Introduction
The use of ultrasonic processing as a novel non-thermal technology has been intensively developed in recent years due to its potential benefits in diverse fields including pharmaceutical, biological and food industries (Knorr et al., Reference Knorr, Zenker, Heinz and Lee2004; Levina et al., Reference Levina, Rubinstein and Rajabi-Siahboomi2000; Paniwnyk, Reference Paniwnyk2017; Patist and Bates, Reference Patist and Bates2008; Suzuki et al., Reference Suzuki, Lee, Padilla and Martini2010). In the dairy field, ultrasound has been widely applied as an emerging technology to improve process efficiency and functional properties of milk and milk products (Deeth et al., Reference Deeth, Datta, Versteeg, Smithers and Augustin2013; Paniwnyk, Reference Paniwnyk2017). The use of ultrasound as an alternative or cooperative method to conventional treatments has offered potential advantages in emulsification, homogenization of milk, crystallization of lactose/fat, alteration of the physical and functional properties of dairy products, fat separation, degassing and foaming (McClements, Reference McClements1995; De Gennaro et al., Reference De Gennaro, Cavella, Romano and Masi1999; Marchioni et al., Reference Marchioni, Riccardi, Spinelli, Dell'Unto, Grimaldi, Bedini, Giliberti, Giuliani, Palomba and Castellano2009; Chandrapala and Leong, Reference Chandrapala and Leong2015).
The most common use of ultrasound in dairy processing has focused on the low frequency range from 20–25 kHz (Deeth et al., Reference Deeth, Datta, Versteeg, Smithers and Augustin2013; Paniwnyk, Reference Paniwnyk2017). Recently, the use of high frequency ultrasound up to 2 MHz has also been explored in dairy processing (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014; Paniwnyk, Reference Paniwnyk2017). The primary mechanism of ultrasound in liquid food application is attributed mostly to acoustic cavitation. This phenomenon occurs when sound waves propagate into liquid food and lead to the formation, growth and collapse of micro bubbles. The collapse generates extremely high localized temperatures exceeding 5000 K within the vicinity of the imploding cavitation bubbles and creating high pressure up to several hundred MPa (Ashokkumar et al., Reference Ashokkumar, Bhaskaracharya, Kentish, Lee, Palmer and Zisu2010). It causes mechanical and physical effects such as shockwave formation and turbulent motion within the liquid (Ashokkumar et al., Reference Ashokkumar, Lee, Kentish and Grieser2007). Moreover, due to extremely high localized temperatures and pressure conditions caused by acoustic cavitation, free radicals and other reactive species are produced within the bubbles and also transferred from the bubbles to the liquid (Riesz and Kondo, Reference Riesz and Kondo1992). These radicals initiate and participate in a series of oxidation reactions in the food media (Ashokkumar and Mason, Reference Ashokkumar and Mason2007). Low frequency ultrasound is known as power ultrasound and generates strong physical effects whereas high frequency, low intensity ultrasound induces strong chemical reactions with little physical effect in liquid systems.
Despite the many applications of ultrasound in dairy processing which offer great potential, one of the main issues associated with ultrasound technology is the production of off-flavours reported as ‘rubbery’, ‘burnt’ or ‘foreign’ flavours (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). Since flavour is one of the most important indicators of product quality and determines the acceptability to consumers of milk and dairy products, generation of off-flavours may negatively affect the suitability of this technology in certain applications. It has been suggested that ultrasound-induced radicals may promote lipid oxidation reactions in milk systems, leading to the generation of undesirable volatile compounds contributing to off-flavours (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). In addition, the degradation of some proteins leading to the cleavage of amino acid side chains may generate some aromatic hydrocarbons (Wu et al., Reference Wu, Lifka and Ondruschka2005; Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a).
Due to off-flavour concerns attributed to the production of volatile compounds as a function of sonication (the application of ultrasound treatment), volatile compounds have been the focus of investigation in sonicated milk systems, with focus on their mechanism and derivation in response to different ultrasonic processing conditions. Furthermore, the compositional variation in milk systems is an important factor influencing the volatile compound production (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). Therefore, understanding the structural alteration of major milk components is also necessary to predict the production of volatile compounds.
This review will discuss the influence of ultrasound on the production of volatile compounds in milk and dairy products with relation to chemical effects as a function of processing conditions. The correlation between ultrasonic processing conditions and the volatile compounds produced in sonicated milk systems will be presented and possible mechanisms and derivation of volatile profiles reported through literature will be discussed. This work subsequently identifies volatile compounds generated in sonicated milk systems and the possible factors influencing their production. Understanding these factors may help to minimize and eliminate the formation of undesirable volatiles, prevent off-flavour development and promote the growth of ultrasound technology in dairy processing.
Mechanisms of ultrasound and their chemical and physical effects on milk components
Mechanisms of applied ultrasound at high- and low-frequency
Ultrasound basically refers to sound waves with a frequency range above human hearing detection (>20 kHz) (Butz and Tauscher, Reference Butz and Tauscher2002; Rahman, Reference Rahman and Rahman2007). Typically, ultrasound can be divided into two distinct categories: low intensity high frequency, and high intensity low frequency (McClements, Reference McClements1995). The former uses intensity at about 1 W/cm2 at a frequency between 0.1–20 MHz that can be subdivided further: 100 kHz – 1 MHz and >1 MHz (Ashokkumar and Mason, Reference Ashokkumar and Mason2007). The latter operates at 10–1000 W/cm2 with the frequency range between 20–100 kHz (Ashokkumar and Mason, Reference Ashokkumar and Mason2007; Leong et al., Reference Leong, Johansson, Juliano, McArthur and Manasseh2013).
Low intensity ultrasound with frequency >1 MHz is primarily applied for non-destructive analysis such as medical diagnostic imaging, monitoring food materials and the separation of multicomponent mixtures (Leong et al., Reference Leong, Johansson, Juliano, McArthur and Manasseh2013). Low intensity ultrasound in the frequency range from 100 kHz to 1 MHz causes significant chemical effects and can be used to initiate chemical modification in food systems (Ashokkumar and Mason, Reference Ashokkumar and Mason2007; Chandrapala and Leong, Reference Chandrapala and Leong2015). In contrast, high intensity ultrasound (<100 kHz) results in less intense chemical effects, but exerts extremely strong physical and mechanical effects on the food materials and is therefore applied widely in food processing (McClements, Reference McClements1995; Mason and Luche, Reference Mason, Luche and van Eldik1997).
The primary ultrasound mechanism in milk systems is based on acoustic cavitation. This phenomenon occurs in the aqueous medium when the sound waves interact with the liquid and dissolved gas leading to the growth and collapse of pre-existing micro bubbles (Fig. 1) (Ashokkumar et al., Reference Ashokkumar, Bhaskaracharya, Kentish, Lee, Palmer and Zisu2010). In acoustic cavitation, micro bubbles available in solution increase their sizes due to rectified diffusion and bubble-bubble coalescence (Ciawi et al., Reference Ciawi, Rae, Ashokkumar and Grieser2006). In the case of rectified diffusion, more gas or solvent vapour nuclei diffuse into the bubbles as compared to gas nuclei which diffuses out when micro bubbles oscillate under the fluctuating pressure. This, in turn, allows the bubbles to reach the resonance size range. In addition, micro bubbles can associate with other bubbles by bubble-bubble coalescence to reach the resonance size. The cavitation bubbles may then grow to their maximum size within one or more acoustic cycles and violently collapse. The collapse generates extremely high localized temperatures up to 5,000 K within the vicinity of the imploding cavitation bubbles and emitting waves of high pressure up to several hundred atmospheres (tens of MPa: Ashokkumar and Mason, Reference Ashokkumar and Mason2007). This phenomenon causes mechanical and physical effects such as shockwave formation and turbulent motion of the liquid (Ashokkumar et al., Reference Ashokkumar, Lee, Kentish and Grieser2007).

