Introduction
The Bearded Screech-owl Megascops barbarus is rare, strictly nocturnal, and endemic to the montane forests (cloud and pine-oak forest) of 1,800–2,500 m in the central highlands of Chiapas and Guatemala (del Hoyo et al. Reference del Hoyo, Elliott and Sargatal1999, König and Weick Reference König and Weick2008). Limited information about its natural history has been reported. Based on its restricted distribution and the lack of ecological information available, this species has been listed recently as ‘in Peril’ on the Mexican Red List (SEMARNAT 2008) and ‘Near Threatened’ globally (BirdLife International 2008). Montane forests in Chiapas have been reduced to less than 25% of their original area with an annual deforestation rate ≥2.7% in the last 30 yrs (Cayuela et al. Reference Cayuela, Rey Benayas and Echeverría2006).
Diet plays a significant role in the health and viability of organisms while their trophic relationships define their ecological functions (Kupfer et al. Reference Kupfer, Langel, Scheu, Himstedt and Maraun2006). Since the abundance of food types varies greatly in space and time (Recher Reference Recher, Morrison, Ralph, Verne and Jehl1990), a large number of animal species show a wide intra-specific and temporal variation in diet patterns (Dalerum and Angerbjörn Reference Dalerum and Angerbjörn2005).
For most tropical owl species, the diet patterns and trophic relationships are poorly known (König and Weick Reference König and Weick2008). Traditionally, diet composition for owls has been determined mostly by observations of foraging and identification of prey remains at nests, in regurgitated pellets, in faeces, and in stomachs (Rosenberg and Cooper Reference Rosenberg, Cooper, Morrison, Ralph, Verne and Jehl1990, Livezey Reference Livezey2007). However, identification of prey items by direct observation is difficult for nocturnal species. Analysis of stomach contents requires invasive surgery or killing of the individuals, which is not a suitable approach for rare and threatened species. Some insectivorous owls rarely produce pellets, making studying their diets difficult (see Lee and Severinghaus Reference Lee and Severinghaus2004). Fecal analysis is limited to the number of individuals that one can capture. Therefore, stable isotopes analysis could be a better tool for studying diet patterns and trophic relationships in owl species (Hobson and Sealy Reference Hobson and Sealy1991, Hobson and Clark Reference Hobson and Clark1992). Stable isotope analysis will not reflect the bulk diet composition available by other methods but will allow quantification of the diet origin of assimilated nutrients in animal tissues (Kelly Reference Kelly2000). It is a useful tool to evaluate spatial-temporal variation in diets and to establish trophic position in birds and mammals (Hobson and Montevecchi Reference Hobson and Montevecchi1991, Urton and Hobson Reference Urton and Hobson2005). Here, we used analysis of stable isotopes to answer whether diet patterns of the Bearded Screech-owl vary temporally in the short- and long-term, and spatially along the range in the Central highlands of Chiapas. Since remaining montane forest habitats for the owls are simplified in structure and their vegetation composition is associated with a drier and warmer forest interior (Ramírez-Marcial et al. Reference Ramírez-Marcial, González-Espinosa and Williams-Linera2001), we predicted that if there is variation in diet over the period when the feathers were growing there would be intra-feather and inter-feather variations in stable isotopes (Mizutani et al. Reference Mizutani, Fukuda and Wada1990). Furthermore, if the distribution and abundance of food types varied among locations and habitat conversion has reduced the diet quality of Bearded Screech-owl, we would expect to find spatial and temporal differences in isotopic signatures of δ13C and δ15N.
Methods
Study area
This study was conducted in the central highlands of Chiapas, Southern Mexico (17°11’N; 92°53’W), which are located from 1,500 to 2,000 m in altitude, and only < 4% is at elevations above 2,500 m. The climate is temperate sub-humid with a mean annual temperature of 13°–15°C. Mean annual precipitation is 1,150 mm (>80% occurs between May and October). The main forest types in the region consist of a wide diversity of pines Pinus spp. and oaks Quercus spp. and patches of diverse secondary communities associated with pine-oak forests (Rzedowski Reference Rzedowski1978).
