age 37.0 ± 6.8. The average duration of disease was 6.7 ± 5.4 years. Twenty-five patients (27.7%) had never received an antiretroviral therapy (ART). The CD4 counts were variable, ranging from 39.6 to 229.6 cells/μl, with a mean of 69.0 cells/μl. The mean viral load burden was 41.743 copies/ml.
The frequency of cerebral toxoplasmosis in relation to the degree of immunosuppression, viral load and antiretroviral treatment duration
In the total cohort of HIV-infected, cerebral toxoplasmosis at the CD4 count ⩽100 cells/μl was more frequent (P < 0.001) compared to CD4 count above 101 cells/μl and above 350 cells/μl (Fig. 1).

Fig. 1. Frequency of cerebral toxoplasmosis in terms of CD4 lymphocyte count, n = 90.
Among patients diagnosed with toxoplasmosis, the proportion of individuals with suppressed viral load was lower than of those with a viral load above 50 copies/μl (P < 0.05) (Fig. 2).

Fig. 2. Frequency of cerebral toxoplasmosis in terms of viremia level, n = 90.
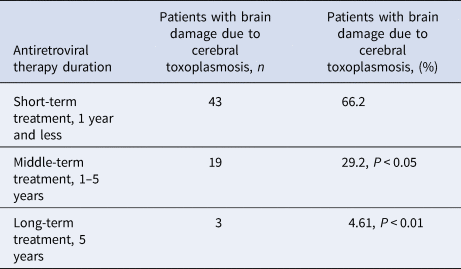
With a shorter ART, cerebral toxoplasmosis is more likely to result in a serious brain damage compared to longer treatment (Table 1).
Table 1. Frequency of cerebral toxoplasmosis depending on the duration of antiretroviral treatment, n = 90
Cerebral toxoplasmosis manifested in the form of Toxoplasma meningoencephalitis and Toxoplasma encephalitis.