Fig. 1. The growth and collapse of bubbles in acoustic cavitation process modified from Abbas et al. (Reference Abbas, Hayat, Karangwa, Bashari and Zhang2013); (Leong et al., Reference Leong, Ashokkumar and Kentish2011).
In addition to physical effects, cavitation can also generate chemical changes in liquid milk. Due to localized high temperatures (primarily) and pressure generated by acoustic cavitation (to a small extent), free radicals and other reactive species are created within the bubbles and also transfer from the bubbles to the liquid, inducing a chain of chemical reactions including redox reactions (Riesz and Kondo, Reference Riesz and Kondo1992). In the case of milk liquid, where the common solvent is water, the water vapour can be split into hydrogen (H•) and hydroxyl (OH•) radicals under extremely high temperature conditions. The (OH•) radicals may recombine to form reactive oxygen species such as H2O2. These radicals initiate and participate in a series of oxidation reactions in the food media (Ashokkumar and Mason, Reference Ashokkumar and Mason2007).
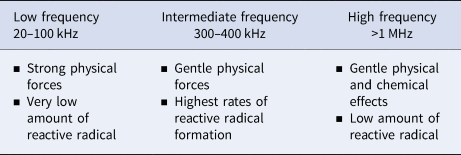
Depending on the specific frequency range of ultrasound, it can cause different physical and chemical effects in milk systems. Particularly in the low frequency range (20–100 kHz), ultrasound generates strong physical forces and very low amounts of reactive radicals (Ashokkumar and Mason, Reference Ashokkumar and Mason2007). Acoustic cavitation at high frequencies in the range 300–500 kHz, however, induces weaker physical effects and significantly greater amounts of chemically active radicals (Ashokkumar et al., Reference Ashokkumar, Bhaskaracharya, Kentish, Lee, Palmer and Zisu2010). For instance, many more hydroxyl radicals (•OH) were produced at higher frequencies as compared to lower frequencies (Fig. 2) (Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008). It has been shown that frequency in the range of 300–400 kHz results in optimal sonochemical reactions with the highest rates of hydroxyl radical formation (Koda et al., Reference Koda, Kimura, Kondo and Mitome2003; Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008). The experiment from Mason et al. (Reference Mason, Cobley, Graves and Morgan2011) demonstrated that a high rate of hydroxyl radical formation takes place between 400–800 kHz, whereas higher ultrasonic frequency above 1 MHz cause insignificant formation of free radicals (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). Strong physical and mechanical effects generate intense shear forces, shock waves and turbulence (Ashokkumar et al., Reference Ashokkumar, Bhaskaracharya, Kentish, Lee, Palmer and Zisu2010), while chemical effects can lead to a chain of chemical reactions, i.e. redox reactions of lipids or proteins, in either the interfacial areas between collapsing gas bubbles and the aqueous phase surrounding or in the liquid phase (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). The differences of physical and chemical effects caused by various ultrasonic frequencies are summarized in Table 1.

Fig. 2. Production of Hydroxyl (•OH) radicals in water during sonication at different ultrasonic frequencies (0.90 W/cm2). Data shown are means ± standard deviation of 3 experiments. (■ 358 kHz, ▾ 1062 kHz, ● 20 kHz) (Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008). The figure is pre-printed with the permission from Elsevier.
Table 1. Physical and chemical effects of different ultrasonic frequencies on milk systems
Physical effects of ultrasound on milk components
Effects on milk proteins
Milk proteins contain primarily 80% casein and 20% whey proteins (Fox, Reference Fox, Fox and McSweeney2003). These two milk protein types are affected differently by ultrasound in ways that depend on ultrasonic processing parameters, composition of milk and pH.
The effects of ultrasound on whey proteins have been extensively investigated. The physical effects caused by ultrasound, especially in low frequency applications, could denature whey proteins to differing extents depending on the energy applied. The denaturation of whey proteins has been of particular interest as it contributes to improvements in the physical and functional properties of sonicated milk and milk products (Villamiel and de Jong, Reference Villamiel and de Jong2000; Nguyen and Anema, Reference Nguyen and Anema2010, Reference Nguyen and Anema2017; Chandrapala et al., Reference Chandrapala, Zisu, Palmer, Kentish and Ashokkumar2011; Arzeni et al., Reference Arzeni, Martínez, Zema, Arias, Pérez and Pilosof2012; Silva et al., Reference Silva, Zisu and Chandrapala2018). It was reported that whey proteins were denatured by the combined effects of low frequency ultrasound and heat treatment (20 kHz, 55–75°C) (Villamiel and de Jong, Reference Villamiel and de Jong2000). Similarly, the application of ultrasound (22.5 kHz, 50 W, 10 min) on skim milk without temperature control was also found to cause denaturation of whey proteins (Nguyen and Anema, Reference Nguyen and Anema2010). In the latter study, Nguyen and Anema (Reference Nguyen and Anema2017) also observed the denaturation of whey proteins and subsequent aggregation of milk proteins and fat globules in reconstituted whole milk when low frequency ultrasound was applied at 22.5 kHz at temperatures higher than 60°C. These changes were assumed to be mostly associated with the heat treatment processing step rather than ultrasound alone as only minor effects were observed when ultrasound was applied at controlled temperature conditions (Nguyen and Anema, Reference Nguyen and Anema2010, Reference Nguyen and Anema2017). In contrast, findings from other studies showed that the strong shear forces caused by acoustic cavitation resulted in the denaturation of whey proteins (Chandrapala et al., Reference Chandrapala, Zisu, Palmer, Kentish and Ashokkumar2011, Reference Chandrapala, Zisu, Kentish and Ashokkumar2012b; Arzeni et al., Reference Arzeni, Martínez, Zema, Arias, Pérez and Pilosof2012; Silva et al., Reference Silva, Zisu and Chandrapala2018). The shear forces from ultrasound (20 kHz) could unfold whey proteins, especially α-la and β-lg, and subsequently generate protein aggregation via hydrophobic interactions and disulphide bonds (Chandrapala et al., Reference Chandrapala, Zisu, Palmer, Kentish and Ashokkumar2011; Silva et al., Reference Silva, Zisu and Chandrapala2018). The unfolding of whey proteins may lead to the exposure of the free sulphydryl groups which are initially buried in the native structure (Silva et al., Reference Silva, Zisu and Chandrapala2018). These groups subsequently play an important role as precursors for the formation of sulphur volatile compounds (Al-Attabi et al., Reference Al-Attabi, D'arcy and Deeth2008). Further, the denatured whey proteins with open structure are susceptible to free radical initiating oxidation reactions (Roberts et al., Reference Roberts, Kehrer and Klotz2015).
