INTRODUCTION
Status epilepticus (SE) is the most common neurological emergency in the intensive care unit (ICU) after a stroke, and it carries with it a high mortality rate.Reference Lowenstein and Alldredge 1 The incidence of SE is estimated at 18.3-41.0 per 100,000.Reference Hesdorffer, Logroscino, Cascino, Annegers and Hauser 2 , Reference DeLorenzo, Hauser and Towne 3 A study from Thailand estimated the incidence of SE at 5.10 per 100,000.Reference Tiamkao, Pranbul, Sawanyawisuth and Thepsuthammarat 4 Although there are no epidemiological studies, the incidence of SE is likely to be higher in developing countries because of the higher incidence of central nervous system (CNS) infections, trauma, stroke, poor infrastructure and the existence of a treatment gap.Reference Kalita, Nair and Misra 5 Refractory status epilepticus (RSE) is defined as SE that does not respond after administration of two intravenous (IV) antiepileptic drugs (AEDs), one of which is a benzodiazepine.Reference Rossetti and Lowenstein 6 RSE occurs in 23-43% of patients with SE.Reference Novy, Logroscino and Rossetti 7 , Reference N air, Kalita and Misra 8 The outcome of SE is determined by the underlying aetiology, and the refractoriness of SE may also influence its outcome.Reference Rossetti, Logroscino and Bromfield 9 The morbidity of SE increases with its refractoriness to medical therapy.Reference Misra, Kalita, Bhoi and Dubey 10 Super-refractory SE (SRSE) refers to SE that is resistant to treatment with IV anaesthetic agents, and it has been reported in 22% of patients with SE.Reference Ferlisi and Shorvon 11
The spectrum of SE is different in developing countries as compared to the developed world. CNS infections contribute to nearly half the patients with SE in developing countries, and this SE group is difficult to control.Reference Kalita, Nair and Misra 5 , Reference Misra, Kalita and Nair 12 There is a paucity of studies on refractory SE in developing countries, so we undertook this research to report on the aetiology and predictors of the outcome of refractory SE in a tertiary care teaching hospital in India.
Subjects and Methods
This prospective hospital-based observational study was conducted from September of 2013 to July of 2015 in a tertiary care postgraduate teaching hospital located in the state of Uttar Pradesh in India. This institution caters to patients from the states of Uttar Pradesh, Bihar and Madhya Pradesh, as well as patients from adjoining Nepal. Patients of all ages (including paediatric patients) are treated in the hospital. Our study was approved by the institutional ethics committee, and the participants or an authorized representative gave written consent for their participation.
Definitions
SE was defined as continuous seizures for ≥5 minutes or recurrent seizures without recovery of consciousness to baseline between attacks. Subtle SE was defined as the presence of coma and ictal discharges on electroencephalogram (EEG), along with subtle convulsive movements.Reference Rossetti and Bleck 13 Regardless of timeframe, SE episodes persisting despite sufficient doses of benzodiazepines (BZDs) and at least one other antiepileptic drug (AED) were diagnosed as “refractory SE” (RSE).Reference Rossetti and Lowenstein 6 Patients’ records of treatment received prior to hospitalization were reviewed where available. SRSE was defined as SE that continued for 24 hours or more after the onset of anaesthetic therapy, including those cases that recurred upon reduction or withdrawal of anaesthesia.Reference Shorvon and Ferlisi 14
Management
Patients with SE were initially treated using a predefined protocol, which was the same for all patients.Reference Brophy, Bell and Claassen 15 , Reference Glauser, Shinnar and Gloss 16 The protocol included IV lorazepam (LZP) 0.1 mg /kg IV, which was repeated if the seizures were not controlled within 10 minutes of the first dose. Second-line AEDs included phenytoin (PHT) 18 mg/kg IV (at the rate of 50 mg/min), sodium valproate (VPA) 30 mg/kg IV (at 100 mg/min) or levetiracetam (LEV) 20 mg/kg IV (at 100 mg/min). If the SE was refractory to the second-line AEDs, patients were managed in the ICU and were given IV anaesthetics such as midazolam (loading dose of 0.1 mg/kg, followed by infusion starting at a rate of 0.1 mg/kg/hour); propofol (loading dose of 2 mg/kg, followed by continuous infusion at 2.5 mg/kg/hour); ketamine (2 mg/kg bolus, followed by continuous infusion at 0.5 mg/kg/hour); or phenobarbital (loading dose of 10 mg/kg, with 100 mg/min up to 700 mg)—and other AEDs as indicated. The treatment protocol was the same no matter what treatment was received before hospital admission. Burst suppression in the EEG was achieved when patients continued to have generalized seizures despite having received three AEDs in addition to BZDs, subject to availability of a ventilator. Infusion of IV anaesthetic was started along with EEG and intra-arterial blood pressure monitoring. Withdrawal of the anaesthetic was attempted 48 hours after remission of seizure. Response to treatment was defined as seizure cessation. An EEG was done an hour after seizure control if the patient’s consciousness did not normalize. The EEG was reviewed by the authors themselves, who are neurologists, and decisions were taken by consensus. Burst suppression was defined as an inter-burst difference in the EEG of 8-20 msec.