The clinical picture of cerebral toxoplasmosis involved focal symptoms (frequency rate, 100%) such as hemiparesis, aphasia, ataxia, alexia and cranial nerve palsies; and cognitive impairment (frequency rate, 100%) such as the impaired memory, concentration, and perception, temporal and spatial orientation. Cognitive disorders developed at the onset of toxoplasmosis in some patients and against the background of neurological damage in other patients. Apart from that, there were reports on the toxic syndrome (frequency rate, 100%), mild cerebral symptoms (frequency rate, 87.8%) and the meningeal symptom (frequency rate, 70%).
Radiological characteristics of cerebral toxoplasmosis in HIV-infected patients
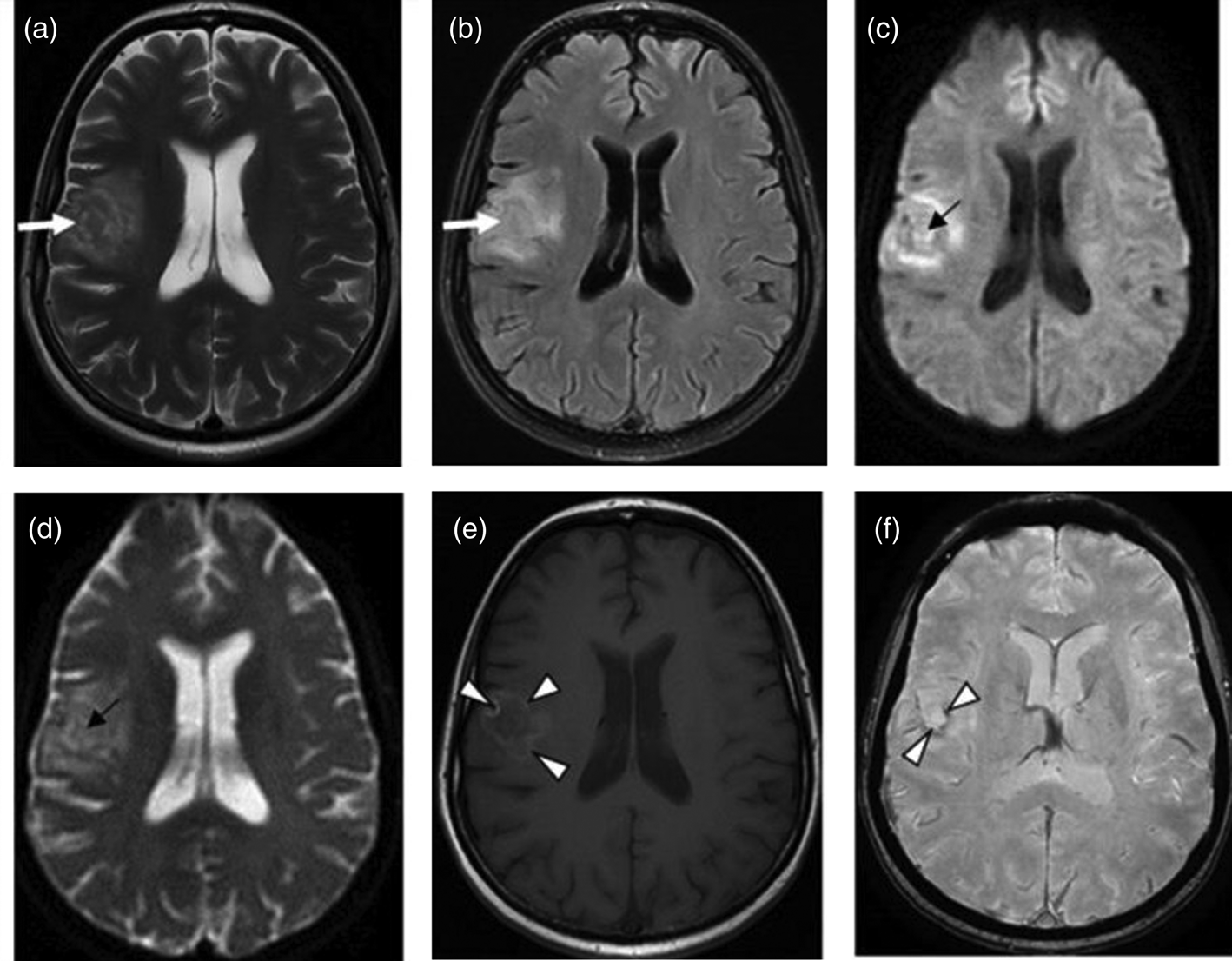
The presence of multiple, less often single, lesions on MRI, with ring and/or nodular enhancement on contrast. Lesions were found in the basal ganglia, thalamus and at the cortical/white matter border, with perifocal oedema and mass effect (Figs 3–4). These lesions had high or mixed signal intensity on T2-weighted and FLAIR images, and low signal intensity on T1-weighted images. Some abscesses demonstrated the involvement of haemorrhage (Fig. 5). The central diffusion restrictions of the pus abscesses were not observed (Fig. 6).
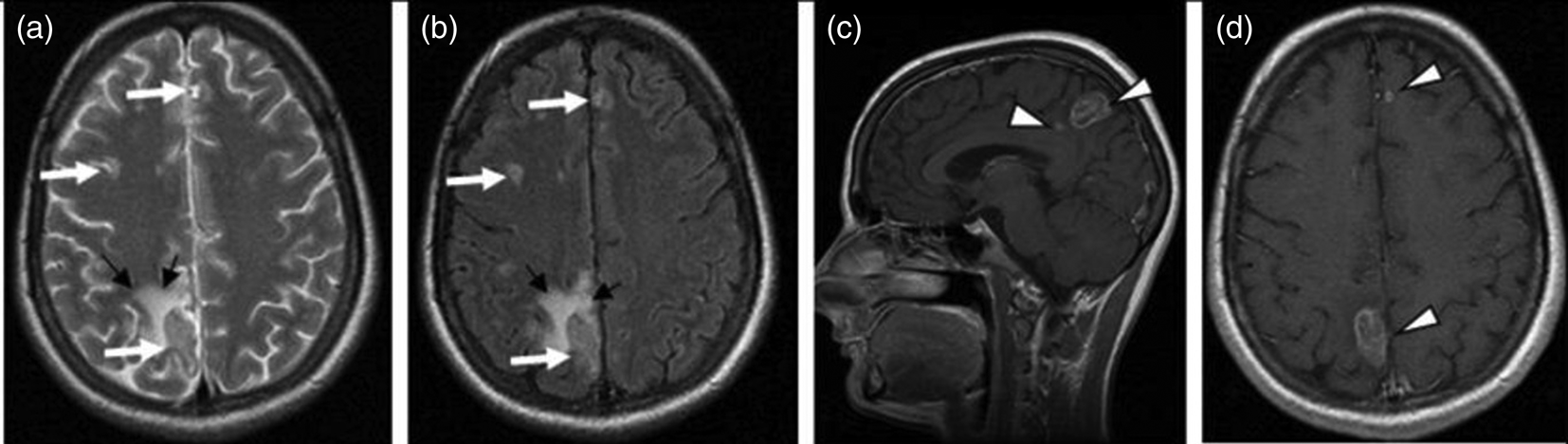
Fig. 3. Cerebral toxoplasmosis. A – T2-VI; B – Т2 FLAIR; C, D – T1-VI. Multifocal brain damage (white arrows), with perifocal oedema (black arrows), and a ring accumulation pattern of the contrast agent (arrowheads).