The effects of ultrasound on casein micelles were reported with diverse results. Taylor and Richardson (Reference Taylor and Richardson1980) showed that casein micelles were disrupted to release free casein in sonicated skim milk, while the reactive sulphydryl groups of proteins were not affected. Another study on re-assembled casein micelles by Madadlou et al. (Reference Madadlou, Mousavi, Emam-djomeh, Ehsani and Sheehan2009) demonstrated that the casein micelle size significantly reduced when subjected to low frequency ultrasound at 35 kHz for 6 h. However, this effect was attributed to the combination of sonication with high pH values (Madadlou et al., Reference Madadlou, Mousavi, Emam-djomeh, Ehsani and Sheehan2009). This result is in agreement with later results reported by Liu et al. (Reference Liu, Juliano, Williams, Niere and Augustin2014) where the casein micelle integrity was reduced when sonicated (20 kHz, 286 kJ/kg, 15 min) at pH 8. A reduction in casein micelle size in skim milk was also observed in the early stage of ultrasound treatment (22.5 kHz, 50 W for up to 10 min) (Nguyen and Anema, Reference Nguyen and Anema2010). In contrast, no change in the casein micelles was observed by Villamiel and de Jong (Reference Villamiel and de Jong2000) after treatment at any of the condition examined (20 kHz, for 102.3 s, up to 75.5°C). The research by Chandrapala et al. (Reference Chandrapala, Martin, Zisu, Kentish and Ashokkumar2012a) on casein micelles integrity also observed that ultrasound treatment (20 kHz, 31 W, 60 min) did not reduce the casein micelle size.
The effect of ultrasound on protein aggregates has been investigated. Ultrasound appears to interrupt the aggregated structures and subsequently reduce their particle size. Ashokkumar et al. (Reference Ashokkumar, Kentish, Lee, Zisu, Martin and Augustin2009) suggest that low frequency ultrasound treatment at 20 kHz disrupts the protein aggregates in preheated whey protein concentrate, resulting in changes in the particle size distribution. Chandrapala et al. (Reference Chandrapala, Martin, Zisu, Kentish and Ashokkumar2012a) suggest that ultrasonication can break whey protein aggregates and large casein-whey protein aggregate structures without causing changes to the casein micelle structure (20 kHz, up to 60 min). Degradation of whey protein aggregates as a function of ultrasound was also reported by Shanmugam et al. (Reference Shanmugam, Chandrapala and Ashokkumar2012). Similarly, aggregate reductions were also reported in sonicated milk protein concentrate (20 kHz, 300 W) (Yanjun et al., Reference Yanjun, Jianhang, Shuwen, Hongjuan, Jing, Lu, Uluko, Yanling, Wenming, Wupeng and Jiaping2014), reconstituted whey protein isolate (20 kHz, 34 W/cm2) (O'Sullivan et al., Reference O'Sullivan, Arellano, Pichot and Norton2014), and denatured casein-whey protein complexes (Leong et al., Reference Leong, Walter, Gamlath, Yang, Martin and Ashokkumar2018). In addition, a larger reduction in the aggregate size was also observed in whey protein suspension sonicated at 20 kHz and 43–48 W/cm2 (Jambrak et al., Reference Jambrak, Mason, Lelas, Herceg and Herceg2008, Reference Jambrak, Mason, Lelas, Paniwnyk and Herceg2014).
Effects on milk fat globules and milk fat globule membranes
Low frequency ultrasound was reported to disrupt the milk fat globules and milk fat globule membrane (MFGM), leading to reductions in fat globule size and improving the homogenization of milk (Villamiel and de Jong, Reference Villamiel and de Jong2000; Wu et al., Reference Wu, Hulbert and Mount2000; Ertugay et al., Reference Ertugay, Şengul and Şengul2004; Bermúdez-Aguirre et al., Reference Bermúdez-Aguirre, Mawson and Barbosa-Cánovas2008). The disruption was caused by the shear forces from acoustic cavitation (Bermúdez-Aguirre et al., Reference Bermúdez-Aguirre, Mawson and Barbosa-Cánovas2008). Fat globule size and distribution, therefore, reduces in respond to ultrasonic power and sonication time (Villamiel and de Jong, Reference Villamiel and de Jong2000; Ertugay et al., Reference Ertugay, Şengul and Şengul2004; Zisu and Chandrapala, Reference Zisu, Chandrapala, Datta and Tomasula2015). In addition, Bermúdez-Aguirre et al. (Reference Bermúdez-Aguirre, Mawson and Barbosa-Cánovas2008) observed significant changes in the microstructure of fat globules in whole milk treated by thermo-sonication (24 kHz, 400 W, 30 min, at 63°C), and suggested that modified MFGM contains more binding sites which favour the interaction between fat membrane to casein and whey proteins.
The disruption of fat globules leads to a reduction in fat globule size and releases more free fatty acids and triglycerides into the surrounding aqueous phase, which are the main targets of lipase and esterase enzyme initiating lipid oxidation reaction chains (Villamiel and de Jong, Reference Villamiel and de Jong2000; Bermúdez-Aguirre et al., Reference Bermúdez-Aguirre, Mawson and Barbosa-Cánovas2008). In high fat content milk, ultrasound can cause coalescence of fat globules, forming fat aggregates, especially at cold ultrasonic conditions (50 W, <10°C) (Zisu and Chandrapala, Reference Zisu, Chandrapala, Datta and Tomasula2015; Chandrapala et al., Reference Chandrapala, Ong, Zisu, Gras, Ashokkumar and Kentish2016). However, on prolonged sonication, the strong shear forces appeared to disrupt the fat clusters to smaller agglomerates and eventually to tiny individual fat globules (Zisu and Chandrapala, Reference Zisu, Chandrapala, Datta and Tomasula2015; Chandrapala et al., Reference Chandrapala, Ong, Zisu, Gras, Ashokkumar and Kentish2016).
Chemical effects of ultrasound on milk components
Lipid oxidation
Lipid oxidation is typically a free-radical chain reaction initially occurring in polyunsaturated fatty acids. Due to the effects of acoustic cavitation, ultrasound can generate free radicals, which in turn initiate the lipid oxidation chain of reactions in milk. Free radicals start attacking unsaturated milk lipids, removing a hydrogen from the methylene group adjacent to the double bond. The resulting free radicals react with oxygen to form peroxide free radicals, which in turn react with another unsaturated lipid and generate a hydroperoxide (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006; Roberts et al., Reference Roberts, Kehrer and Klotz2015). A lipid oxidation chain has been proposed to involve initiation, propagation and termination reactions as shown in Fig. 3. Due to the oxidation reaction, unsaturated fatty acids in milk are oxidized to form hydroperoxides which are unstable and subsequently degraded to form carbonyls such as aldehydes, ketones, acids, alcohols and other compounds as illustrated in Fig. 4 (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006).

Fig. 3. The formation of free radical and hydroperoxides in the pathway for the chain reaction of the autooxidation of lipids (modified from O'Connor and O'Brien (Reference O'Connor, O'Brien, Fox and McSweeney2006)).