Evaluation
The patients’ demographic details (e.g., age, area of residence and gender) were collected. The duration of seizures (time including treatment outside the hospital) and the nature of treatment before hospitalization in our centre were also noted. SE severity upon admission was assessed by the STESS,Reference Rossetti, Logroscino, Milligan, Michaelides, Ruffieux and Bromfield 17 which was calculated by level of consciousness (0 for alert, 1 for stupor or coma), seizure type (1 for generalized, 2 for non-convulsive in coma, 0 for others), patient age (0 for <65 and 1 for ≥65) and history of previous seizures (0 if yes, 1 if no or unknown). The STESS ranged between 0 and 6, where a score of 0-2 was regarded as favourable and 3-6 as unfavourable.Reference Rossetti, Logroscino, Milligan, Michaelides, Ruffieux and Bromfield 17 We also documented medical comorbidities, SE aetiology, cranial CT or MRI findings, type of SE, and CSF findings. We also made note of the type of SE: generalized, secondary generalized, simple partial and non-convulsive. The aetiology of the SE was recorded, and we recorded the imaging findings (CT and/or MRI), cerebrospinal fluid (CSF) findings, blood counts and serum chemistry (blood urea nitrogen, creatinine, bilirubin, transaminases, alkaline phosphatase and electrolytes). We also performed chest radiography and EEGs, and tested for arterial blood gas. The aetiology of the SE was categorized as stroke (arterial or venous), CNS infection, tumour, drug withdrawal, metabolic and other causes. The outcome of RSE was defined as clinical cessation of seizures. Death in hospital and its cause were also recorded. The patient’s condition at the time of discharge was recorded using the modified Rankin Scale (mRS), where mRS ≤3 was considered a good and >3 a poor outcome.
Statistical Analysis
The aetiology of SE, RSE, and SRSE was compared with respect to demographic information, clinical data, STESS results and outcomes using the parametric test for categorical variables and analysis of variance (ANOVA) for continuous variables. The predictors of SE and RSE were evaluated using univariate regression analysis, followed by multivariate regression. Variables with a two-tailed value of p<0.1 were included in the multivariate regression analysis. The outcome of SE, RSE and SRSE in different age groups was evaluated using the chi-squared test for categorical variables and ANOVA for continuous variables. A two-tailed value of p<0.05 was considered significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, v. 16, SPSS Inc., Chicago, USA).
RESULTS
A total of 81 consecutive patients with SE were included in our study. The median age of patients was 38 years (range=8-90), 27 (32.5%) of whom were females and 13 (16%) below 18 years of age. The aetiologies of SE were CNS infections in 24 (29.6%), stroke in 19 (23.5%), metabolic disorders in 17 (21%), drug withdrawal in 3 (3.6%) and miscellaneous aetiologies in 11 (13.2%). Some 68 (84%) patients did not have a prior history of seizure or epilepsy. The STESS was favourable (0-2) in 25 (30.1%) participants. A single comorbidity was present in 42 (51.9%) and more than one comorbidity in 17 (21%) (see Table 1 and Figure 1). A total of 39 (48.1%) patients were treated for SE before admission to our hospital, but the complete details of outside treatment were available for only 30 participants. All patients for whom pre-hospital treatment was inadequate or whose hospital records did not mention treatment were retreated.

Figure 1 Flow chart showing the subgroups of status epilepticus, their etiologies and outcome. CNS=central nervous system; mRS=modified Rankin Scale; RSE=refractory status epilepticus; SRSE=super refractory status epilepticus.