Fig. 4. Cerebral toxoplasmosis. A – T1-VI; B – Т2 FLAIR; C, D – T1-VI. Multifocal brain damage (white arrows), with perifocal oedema (black arrows), ring and nodular accumulation patterns of the contrast agent (arrowheads).

Fig. 5. Cerebral toxoplasmosis. A – Т2 FLAIR; B – SWI; C – T1-VI. Multifocal brain damage with perifocal oedema (white arrows) and haemoglobin breakdown products (black arrows).
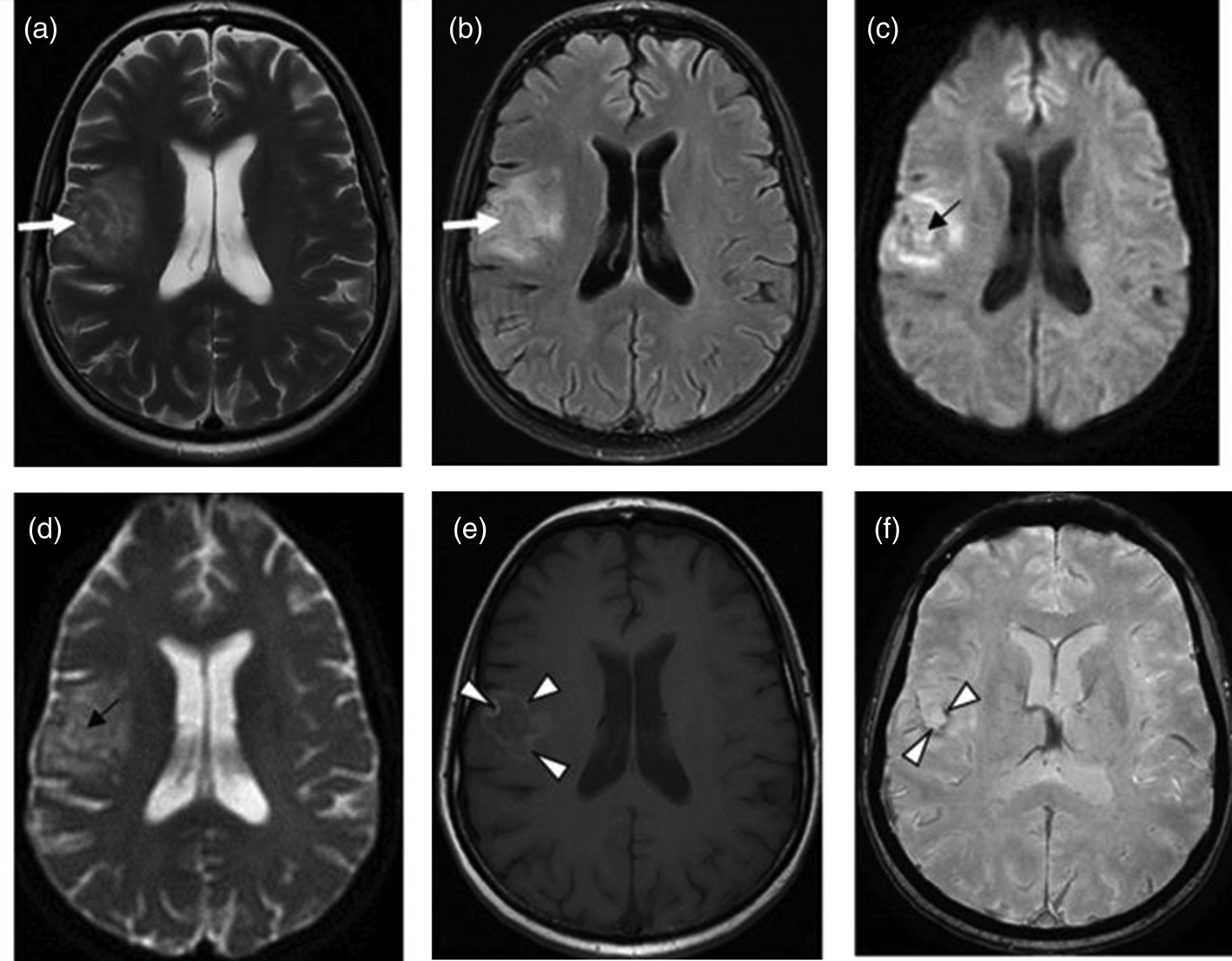
Fig. 6. Cerebral toxoplasmosis. A – T2-VI; B – Т2 FLAIR; C – DWI; D – DCI; E – T1-VI; F – SWI. Multifocal brain damage with perifocal oedema (white arrows), with the absence of reduced diffusion in the central part (black arrows), and with the haemoglobin breakdown products (arrowheads).