Fig. 4. General pathways for the metabolism of milk triglycerides and fatty acids. The figure is pre-printed from Cadwallader and Singh (Reference Cadwallader, Singh, McSweeney and Fox2009) with the permission from John Wiley and Sons.
Milk fat globules are normally covered by the fat globule membrane, which protects the fat globules against oxidation reactions. However, physical forces from ultrasound, especially low frequency ultrasound, leads to the disruption of milk fat globule membranes (Bermúdez-Aguirre et al., Reference Bermúdez-Aguirre, Mawson and Barbosa-Cánovas2008). This will facilitate the attack of free radicals to the polyunsaturated phospholipid fraction of the membrane, followed by the main triacylglycerol fraction inside the fat globules (Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). Therefore, physical and chemical effects of ultrasound possibly promote the lipid oxidation processes in milk.
Protein oxidation
As aforementioned, the generation of free radicals is strong in high frequency ultrasound and affects the proteolysis process (Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008; Munir et al., Reference Munir, Nadeem, Qureshi, Leong, Gamlath, Martin and Ashokkumar2019). Proteins are one of the main targets for oxidative modification due to the high rate constants for reactions of radicals with proteins (Roberts et al., Reference Roberts, Kehrer and Klotz2015). Despite this, there is a lack of research specifically examining the oxidation of proteins as affected by ultrasound. It has been suggested that milk proteins may be prone to oxidative modifications under conditions of high temperature, high pressure, shear and radiation. Oxidative damage of proteins results in a partial unfolding and loss of function while most oxidative protein modifications are irreversible. These oxidized proteins accumulate and tend to aggregate (Roberts et al., Reference Roberts, Kehrer and Klotz2015). The oxidation of protein caused by radicals may lead to the damage of either the protein backbone or side-chains (Roberts et al., Reference Roberts, Kehrer and Klotz2015). Radicals tend to rapidly attack the protein backbones and eventually generate peroxyl radicals, leading to the fragmentation of the protein backbones, along with the formation of hydroperoxides. Hydroperoxide subsequently degrades to alkoxyl-radicals and experiences further reactions (Davies, Reference Davies2005). Attack by free radicals also occurs in amino acid side-chains, in ways that are more complicated than the protein backbone oxidative reactions. Free radicals can attack either aromatic amino acid residues (tyrosine, tryptophan, phenylalanine, and histidine) leading to the formation of dityrosine, kynurenine, N-formylkynurenine, 5- and 7-hydroxytryptophan 2-oxohistidine formation, or sulphur-containing amino acid residues resulting in the formation of oxyacids or mixed disulphides (Roberts et al., Reference Roberts, Kehrer and Klotz2015). Moreover, carbonyl groups involving aldehydes and ketones are formed directly via oxidation of proline, arginine, lysine, threonine and other amino acid residues, as well as in oxidative backbone fragmentation. In addition, secondary reactions of cysteine, histidine and lysine residues lead to formation of protein carbonyls. These residues may react with bi-functional aldehydes, generated in lipid peroxidation, and carbonyl derivatives (keto-amines, keto-aldehydes) (Dalle-Donne et al., Reference Dalle-Donne, Rossi, Giustarini, Milzani and Colombo2003; Roberts et al., Reference Roberts, Kehrer and Klotz2015).
As a consequence of all this, chemical effects caused by ultrasound may promote the proteolysis process occurring in milk, leading to the formation of undesirable volatile compounds such as sulphur-compounds, aromatic hydrocarbons, or some carbonyl volatile compounds. If uncontrolled, the combination of physical and chemical effect could potentially denature and degrade milk proteins. While physical effects offer great potential improvement to the physicochemical and functional properties of milk proteins and milk products, the chemical effects and possible formation of undesirable volatile compounds is a concern and may affect sensorial properties by contributing to off-flavour development. Ultrasound processing conditions including treatment time and frequency should be considered to minimize the negative effects of free radicals on milk components.
Influence of ultrasound on the production of volatiles in milk
A combination of naturally occurring volatile compounds exist in milk and these contribute to the typical flavour identified in milk (Brockman and Beeren, Reference Brockman, Beeren and Fuquay2011). Most of the natural volatile compounds found in unprocessed bovine milk are desirable such as ethyl butanoate and ethyl hexanoate, which are the dominant odour-impact chemicals (Marsili, Reference Marsili and Fuquay2011). In addition to esters, the presence of alcohols including 1-octen-3-ol and phenylethanol crucially contribute to the aroma and flavour of milk (Moio et al., Reference Moio, Dekimpe, Etievant and Addeo1993). However, these naturally desirable volatiles are affected by processing, leading to the loss of these compounds in milk (Marsili, Reference Marsili and Fuquay2011). Since the physical and chemical effects of sonication also generate volatile compounds, these change the flavour of milk (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). Moreover, milk flavour is generally bland or slightly sweet in taste with no unpleasant after taste providing a pleasant mouthfeel (Marsili, Reference Marsili and Fuquay2011). Due to its relative blandness, milk is susceptible to many flavour defects resulting from the formation of undesirable volatile compounds (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006). The generation of volatile compounds and their respective off-flavours in sonicated milk as the function of either low or high frequency ultrasound is a concern. However, only a few researches have examined the production of volatiles in milk systems as affected by sonication (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010; Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014).
Low frequency–high intensity ultrasound
The application of low frequency ultrasound causes gentle chemical reactions with a lesser extent of free radical generation compared to high frequency (Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008; Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). Regardless of the extent of free radical generation, the production of volatile compounds was detected in low frequency sonicated milk. These volatiles appeared to be associated to lipolysis and proteolysis reactions and subsequent oxidation reactions caused by free radicals, high local temperature and pressure generated from acoustic cavitation (Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009; Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). In this context, lipolysis and proteolysis could be initiated by the endogenous milk enzymes, which catalyse triglycerides forming free fatty acids and milk proteins forming small peptides and amino acids (Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). Due to free radicals and physical effects caused by low frequency ultrasound, fatty acids experience further oxidation reactions, eventually producing volatile compounds such as methyl ketones, aldehydes, esters, and secondary alcohols as shown in Fig. 4 (Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). In proteolysis, further reactions occur with amino acids, eventually leading to the formation of aldehydes, alcohols, esters or sulphur compounds (Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). However, controversial findings were also presented in the absence of lipid oxidative volatile compounds after sonication at low frequency and selected energy density (<230 kJ/kg) in the study by Juliano et al. (Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014).