Field and museum reference feathers (sample collection)
Studying stable isotopes in metabolically non-active tissues such as feathers (Duxbury and Holroyd Reference Duxbury, Holroyd, Duncan, Johnson and Nicholls1997) only reflects the dietary habits during the feather-growth period (Mizutani et al. Reference Mizutani, Fukuda and Wada1990). Nevertheless, it is possible to assess short- and long-term variation in diet patterns by comparing progressive sections of a single feather (Thompson and Furness Reference Thompson and Furness1995), among feathers grown at different stages of the moult cycle (Dalerum and Angerbjörn Reference Dalerum and Angerbjörn2005), and by analysing feathers collected in different locations and periods of time within the species range (Kelly Reference Kelly2000).
Owls were captured using a procedure combining broadcasting of pre-recorded calls and mist-netting. Each captured owl was weighed, measured and banded with a numbered aluminium band. Prior to release, body and tail feathers were collected (two each), stored in a paper envelope, and kept at ambient temperature before analysis. The date of capture, location, and sex were recorded. Additionally, we obtained Bearded Screech-owl body feathers from bird collections (Table 1).
Table 1. Feathers used from Bearded Screech-owl specimens located in museums and bird collections.

*KUNHM (University of Kansas-Natural History Museum), WFVZ (Western Foundation of Vertebrate Zoology), CZRAV (Colección Zoológica Regional Aves-Instituto de Historia Natural y Ecología de Chiapas), and AMNH (American Museum of Natural History). **Ni (No information).
Stable isotope measurements
Because C3 and C4 plants have distinct carbon-isotope signatures (Hobson and Sealy Reference Hobson and Sealy1991), the stable-carbon isotope analysis can distinguish C3 plants, which are associated with humid habitats and have low δ13C values, from C4 plants and CAM (Crassulacean Acid Metabolism) that are associated with drier habitats and exhibit higher δ13C values (Marra et al. Reference Marra, Hobson and Holmes1998, Kelly Reference Kelly2000). There is an enrichment of δ15N at each trophic level (i.e., more positive nitrogen values will be present at higher levels of the food chain than animals at lower levels; Gannes et al. Reference Gannes, O'Brien and Martínez del Río1997), which is detectable by stable-nitrogen isotope analysis.
We removed oil and dirt from feathers by washing them with liquid soap and then with chloroform-methanol (2:1) solution (Wassenaar and Hobson Reference Wassenaar and Hobson2000). After drying at room temperature, we cut the distal end (calamus) and the upper part of each feather into small fragments and packed each part separately (0.7–1.1 mg) in clipping silver capsules for solid samples (5 x 9 mm; Costech, Valencia, CA, USA). Whenever possible, pairs of body feathers and rectrices were analysed to confirm that these feathers contained similar carbon and nitrogen isotope ratios. Stable isotope ratios of carbon and nitrogen were measured by continuous flow isotope ratio mass spectrometry (IRMS; 20–20 mass spectrometer, PDZ Europa, Northwich, UK) after sample combustion to CO2 and N2 at 1,0000 C in an on-line elemental analyzer (PDZ Europa ANCA-GSL). Gases were separated on a Carbosieve G column (Supelco, Bellefonte, PA, USA) before introducing them to the IRMS. Sample isotope ratios were compared to those of standard gases injected directly into the IRSM before and after the sample peaks, and delta 13C (PDB) and delta 15N (AIR) values were calculated. Final isotope values were adjusted to the mean values of standard samples, which was a mixture of ammonium sulphate and sucrose with delta 13C = −23.83 and delta 15N = 1.33 distributed at intervals in each analytical run to the correct values of the working standards. The working standards were periodically calibrated against international isotope standards. The standard model to calculate the whole sample and report the 13C and 15N in parts per mil (‰) in delta (δ) notation was:
Where X is the 13C or 15N, R sample is the ratio of 13C/12C or 15N/14N and R standard is the Pee Dee Belemnite (PDB) standard for Carbon and AIR standard for nitrogen. The standard deviation of the measurements for C samples was ±0.22‰ and for N ± 0.14‰.