Table 1 Comparison of demographic, clinical, imaging and laboratory parameters in patients with refractory status epilepticus (RSE) and non-refractory status epilepticus (NRSE)

mRS=modified Rankin Scale; NRSE=non-refractory status epilepticus; RSE=refractory status epilepticus; STESS=status epilepticus severity score.
Refractory SE
Some 35 of the 81 (42.2%) patients had RSE. The median age of patients with RSE was 38 years (range=9-90), and 10 (28.6%) were females. The median duration of SE before initiation of AEDs was longer in RSE compared to non-refractory status epilepticus (NRSE) patients (p=0.01). Some 21 patients (60%) had associated comorbidities, and 11 (31.4%) had more than one comorbidity. RSE patients had more frequent multiple comorbidities (31.4 vs. 13%, p=0.0.04), hypertension (42.9 vs. 19.6%, p=0.02) and renal failure (25.7 vs. 6.5%;, p=0.02) compared to the NRSE group. The underlying aetiology of RSE was stroke in 5 (14.3%) (venous in 1, arterial ischemic in 2 and intra-cerebral haemorrhage in 2). Four of these patients had early SE, and one had SE 7 days following the stroke. RSE was associated with CNS infections in 12 (34.3%) (encephalitis in 5, meningitis in 6, neurocysticercosis in 1); metabolic aetiologies in 13 (37.1%); renal failure in 9, 8 of whom were dialysis-dependent; liver failure in 1; hyponatremia in 1; prolonged hypoglycemia in 1; hyperglycemic hyperosmolar state in 1; drug withdrawal in 1 (2.9%); and miscellaneous causes in 3 (8.6%). A total of 19 (54.3%) patients had abnormal brain imaging, which included cortical involvement in 11 and subcortical involvement in 8 patients. The RSE patients more frequently had a metabolic aetiology (37.1 vs. 8.7%, p<0.001). Admission STESS was more frequently favourable in NRSE than in RSE patients. Upon multivariate analysis, the predictors of RSE were unfavourable STESS (OR=, CI 95%=1-36.33, p=0.05) and duration of SE before treatment (OR=3.35, CI 95%=0.243-0.779, p=0.01).
There were 13 SE patients whose age was <18 years, 8 (61.5%) of whom developed RSE and 3 (23.07%) of whom had SRSE. Two patients (25%) in each group had CNS infections and a metabolic aetiology of RSE. Outcome in the paediatric RSE group was better compared to that of adults, as only one patient (12.5%) died, but this result was not statistically significant (p=0.12) (Table 2).
Table 2 Comparison of demographic, clinical, imaging and laboratory findings in patients with refractory status epilepticus (RSE) and non-refractory status epilepticus (NRSE) in paediatric (<18 years) patients (n=13)

Treatment
The median dose of LZP was 8 mg (range=4-12), LEV 1500 mg (range=600-1800), VPA 1500 mg (range=600-1800), PHT 900 mg (range=300-1000), and lacosamide 400 mg (range=200-400) in patients with RSE. The third-line AEDs administered included midazolam in 13 (37.1%), LEV in 15 (42.9%), VPA in 4 (11.4%), and lacosamide in 1 (2.9%). SE was controlled in 18 (51.4%) patients after administration of the third-line AED.
Outcome
More patients with RSE died compared to those with NRSE (14 vs. 6, p<0.001), and RSE patients had worse outcomes upon discharge (62.9 vs. 32.6%;, p=0.01). Cause of death was directly related to prolonged SE in one patient only, who had encephalitis with thrombocytopenia, renal failure, liver failure and hypotension. The median length of hospital stay of the patients who died was 9 days (range=1-57), and SE persisted for a median of 5.75 hours (range=1-42) of treatment. Death in the remaining patients was due to underlying causes rather than the SE per se. The clinical and radiological results and outcomes of the RSE and NRSE patients are presented in Table 1.
Super-Refractory SE
A total of 10 (12%) patients were categorized as having SRSE. The mean age of these patients was 32±22.1 years, and 4 (40%) of them were females; 5 (50%) had encephalitis and 3 (30%) chronic renal failure. Brain imaging findings in this group revealed characteristic fronto-temporal involvement in patients with herpes simplex encephalitis (HSE). MRI changes were present in two patients from the metabolic group. The MRI changes in three patients were regarded to be due to SE per se. Brain imaging was more frequently abnormal in SRSE compared to RSE (60 vs. 52%, p=0.04). Midazolam was prescribed for all these patients, propofol to 4 (40%), ketamine to 3 (30%) and phenobarbitone to 3 (30%). One patient with SRSE responded to a ketogenic diet, which was prescribed when their SE was refractory to seven AEDs. A total of 4 (40%) patients with SRSE died, and 4 (40%) had a good outcome at the time of discharge (Table 3).