The production of volatiles is dependent on the energy density applied to the milk system and the properties of milk components. These factors have considerable effects on both volatile profiles and the extent of volatiles detected (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010; Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). The earliest research reporting volatile production and off-flavour development in low frequency sonicated milk was conducted by Riener et al. (Reference Riener, Noci, Cronin, Morgan and Lyng2009a). The authors detected ‘rubbery’ off-flavour in 200 ml pasteurized and homogenized reduced fat milk (1.5% fat) after sonication at low frequency (24 kHz, 45°C, treatment time from 2.5–20 min) (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). The extent of this undesirable flavour increased as the energy density increased from 100 to 400 W. Accordingly, ultrasound in this case produced mostly undesirable volatile compounds including hydrocarbons, aldehydes, ketones, sulphur-compounds as illustrated in Table 2 (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). The formation of hydrocarbons (such as 1-hexene, 1-octene, 1,3-butadiene, 1-nonene, 5-methyl-1,3-cyclopentadiene, benzene, toluene, p-xylene) and carbonyl volatiles such as aldehyde (n-hexanal and n-heptanal) and ketones (2-butanone and 2-pentanone) was only detected in sonicated samples (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). Among these volatiles, the extent of hydrocarbons and aldehydes increased in the first 5 min of sonication and remained nearly stable afterwards (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). Moreover, the authors suggested that the yield of volatiles accelerated with sonication time during the first 8 min, and after that, the total volatile content remained unchanged due to reaching an equilibrium between the generated and degraded products in sonicated milk (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). However, it is important to note that the temperature of samples in this study was not controlled during ultrasound processing. Therefore, the increase of temperature may also have contributed to the production of these volatile compounds. Furthermore, it was noted that no ester compounds were detected, especially the desirable esters such as ethyl butanoate and ethyl hexanoate which are naturally available in raw milk (Marsili, Reference Marsili and Fuquay2011). These esters are heat sensitive and were likely lost during thermal processing of the pasteurized milk used in this study (Marsili, Reference Marsili and Fuquay2011). These esters were undetected in both the sonicated and unsonicated samples. Compounds such as acetone, 2-butanone, chloroform, and dimethylsulphide already existed in non-sonicated pasteurized milk and appeared to be mostly unaffected by the ultrasound treatment. Among these compounds, acetone and 2-butanone were the predominant components (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a), and were thought to be derived from cow feed and commonly detected in raw milk (Gordon and Morgan, Reference Gordon and Morgan1972; Señorans et al., Reference Señorans, Tabera, Herraiz and Reglero1996). Meanwhile, the sulphur compound dimethylsulphide which is related to thermal denaturation of milk proteins is unlikely to have been generated by the ultrasound treatment as it was also detected in unsonicated samples and its concentration remained unchanged during processing.
Table 2. Production of volatiles in sonicated milk and sonication-derived milk products

* GC-gas chromatography; SPME-solid phase microextraction; DVB/CAR/PDMS- Divinylbenzene/Carboxen/Polydimethylsiloxane; CAR/PDMS-Carboxen/Polydimethylsiloxane.
Despite Riener et al. (Reference Riener, Noci, Cronin, Morgan and Lyng2009a) identifying volatile compounds, the contributions of volatiles to the ‘rubbery’ off-flavour was not clearly defined by gas chromatography-olfactometry (GC-O) through a sniff port. Keeping in mind that off-flavours were detected in an uncontrolled temperature environment, the volatile profile associated with ‘rubbery’ flavour has not been ascertained yet. Juliano et al. (Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014) estimated that energy density in this case can reach up to 288 kJ/kg after 6 min of sonication with the temperature of either 70°C or above 100°C. In this context, the increase in temperature of samples with prolonged sonication time may also contribute to the off-flavour. The intensity of rubbery flavour was reduced by decreasing sonication power, however, it was not eliminated completely even at 100 W (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a).
Chouliara et al. (Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010) attempted to characterize the volatile compounds and the corresponding off-flavours in full fat milk (3.5% fat, raw, pasteurized, thermized milk) sonicated at 24 kHz and 200 W for up to 16 min with a 22 mm diameter probe at temperatures between 15–25°C. Although the temperature in this study was controlled to prevent thermal change, oxidation reaction appeared to be enhanced by sonication. Burnt and foreign flavours were detected in sonicated samples (Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010) and most of the volatiles identified were similar to those detected in the study of Riener et al. (Reference Riener, Noci, Cronin, Morgan and Lyng2009a). For example, the authors reported aldehydes (pentanal, hexanal, heptanal, octanal), ketones (2-pentanone), aromatic hydrocarbons (benzene, toluene), short-chain alkanes (2,2,4-trimethyl pentane) and sulphur compound (dimethylsulphide) as shown in Table 2. However, according to Chouliara et al. (Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010), other volatiles including alcohols (1-propanol, 2-butanol) and especially some esters (acetic acid ethyl ester, propanoic acid ethyl ester), along with ethyl benzene and octanal were additionally identified in all samples which were not detected in the study of Riener et al. (Reference Riener, Noci, Cronin, Morgan and Lyng2009a). Unlike the findings of Riener et al. (Reference Riener, Noci, Cronin, Morgan and Lyng2009a), n-alkenes (such as 1-hexene, 1-heptene, 1-octene and 1-nonene) were not detected in this study. Although similar volatile compounds were identified between unsonicated and sonicated samples, the concentration of most of these volatiles increased with longer sonication times. The authors suggest that ultrasound treatment enhances the degree of lipid oxidation in sonicated milk, resulting in the rapid deterioration of milk quality during storage (Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). When evaluating lipid oxidation values, the level of malondialdehyde should be considered. Malondialdehyde is a fat oxidation product and well known as a biomarker of oxidative stress as it contributes to the sensorial acceptability of products (Li et al., Reference Li, Mo, Yang, Zhang, Xu, Ge, Xu, Shi and Le2019). The authors observed that the levels of malondialdehyde were equal to or lower than 1.95 mg/kg, which is lower than the detected thresholds (2 mg/kg) of unacceptable sensory attributes caused by lipid oxidation. Therefore, oxidation is unlikely the origin of dominant milk off-flavour development with sonication (Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). Consequently, none of the detected lipid oxidative volatile compounds were correlated to off-flavours observed in sonicated milk (Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010).
Even though the correlation between volatile profiles and detected off-flavours was not clarified, Chouliara et al. (Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010) indicated that sonicated thermized and pasteurized milk had a sensory score equal to or lower than that of untreated milk. Prolonged exposure to sonication up to 16 min caused unacceptable odour and taste in sonicated thermized milk and unacceptable taste in sonicated pasteurized milk (Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). Sonication of thermized milk for 2 min is the only occasion in which sonication resulted in a higher taste score than untreated milk, indeed, this treatment condition maintained acceptable taste and odour in sonicated thermized milk after 4 d of storage. Hence, it was suggested that the longest sonication time which can provide a positive effect on the sensory acceptability of milk was only 2 min (Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). This finding is important as it clearly defines the energy density threshold beyond which the negative sensorial attributes associated with ultrasound can be expected in full-fat milk.
Juliano et al. (Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014) did not detect lipid oxidation volatiles, such as aldehydes, ketones, or n-alkenes in sonicated raw non-homogenized milk when applying ultrasound (20 kHz, 20°C, batch horn transducer with energy density <230 kJ/kg) at similar conditions to those used in the study of Chouliara et al. (Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). The authors argued that the compounds detected in sonicated milks resulted from biochemical reactions and have similar amounts to those quantified in non-sonicated milks (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). However, the use of sonication at 20 kHz (in 0.3 l continuous through flow unit, operating above 90 kJ/kg) produced oxidative volatiles including pentanal, nonanal, and 2,3-octanedione in full milk and skim milk (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). The level of nonanal exceeded its threshold odour value after sonication of full cream milk for 5 min (above 73 kJ/kg) and skim milk for 16 min (261 kJ/kg) (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014).