Prior to statistical analysis, data residuals were checked for statistical normality with Shapiro-Wilks (W) test, and for equal variance with Bartlett's test (Gotelli and Ellison Reference Gotelli and Ellison2004). We compared means of the δ13C and δ15N signature concentration using ANOVA or χ 2 statistical analysis. We evaluated the variation in isotopic values within feathers, between feathers of the same individual, sex, locations, and years. All statistical analyses were performed using JMP IN-SAS 5.1 (Sall et al. Reference Sall, Creighton and Lehman2005). All means are presented (±1 SD) and tests were considered significant at α = 0.05.
Results
We obtained feathers from 24 individuals (12 individuals captured in the field and 12 museum specimens) of the Bearded Screech-owl from the highlands of Chiapas and Guatemala. The mean feather isotope signature values for δ13C was −22.53 ± 1.29 (range −24.74 to −19.77‰), and that for δ15N was 5.87 ± 0.71 (range 3.96–7.13‰). Figure 1 shows the distribution and correlation between stable-isotope ratios of carbon and nitrogen in body feathers (calamus) of the Bearded Screech-owl. There were no significant differences in δ13C values (![]() = 17.75, P = 0.47) and δ15N values (
= 17.75, P = 0.47) and δ15N values (![]() = 20.2, P = 0.33) among feather samples.
= 20.2, P = 0.33) among feather samples.
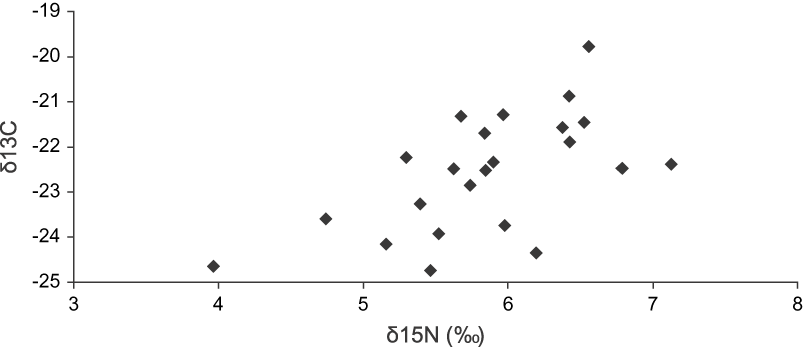
Figure 1. Distribution of the stable-nitrogen (15N) and carbon (13C) isotopes values from body feathers (calamus) of the Bearded Screech-owl. Each point represents an individual owl.
Intra- and inter-feather variations in stable isotopes
Stable isotope analysis of intra-body feathers (calamus and the upper part of the feathers) showed an enriched trend in assimilation time in δ13C (F 1, 31 = 3.68, P = 0.06) but not in δ15N (F 1, 31 = 0.27, P = 0.6). Neither δ13C (F 1, 16 = 3.88, P = 0.07) nor δ15N (F 1, 16 = 0.35, P = 0.56) showed intra-rectrices variation. There was a high and positive correlation between body feathers and rectrices in δ13C (r 2 = 0.77, P = 0.004) and in δ15N (r 2 = 0.67, P = 0.01).
Spatial differences in stable isotope measurements
Values of δ13C and δ15N did not vary strongly across nine locations where Bearded Screech-owls have been recorded (Figure 2). Teopisca showed the highest values of δ13C (−20.87) and δ15N (6.42). Huitepec and El Callejón showed the most depleted values of δ13C; these two locations had the highest occurrence of Bearded Screech-owls (Enríquez Reference Enríquez2007) and these locations were highly significant than the other sample locations (F 1,20 = 15.68, P < 0.001). No significant difference was observed for δ15N values (F 1,20 = 2.87, P = 0.10) between these same areas. Elevation was not related to δ15N or δ13C values (δ15N: r 2 = 0.38, F 9,12 = 0.83, P = 0.60; δ13C: r 2 = 0.58, F 9,12 = 1.84, P = 0.16; Figure 2).