Table 3 Comparison of demographic, clinical, imaging and laboratory parameters in patients with refractory status epilepticus (RSE) and super-refractory status epilepticus (SRSE)

mRS=modified Rankin Scale; RSE=refractory status epilepticus; SRSE=super refractory status epilepticus; STESS=status epilepticus severity score.
Complications
A total of 23 (65.7%) patients required mechanical ventilation (MV), 24 (68.6%) developed sepsis, 21 (60%) had hypotension, 12 (34.3%) developed ventilator-associated pneumonia (VAP), 7 (20%) required tracheostomy and 1 (2.9%) had cardiac arrhythmias. Some 6 patients with RSE (17.1%) developed recurrent SE. A comparison of the complications among patients with NRSE, RSE and SRSE are presented in Table 4.
Table 4 Complications of ICU-related complications in patients with refractory status epilepticus (RSE) and super-refractory status epilepticus (SRSE)

NRSE=non-refractory status epilepticus; RSE=refractory status epilepticus, SE=status epilepticus, SRSE=super-refractory status epilepticus, VAP=ventilator-associated pneumonia.
Outcome
Out of the 81 patients with SE, 20 (24.7%) died in the hospital, 37 (47%) had poor outcomes and 44 (53%) had good outcomes upon discharge. The predictors of poor outcome in RSE (including death) were as follows: age above 60 years (OR=1.95, CI 95%=0.10-4.66, p=0.02); mechanical ventilation (OR=6.0, CI 95%=0.973-37.5, p=0.05); and metabolic aetiology (OR=3.85, CI 95%=0.241-61.6, p=0.05). Table 5 presents the outcome predictors for RSE patients of different age groups.
Table 5 Outcome of refractory status epilepticus (RSE) in different age groups
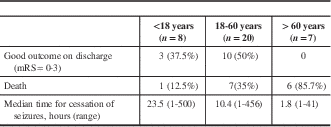
mRS=modified Rankin scale.
DISCUSSION
A total of 35 (42.2%) of our patients with SE developed RSE. Metabolic encephalopathy and CNS infections were the most common aetiologies associated with RSE. Delay in treatment and high STESS were found to be predictors of RSE. Some 40% of patients with RSE died, and 47% had poor outcomes, which was related to age above 60 years, MV and metabolic aetiology.
There is a paucity of studies on RSE in developing countries. Our results are in agreement with another study from India, in which delay in initiating treatment for SE resulted in RSE. VPA and LEV had similar response rates in these patients and resulted in similar side effects.Reference Tripathi, Vibha and Choudhary 18 There was a wide variation in the frequency of RSE in different studies, ranging from 9 to 44%.Reference Rossetti, Logroscino and Bromfield 9 , Reference Mayer, Claassen, Lokin, Mendelsohn, Dennis and Fitzsimmons 19 - Reference Treiman, Meyers and Walton 21 The reason for this broad variance may be due to differences in how they defined RSE and different underlying aetiologies. We defined RSE as resistance of SE to two AEDs, one of which being a BZD.Reference Tiamkao, Pranbul, Sawanyawisuth and Thepsuthammarat 4 The type of SE and aetiology of SE will also predict RSE. Generalized SE due to drug noncompliance may be more responsive to therapy compared to secondary generalized seizures following CNS infection or necrotizing lesions. The International League Against Epilepsy Task Force on SE recently provided a new classification of SE that includes four axes: semiology, EEG correlate, aetiology and age. The duration of convulsive SE includes two main timepoints: (1) t1=5 min (the earliest time when treatment should be begun), which is concerned with the lack of a mechanism for terminating seizures and the mechanisms responsible for abnormally prolonged seizures; and (2) t2, when such long-term complications as neuronal injury, neuronal death, alteration of neuronal networks and functional deficits are increasingly likely.Reference Trinka, Cock and Hesdorffer 22 Such a classification may help to develop a more comprehensive and uniform documentation of SE in future studies.