In addition to the ‘rubbery’, ‘burnt’ and ‘foreign’ flavours mentioned previously, rancid off-flavours are also associated with sonicated milk due to lipid oxidation (Chandrapala and Leong, Reference Chandrapala and Leong2015). It has been suggested that high levels of hexanoic and butanoic acids are responsible for the ‘rancid’ flavour in milk (Pereda et al., Reference Pereda, Jaramillo, Quevedo, Ferragut, Guamis and Trujillo2008; Delgado et al., Reference Delgado, González-Crespo, Cava and Ramírez2011). Engin and Karagul Yuceer (Reference Engin and Karagul Yuceer2012) reported that greater concentrations of butyric, pentanoic and hexanoic acids were found in ultrasound treated milk (20 kHz, controlled temperature at 5°C) compared to ultraviolet light treatment. These acidic compounds might be induced by ultrasonic cavitation and the disruption of the fat globule membrane.
The mechanisms and pathways for volatile production can be attributed to several chemical reactions of different milk components. The effects of sonication on volatile production in milk are likely associated with many components in the complex milk matrix, while the mechanisms and pathways of volatile compound generation appear to be complicated (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a). Due to high localized temperature and pressure caused by acoustic cavitation, free radicals are generated in milk, promoting the lipid oxidation, degradation of fatty acid chains, and possible cleavage of side chains of amino acids. This leads to the formation of carbonyl compounds such as aldehydes, ketones, alcohols, acids, and the production of hydrocarbon compounds including n-alkanes, n-alkenes, aromatic hydrocarbons (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010).
Carbonyl compounds such as aldehydes, ketones, alcohols are largely recognized as secondary products of free radical-induced lipid oxidation resulting from the decomposition of unsaturated fatty acid hydroperoxides as shown in Fig. 4 (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009; Vagenas and Roussis, Reference Vagenas and Roussis2012). Therefore, lipid oxidation induced by sonication is one likely mechanism in the production of these volatiles. The mechanism and pathway of lipid oxidation is shown in Figs 3 and 4. Particularly, as aforementioned, the milk fat globule membrane protects the triglycerides inside the globules from lipolytic attack. However, physical effects from ultrasound can disrupt the fat globule membrane (Villamiel and de Jong, Reference Villamiel and de Jong2000; Bermúdez-Aguirre et al., Reference Bermúdez-Aguirre, Mawson and Barbosa-Cánovas2008). This promotes the lipolysis process, and subsequently leads to the production of free fatty acids (Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). Free fatty acids are either individual aroma compounds or they undergo further oxidization with free radicals to form volatile compounds such as methyl ketones, aldehydes, esters, and secondary alcohols (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). These volatile compounds produce very potent flavours (Vagenas and Roussis, Reference Vagenas and Roussis2012). Ketones are commonly produced in the form of methylketones after fatty acids are oxidized to β-keto acids and then decarboxylated to the corresponding methylketones. Various ketones such as 2-heptanone, 2-octanone, 2-nonanone, 2-decanone, and 2-undecanon contribute to the flavour of milk products and they possess low perception thresholds (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009; Vagenas and Roussis, Reference Vagenas and Roussis2012). Meanwhile, aldehydes such as n-butanal, n-pentanal, n-hexanal, and n-nonanal may be generated from β-oxidation of unsaturated fatty acids. Aldehydes are very unpleasant when their concentrations exceed certain thresholds (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009; Vagenas and Roussis, Reference Vagenas and Roussis2012). The formation of secondary alcohols is likely to result from enzymatic reduction of the corresponding methyl ketones and esters are known products of free fatty acids esterification with alcohols. Esters play an important role in determining the aroma of milk but high concentration of esters can have a negative impact on milk flavour (O'Connor and O'Brien, Reference O'Connor, O'Brien, Fox and McSweeney2006; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009; Vagenas and Roussis, Reference Vagenas and Roussis2012).
Alkanes and alkenes with C6–C9 chain length could result from pyrolytic cleavage of fatty acid chains (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). The production of aromatic hydrocarbons such as benzene and toluene in milk system could result from the cleavage of amino acid side chains, i.e. phenylalanine (Wu et al., Reference Wu, Lifka and Ondruschka2005).
Sulphur-compounds are more likely the product of protein degradation as an effect of heat (Al-Attabi et al., Reference Al-Attabi, D'arcy and Deeth2008; Al-Attabi et al., Reference Al-Attabi, D'Arcy and Deeth2014). In addition to sulphur-compounds, protein degradation action may also be a pathway for the formation aldehydes, esters, alcohols (Singh et al., Reference Singh, Drake and Cadwallader2003; Cadwallader and Singh, Reference Cadwallader, Singh, McSweeney and Fox2009). Peptides and amino acids resulting from proteolysis can be catalysed to form α-keto acids and then decarboxylated to form aldehydes, which may eventually be dehydrogenized to generate alcohols (Singh et al., Reference Singh, Drake and Cadwallader2003). The degradation of α-keto acids also produces methanethiol which is subsequently oxidized to form sulphur compounds (Singh et al., Reference Singh, Drake and Cadwallader2003).
Even though off-flavours have been reported in milk sonicated at specific conditions including frequency, time, and temperature, these reports make no correlation between the off-flavour and specific volatile compounds using sniffing port GC-O or by sensory evaluation (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). Therefore, the volatile constituents contributing to the undesirable flavours still have not been clearly identified. Moreover, it has been suggested that the chemical reactions occurring during sonication are related to not only one or two of the components in milk (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a), and several chemical reactions related to milk fat and proteins should be considered instead.
High frequency–low intensity ultrasound
High frequency ultrasound can generate large amounts of free radicals which can promote the oxidation of milk components, especially milk fat (Ashokkumar et al., Reference Ashokkumar, Sunartio, Kentish, Mawson, Simons, Vilkhu and Versteeg2008; Johansson et al., Reference Johansson, Singh, Leong, Mawson, McArthur, Manasseh and Juliano2016). The formation of lipid oxidation volatiles relies on several factors such as frequency, power, processing time, temperature, fat content and the type of transducer (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). The study by Juliano et al. (Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014) showed that sonication at frequencies of 400 and 1000 kHz and energies greater than 271 kJ/kg produced lipid oxidation volatile compounds which exceeded their odour threshold value in raw milk (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). This high frequency range is often used in applications targeting milk fat separation. Aldehydes including hexanal and nonanal were the major compounds targeted for their significant role in milk flavour development (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014; Torkamani et al., Reference Torkamani, Juliano, Ajlouni and Singh2014). Nonanal was found to exceed the threshold odour value in skim milk and raw milk diversely depending on the applied frequency and system temperature. The highest value of nonanal was obtained when skim milk was sonicated at 0.4 MHz for 20 min at 271 kJ/kg, in which nonanal exceeded its sensory threshold value by 80 times (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). At 1 MHz sonication, nonanal was detected over its threshold values after 5 min of sonication in skim milk at both 4°C and 45°C (102–409 kJ/kg), whereas in raw milk similar increase was obtained after sonication for 20 min at 4°C (409 kJ/kg) and after 5 min at 45°C (102–409 kJ/kg). Temperature affects nonanal production; the formation of nonanal at higher temperature (45°C) was greater than at refrigerated conditions (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). The concentration of nonanal in skim milk was greater by 3–5 times than that in skim milk when sonication processing was done at 45°C (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014). In contrast, at lower energy densities (<230 kJ/kg) and high frequency (0.4–2 MHz), milk oxidation volatiles were not observed even when sonicated for 20 min at 4°C or 5 min at 63°C. Hence, the authors suggested that the extent of lipid oxidation in milk can be controlled by decreasing the sonication time and system temperature (Juliano et al., Reference Juliano, Torkamani, Leong, Kolb, Watkins, Ajlouni and Singh2014).