Morphology of cerebral toxoplasmosis in HIV-infected patients
Lesions had three distinct zones: a necrotic centre, an intermediate zone with intense inflammatory reaction and a peripheral zone with an encysted form of Toxoplasma. Cerebral toxoplasmosis occupied a leading place among conditions that caused damage to the brain, accounting for 20% [Reference Azovtseva23].
Gross: single or multiple cream coloured foci of necrosis. Histological examination revealed necrotic changes and a mild exudative reaction. In necrotic masses, the Toxoplasma cysts were found (Fig. 7).

Fig. 7. Cerebral toxoplasmosis, haematoxylin-eosin stained. Spherical cyst (arrows) with multiple Toxoplasma.
Discussion
The MRI visualisation of pathological processes that take place in the brain is undoubtedly of great diagnostic value, especially in differential diagnosis or when an adequate laboratory testing is not possible or provides uncertain results [Reference Essa, Song and Chang24–Reference da Silva26]. In cerebral toxoplasmosis, multiple lesions are a common implication. Single foci in the brain were mostly found in patients with acute clinical symptoms. In this case, an oedema surrounding the foci is more observed and, if present, it can contribute to a severe form of toxoplasmosis and a possibly higher mortality.
Dynamic MRI scans are important for monitoring treatment and outcomes. The positive clinical dynamics [Reference Correia, Melo and Costa12, Reference Gubareva15] and a decrease in MRI abnormalities within 2 weeks of etiotropic treatment [Reference Levine16] indicate progress in therapy. These tendencies were not observed in patients who eventually died.
Cerebral toxoplasmosis is the most frequent opportunistic infection of the brain among diagnosed. The incidence of cerebral toxoplasmosis significantly increases at the CD4 count below 100 cells/μl, P < 0.001, and at the HIV viral load above 50 copies/ml, P < 0.05. However, the identification frequency of cerebral toxoplasmosis reduced significantly among patients who had more than 1 year of ART treatment, P < 0.01.
The clinical picture of cerebral toxoplasmosis included focal symptoms (100%), cognitive impairment (100%), toxic syndrome (100%), mild cerebral symptoms (87.8%) and a meningeal symptom (70%). On the contrast-enhanced MRI scans, multiple, less often single, ring- and nodular-enhancing lesions with perifocal oedema and mass effect were observed in the basal ganglia, thalamus, at the cortical/white matter border. The PCR assay revealed 35.5% of T. gondii DNA in the CSF samples (non-specific criterion).
Therefore, cerebral toxoplasmosis is diagnosed through a combination of diagnostic methods (i.e. clinical exаmination, laboratory testing, immunological research, molecular genetic testing and neuroradiological imaging).
Cerebral disturbances in HIV-infected patients can be linked to multiple factors. To begin with, the parasite itself is capable of damaging the brain tissue. The time to brain involvement largely depends on the strength of the immune system, brain health, drug addiction and alcohol abuse [Reference Duarte25, Reference Hategan27]. The brain damage can be associated with the development of opportunistic infections including cerebral toxoplasmosis and secondary diseases, which can have a diverse range of risk factors.
Presently, MRI is regarded as the major radiological method for examining HIV-infected patients, whereas it poses a challenge even to experienced neuroradiologists [Reference Duarte25]. Therefore, the accurate interpretation of cerebral damage requires the combined use of methods such as clinical, laboratory and radiological examinations [Reference Duarte25–Reference Dean28].
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors
Conflict of interest
None.
Ethical standards
This article does not contain any studies with human or animal subjects performed by any of the authors.