There was a high proportion of metabolic aetiologies (37.1%) and CNS infections (34.3%) in our population, in contrast to an earlier study from a developed country which found that low AED levels constituted the major cause.Reference Mayer, Claassen, Lokin, Mendelsohn, Dennis and Fitzsimmons 19 The high frequency of metabolic aetiologies leading to RSE may be due to a referral bias. Our hospital is a tertiary referral center for renal, gastrointestinal and endocrinological diseases, and thus the trends to a high proportion of renal failure, liver failure and hyperosmolar coma leading to SE. Restoration of an underlying transient metabolic alteration (e.g., electrolyte imbalance) might easily control SE, but such chronic progressive disorders as chronic renal failure, liver failure and hyperosmolar states may be more resistant to treatment. Some 22.9% of our RSE patients were dialysis-dependent, 11.4% had associated cirrhosis of the liver, 5.7% had hyponatremia and 2.9% were in a hyperosmolar state. Encephalitis was determined to be the underlying cause of RSE in 34.3% of our patients. Encephalitic conditions like HSE and Japanese encephalitis (JE) can produce necrotizing haemorrhagic lesions in the fronto-temporal cortex and subcortical regions, respectively. In a study of 117 patients with SE,Reference Kalita, Nair and Misra 23 53.8% had a CNS infection, 14.5% had metabolic encephalopathy, 12.8% stroke, 7.9% drug noncompliance, and 11% had miscellaneous causes. Similar results were reported in a studyReference Chen, Zhou and Li 24 on SE in which CNS infections were responsible for 32.7% of the cases. Aside from viral encephalitis, bacterial and fungal CNS infections can also result in SE. In a study of SE caused by CNS infections,Reference Misra, Kalita and Nair 12 the encephalitides responsible for SE included HSE in 3, JE in 4 and nonspecific encephalitis in 12. Meningitides (tuberculosis in 5, pyogenic in 3 and fungal in 1) and CNS granuloma in 7 were also noted. Some 24.3% of these patients had RSE, and 29.7% died.
Seizures are common in CNS infections (e.g., CNS tuberculosis, neurocysticerscosis, fungal infection and viral encephalitis). Encephalitides seem to be notably epileptogenic and have been reported to cause an RSE that requires long-term treatment with antiepileptic drugs.Reference Holtkamp, Othman, Buchheim and Meierkord 20 , Reference Yaffe and Lowenstein 25 , Reference Parent and Lowenstein 26 HSE involves the fronto-temporal cortex, which has a low seizure threshold. Seizures have been reported in acute herpes simplex encephalitis (40-60% of cases) and in JE (7-60% patients).Reference Misra, Kalita and Nair 12 Seizures in encephalitis are related to young age, level of consciousness and cortical involvement upon imaging. Misra et al.Reference Misra, Kalita and Nair 12 also found that 60% of children, 27% of adults and 54% with cortical involvement had seizures, compared to 15% of patients without cortical involvement.Reference Misra, Kalita and Nair 12
Our univariate analysis found that delay in SE treatment, metabolic aetiology, a high STESS and comorbidity were associated with RSE. Our multivariate analysis found that delay in SE treatment and a high STESS were also correlated with RSE. Prolonged persistence of SE produces trafficking of GABA receptors. There is a decreased number of GABA subunits present in the postsynaptic membranes and an increase in their reuptake by the cells. Endocytosis of GABA receptors accounts for progressive pharmaco-resistance to benzodiazepines as the SE proceeds.Reference Naylor, Liu and Wasterlain 27 Moreover, such metabolic alterations as acidosis, hypernatremia and brain oedema initiate a vicious cycle. Poor infrastructure, tardy transport and the scarcity of healthcare facilities lead to delayed treatment of emergencies such as SE in developing countries. The mean duration of SE before treatment in developing countries has ranged from 11.65 to 18.02 hours.Reference Murthy, Jayalaxmi and Kanikannan 28 , Reference Bhalla, Das, Som, Prabhakar and Kharbanda 29 Documentation of pre-hospital treatment in our study was also not adequate, as complete details were available for only 30 (37%) patients. For the remaining patients, drugs were mentioned but without dosages, and there was often no mention of treatment at all. Before being referred, most patients were treated in local primary healthcare facilities or at private hospitals, where proper documentation procedures were not followed.