Similarly, Torkamani et al. (Reference Torkamani, Juliano, Ajlouni and Singh2014) reported the highest amount of hydroxyl radical formation at frequencies between 400–1000 kHz in sonicated cheddar whey. However, the application of high frequency ultrasound at 400 and 1000 kHz did not promote lipid oxidation at the energy input value lower than 390 kJ/kg. The production of lipid oxidation volatiles at these conditions remained below detectable odour thresholds. These volatile compounds were present in both unsonicated and sonicated whey (Torkamani et al., Reference Torkamani, Juliano, Ajlouni and Singh2014). Aldehydes such as hexanal, (E)-2-hexanal, heptanal, nonanal, and (E)-2-heptanal were the primary compounds investigated for their odour active values in this study (Torkamani et al., Reference Torkamani, Juliano, Ajlouni and Singh2014). In addition, the author observed that the use of ultrasound negligibly affects lipid chemistry. Either phospholipid composition or free fatty acid concentration remained unchanged after ultrasound processing at 20, 400, 1000 and 2000 kHz (Torkamani et al., Reference Torkamani, Juliano, Ajlouni and Singh2014). These findings, therefore, show that ultrasonic technology at both low and high frequency has potential application in whey processing.
Johansson et al. (Reference Johansson, Singh, Leong, Mawson, McArthur, Manasseh and Juliano2016) developed a large-scale 1–2 MHz high frequency ultrasonic system operating in non-cavitation or low-cavitation conditions for treating unprocessed fresh milk (at temperatures between 4–27°C, for 1–10 min). The study concluded that lipid oxidation derived volatiles were below the human sensory thresholds. Ultrasound treatment therefore appeared to insignificantly affect the oxidative reactions in milk at either 1 MHz (with ultrasonic power of 46 kJ/l and 464 kJ/l) or 2 MHz (at 37 kJ/l and 373 kJ/l) (Johansson et al., Reference Johansson, Singh, Leong, Mawson, McArthur, Manasseh and Juliano2016).
Influence of ultrasound on the production of volatile compounds in sonicated milk products and their respective off-flavours
The application of ultrasound as a homogenization method in yogurt and soft cheese processing offers great potential for improving physical and functional properties (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009b; Nguyen and Anema, Reference Nguyen and Anema2010, Reference Nguyen and Anema2017; Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2010). However, the production of volatile compounds causing off-flavour derived from ultrasonicated milk must be considered. Thus, the production of volatile compounds which may affect the flavour profile and sensory properties has been examined (Sanchez et al., Reference Sanchez, Simal, Femenia, Benedito and Rosselló2001; Sfakianakis and Tzia, Reference Sfakianakis and Tzia2017; Lacivita et al., Reference Lacivita, Conte, Musavian, Krebs, Zambrini and Del Nobile2018). The off-flavour producing ‘rubbery’, ‘burnt’, plastic, metallic were also detected in cheese made from low-frequency sonicated milk (Marchesini et al., Reference Marchesini, Balzan, Montemurro, Fasolato, Andrighetto, Segato and Novelli2012) or burnt, pungent flavour or fatty attributes in yogurt derived from sonicated milk (Sfakianakis and Tzia, Reference Sfakianakis and Tzia2017). These undesirable flavours appeared to cause negative effects on the sensory properties of these milk products.
Sfakianakis and Tzia (Reference Sfakianakis and Tzia2017) applied ultrasound (20 kHz, <750 W, 10 min) to homogenized milk in yogurt-making process and found that ultrasound generally result in the off-flavours which affect the degree of likeness in the end products. Compared to yogurt from conventional homogenized milk, the yogurt pre-processed by ultrasound had higher sensorial score in most individual properties in term of taste and flavour. These included both positive (sweet, acidic taste whey and milky odour and flavour) and negative (grassy, pungent, burnt off-flavour) attributes (Sfakianakis and Tzia, Reference Sfakianakis and Tzia2017). Although sweetness and acidity grades were close to the panellist's liking, the presence of off-flavour in ultrasound treated samples appeared to be significantly higher than that in conventionally homogenized samples where the off-flavour were almost not detectable. The overall degree of acceptability in ultrasound treated samples, therefore, was lower than that of conventionally homogenized samples. Flavour of yogurt samples were attributed to the volatile compounds produced (Sfakianakis and Tzia, Reference Sfakianakis and Tzia2017). Yoghurt pre-processed by ultrasound had a higher concentration of ketones, aldehydes, hydrocarbons and dimethylsulphide when analysed by GCMS, except for 2,3-butadione, 3-hydroxy-2-butanone, and delta-decalactone which were similar for samples with or without treated by ultrasound. Among these compounds, only 3-hydroxy-2-butanone and delta-decalactone have positive effects on the degree of acceptability whereas hexanal, butyric acid, 2-heptanone, 2-undecanone and hexadecanoic acid negatively affect flavour and subsequently acceptability. However, the authors observed that ultrasound treatment at 150 W did not cause the differences in taste and flavour profiles from pressure homogenized samples, whereas ultrasound at 375 W or at higher energy density resulted in higher intensities of off-flavour and lower acceptability in ultrasound treated samples (Sfakianakis and Tzia, Reference Sfakianakis and Tzia2017). Optimizing the energy density by using lower and medium range ultrasound power allowed the production of yogurt with acceptable flavour (Sfakianakis and Tzia, Reference Sfakianakis and Tzia2017). In addition, potential improvements in texture and on the fermentation process reinforce the usefulness of ultrasound in yogurt manufacture (Sfakianakis and Tzia, Reference Sfakianakis and Tzia2014, Reference Sfakianakis and Tzia2017).
Ultrasound was also applied as a complementary method during the brining period in cheese processing by Sanchez et al. (Reference Sanchez, Simal, Femenia, Benedito and Rosselló2001). In this research, Mahon cheese was brined in the ultrasonic bath at 300 W, 30 kHz and the free fatty acids were examined during ripening at 12°C along with sensory evaluations of the final cheese products. The application of ultrasound during brining significantly increased the extent of lipolysis due to effect of acoustic cavitation resulting in fat globule disruption. As a result, acoustically brined cheese had a higher concentration of free fatty acid, and the final cheese products possessed higher intensities of sensorial characteristics in terms of firmness, aroma, odour and flavour compared to conventionally brined cheese. The ultrasonically brined cheese had a higher degree of sweetness, bitterness and acidity possibly due to the contributions of ethyl esters resulting from the enzymatic or chemical esterification of fatty acid with ethanol, and the increase in concentration of free fatty acids (FFAs). Interestingly, the quality of the final product was acceptable even though the degree of bitterness increased (Sanchez et al., Reference Sanchez, Simal, Femenia, Benedito and Rosselló2001). Therefore, the application of ultrasound in brining of cheese at low frequency appears to be promising in the cheese-making process. Furthermore, the combined application of steam (94–95°C) and ultrasound (25–40 kHz) (so-called Sonosteam) on cheese processing for up to 6 s displayed unchanged sensory attributes in term of odour while it helped to improve the bactericidal inactivation (Lacivita et al., Reference Lacivita, Conte, Musavian, Krebs, Zambrini and Del Nobile2018). The authors suggested that the use of Sonosteam at low frequency for a rapid treatment time (25–40 kHz, 6 s) is potentially useful for cheese processing (Lacivita et al., Reference Lacivita, Conte, Musavian, Krebs, Zambrini and Del Nobile2018). However, in these studies, only general sensory attributes were considered, and no volatile profiles were reported.