Some 65.7% of our RSE patients required MV, 14 (40%) of whom died. MV is required for the management of RSE because most of the IV antiepileptic drugs used in treatment can result in respiratory suppression or hypotension, or both. Due to limited ICU facilities, we used IV anaesthetics with great caution. Midazolam (37.1%), propofol (11.4%), phenobarbitone (11.4%), ketamine (8.6%) and phenobarbital (8.6%) are all utilized sparingly. We employed VPA (42.9%), LEV (57.1%), lacosamide (51.4%) and PHT (11.4%) as a second or third choice because of their safer respiratory and cardiovascular profiles. In a retrospective analysis of RSE,Reference Hocker, Britton, Mandrekar, Wijdicks and Rabinstein 30 MV was needed in 57 of 63 patients (90%), and long duration of MV was associated with death in 31.8% of the population. The MV-related complications in our study included cardiac arrhythmias (2.9%), VAP (34.3%), sepsis (68.6%) and hypotension (60%), and the mortality rate was comparable to that of the abovementioned study (40 vs. 31.8%). We followed a conservative policy when it came to intubation and MV.Reference Misra, Kalita and Bhoi 31 BZDs were the first drugs that patients received before being referred to the hospital, and, as per standard protocol, they received additional doses of BZDs, which may predispose them to hypotension and respiratory suppression. Higher death rates have been reported in RSE patients treated with IV anaesthetics.Reference Sutter, Marsch, Fuhr, Kaplan and Rüegg 32 It is thus prudent to be cautious with BZDs if the history of prior treatment is unknown. In our study, LEV and LZP were equally efficacious, but there were more hypotensive episodes and a greater need for the use of a ventilator in LZP-treated patients.Reference Misra, Kalita and Maurya 33
We performed burst suppression in only three patients. EEG burst suppression has not been associated with good outcomes. In a retrospective analysisReference Hocker, Britton, Mandrekar, Wijdicks and Rabinstein 30 using IV anaesthetics, 85% of SE episodes associated with burst suppression and 100% associated with isoelectric EEGs had poor outcomes. Recording an EEG in a convulsing patient is difficult due to EMG artefacts. We recorded EEGs an hour after cessation of a clinical seizure, when consciousness had normalized sufficiently to differentiate ongoing non-convulsive SE from drug-induced alterations of the sensorium.Reference DeLorenzo, Waterhouse and Towne 34 There is a marked variation in treatment policy at different hospitals.Reference Walker, Smith and Shorvon 35 , Reference Waterhouse, Garnett and Towne 36 The absence of a standard protocol for management of SE results in suboptimal treatment, which leads to poor outcomes. Adapting a treatment protocol for SE would probably help to improve outcomes.Reference Waterhouse, Garnett and Towne 36
A total of 47% of he patients in our study with RSE had poor outcomes as assessed by mRS at the time of discharge. The high mRS score improved upon further follow-up. The mortality rate and poor outcomes in RSE are mainly due to the precipitating illness or comorbidity rather than to the RSE itself. Comorbidities in our patients did predict poor outcomes. We have not as yet evaluated improvement upon long-term follow-up, as has been reported elsewhere.Reference Hocker, Britton, Mandrekar, Wijdicks and Rabinstein 30
STRENGTHS and WEAKNESSES of the STUDY
Ours is a prospective study evaluating the spectrum and outcome of RSE in a developing country. Though located in a developing country, the institution where it was carried out is a tertiary care referral center. The main aetiologies of SE were CNS infection and metabolic encephalopathy. The drugs and ICU facilities are similar to those in developed countries, but economic considerations were important because the medical care system functions based on out-of-pocket expenditures.
The limitations of our study are as follows: (1) it was conducted at a tertiary care teaching hospital with a referral bias for severe cases and a predominance of metabolic encephalopathy and CNS infections; (2) there was limited EEG monitoring; and (3) there was limited use of burst suppression.
We can conclude that RSE occurred in 42.5% of our patients with SE, that it was associated with death in 40% of our patients and that it was correlated with delay in treatment, MV and unfavourable STESS scores.
Acknowledgments
We thank Rakesh Kumar Nigam and Shakti Kumar for secretarial help.
Funding
This study was not financially supported.
Disclosures
Deepanshu Dubey, Sanjeev K. Bhoi, Jayantee Kalita and Usha K. Misra hereby state that they do not have anything to disclose.
Conflicts of Interest
There are no conflicts of interest to declare.
Statement of Authorship
Deepanshu Dubey was responsible for data collection. Sanjeev K. Bhoi performed data interpretation. Jayantee Kalita was involved in planning, writing and data interpretation. Usha K. Misra was responsible for planning, writing and data interpretation.