The application of ultrasound (400 W, 24 kHz, 22 mm diameter probe) on raw milk for cheese-making appeared to result in a significant rise in burnt off-flavour (Marchesini et al., Reference Marchesini, Balzan, Montemurro, Fasolato, Andrighetto, Segato and Novelli2012). In addition, plastic, rubbery and metallic flavours were also detected in ultrasound samples when applying sonication for 50 s to 200 s. The authors suggested that the application of ultrasound for less than 200 s can minimize the generation of burnt flavour. This research was in agreement with findings from Chouliara et al. (Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010), in which the application of ultrasound for 2 min or less was preferred to maintain the sensory acceptability of milk. The burnt flavour was decreased when CO2 was added as a pre-treatment (Marchesini et al., Reference Marchesini, Balzan, Montemurro, Fasolato, Andrighetto, Segato and Novelli2012). The authors suggested that higher gas content affected the collapsing bubbles, and as a result, reduced the intensity of pyrolytic processes and thus the formation of oxidation products. The milk coagulation properties in cheese processing were improved as a function of ultrasound treatment, which thus has a potential in cheese-making industry (Marchesini et al., Reference Marchesini, Balzan, Montemurro, Fasolato, Andrighetto, Segato and Novelli2012). The addition of CO2 to reduce lipid oxidation products may improve the utility of ultrasound not only in cheesemaking but also in other dairy food systems (Marchesini et al., Reference Marchesini, Balzan, Montemurro, Fasolato, Andrighetto, Segato and Novelli2012).
In addition to yogurt and cheese, other uses of low frequency ultrasound have been reported. Guimaraes et al. (Reference Guimaraes, Silva, Ranadheera, Moraes, Raices, Silva, Ferreira, Freitas, Meireles and Cruz2019) applied ultrasound (19 kHz, 3 min, 13 mm diameter probe, uncontrolled temperature) at various power (0, 200, 400, and 600 W) to soursop whey beverage. Various volatile compounds including acids, alcohols, esters, alkanes were produced after any of the low frequency ultrasound treatments (as illustrated in Table 2), which were not detected in unsonicated samples. These compounds include 3-furanmethanol, 1-hexanol, α-methylbutyric acid, isobutylacetic acid, 3,3,5-trimethyl heptane, 1-octanol, 2-nonanone, α-isophorone, methyl octanoate, pyranone, hexanoic acid, β-linalool, octanoic acid and decanoic acid. Some volatile compounds were detected as the negative effects of ultrasound, however, the beneficial effects in improvement of chemical changes were prominent (Guimaraes et al., Reference Guimaraes, Silva, Ranadheera, Moraes, Raices, Silva, Ferreira, Freitas, Meireles and Cruz2019). For example, ultrasound processing was found to induce more beneficial modifications in nutritional attributes (improving the antioxidant and anti-hypertensive activity, decreasing undesired minerals, and increasing phenolic content) than the conventional heat treatment. Concerns regarding volatile production could be managed by controlling the power applied, with medium power (400 W) suggested as the best option to avoid negative effects. This generated fewer number of volatiles (5 compounds among 14 compounds detected) compared to ultrasound at 200 and 600 W, which produced 9 volatile compounds (Guimaraes et al., Reference Guimaraes, Silva, Ranadheera, Moraes, Raices, Silva, Ferreira, Freitas, Meireles and Cruz2019).
The application of ultrasound in beverage-making process has also been attempted by Monteiro et al. (Reference Monteiro, Silva, Alvarenga, Moraes, Freitas, Silva, Raices, Sant'Ana, Meireles and Cruz2018). Twenty nine volatile compounds including 12 ketones, 1 terpene, 4 alcohols, 3 aldehydes and 9 acids were identified in chocolate milk treated by either conventional heat treatment or low frequency ultrasound (19 kHz, 13 mm diameter probe, 0.3–3 kJ/cm3) as listed in Table 2. These volatiles appeared to be similar to those identified for low frequency sonicated milk (Riener et al., Reference Riener, Noci, Cronin, Morgan and Lyng2009a; Chouliara et al., Reference Chouliara, Georgogianni, Kanellopoulou and Kontominas2010). The application of ultrasound instead of heat treatment appeared to avoid the heat degradation of large amount of desirable volatile compounds as more volatile compounds were identified in all ultrasound treated samples (16–24 compounds) compared to heat treated samples (14 compounds). The author suggested that the application of ultrasound with various energy densities allowed a qualitative identification of volatile compounds compared to the conventional heat treatment and when applying energy density at the highest level (2.4 and 3.0 kJ/cm3) allowed the larger number of volatile compounds detected (Monteiro et al., Reference Monteiro, Silva, Alvarenga, Moraes, Freitas, Silva, Raices, Sant'Ana, Meireles and Cruz2018). Interestingly, the application in this case provided beneficial effects on the microbiological and physicochemical quality of the chocolate milk beverage along with maintaining the nutritional value of the products (Monteiro et al., Reference Monteiro, Silva, Alvarenga, Moraes, Freitas, Silva, Raices, Sant'Ana, Meireles and Cruz2018). Therefore, the author suggested that ultrasonic technology could be considered as promising and effective alternative to conventional heat treatment in development of new products.
Final considerations and further directions
The application of ultrasound provides great potential benefits to wide aspects of dairy processing in enhancing functional properties and process efficiencies. However, the production of undesirable volatiles from lipolysis and proteolysis of milk fat and milk proteins is of concern. Fortunately, these detrimental effects can be controlled by optimizing ultrasonic processing conditions. The optimal ultrasonic method can be achieved by decreasing the sonication time, temperature and selection of a suitable ultrasonic frequency, which helps to reduce the oxidation reactions caused by free radicals. Importantly, the benefits of ultrasound regarding homogenization, emulsification and indeed most of its positive effects can be achieved by ultrasound over short times and at low frequencies. Under these ultrasound conditions, the production of undesirable compounds and generation of off-flavours is insignificant and therefore, no marked influence on the sensory value of milk systems. Moreover, the advantage of using ultrasound in combination with a heat treatment provides sterilization at a much lower temperature which then helps to significantly reduce off-flavour from conventional heat processing while still maintaining the sensitive nutritional ingredients. Ultrasound technology thus appears to be a promising innovative method not only to improve the quality and safety of processed milk but also offering potential economic and environmental benefits. More research is needed to improve ultrasonic equipment for large scale applications, and to restrict the generation of off flavours in such systems, so as to promote the development of ultrasound technology in the future dairy industry.
Acknowledgements
The authors gratefully acknowledge the support from Royal Melbourne Institute of Technology, Australia and Vietnam Institute of Education Department (VIED) scholarship.