Figure 2. Isotope ratios of δ15N (squares) and δ13C (triangles) of body feathers from the Bearded Screech-owl in nine locations in the central highlands of Chiapas and Guatemala. Locations are in elevation gradient (metres above sea level) from minor to major (range 1,000 m), and sample size given in parenthesis. SD values given only for more than one sample.
Temporal differences in stable isotope values
There was no observable pattern or trend in the stable isotope values through the years analysed (n = 22; Figure 3). The stable isotopes in feathers collected over the years showed values remarkably constant for δ13C (F 9,12, = 1.10, P = 0.45; Figure 4a) with the value for 1897 virtually identical to the recent measurements. However, the δ15N signature values showed significant temporal differences (F 9, 12 = 4.52, P < 0.01; Figure 4b).
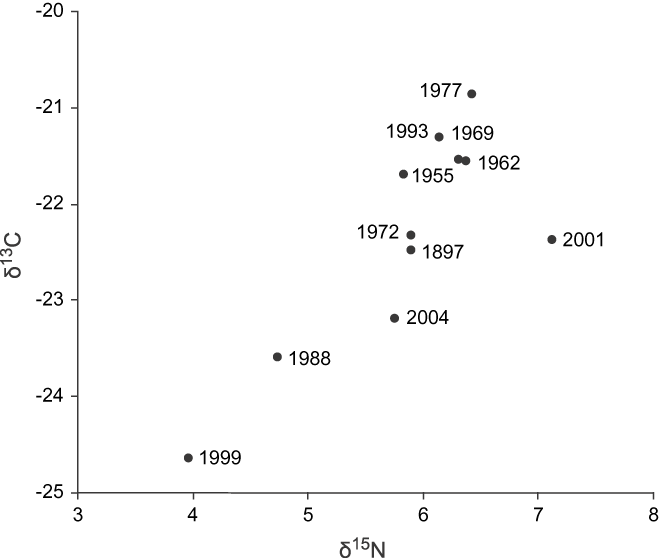
Figure 3. Annual distribution of the isotope ratios of δ13C and δ15N of body feathers from Bearded Screech-owl in the Central Highlands of Chiapas and Guatemala.

Figure 4. a) Isotope ratios of δ13C (‰) and b) Isotope ratios of δ15N (‰) of body feathers from Bearded Screech-owl per year (1955–2004), as well as one sample from 1897 in the Central Highlands of Chiapas and Guatemala. SD values given for years with more than one sample/yr.
Discussion
Feather analysis reflected that the Bearded Screech-owl inhabits a terrestrial C3-dominated ecosystem (Kelly Reference Kelly2000), which matches with their preferred habitat of humid oak forests. The mean nitrogen values for our species are similar to that reported for Northern Hawk Owl Surnia ulula (3.77%), Barred Owl Strix varia (4.29%), and Great Horned Owl Bubo virginianus (8.89%) (Duxbury and Holroyd Reference Duxbury, Holroyd, Duncan, Johnson and Nicholls1997). The diets of these species include a variety of vertebrates and invertebrates (König and Weick Reference König and Weick2008). On the other hand, the Great Horned Owl, considered a generalist (del Hoyo et al. Reference del Hoyo, Elliott and Sargatal1999), showed the greatest dietary diversity among owl species and also the largest range of isotope values (Duxbury and Holroyd Reference Duxbury, Holroyd, Duncan, Johnson and Nicholls1997). The variation of isotopic values found in Bearded Screech-owl was not to the degree found in a true generalist as the Great Horned Owl. The Bearded Screech-owl is mainly insectivorous (Enríquez and Cheng Reference Enríquez and Cheng2008) and the range of nitrogen isotopes values found overlapped those reported for forest birds that are mainly insectivorous with fruit supplements (e.g. Bright-rumped Attila Attila spadiceus; Herrera et al. Reference Herrera, Hobson, Rodríguez and Hernández2003).
The moulting period for the Bearded Screech-owl is prebasic (Pyle Reference Pyle1997a) and occurs during the rainy season (Enríquez and Cheng Reference Enríquez and Cheng2008). Analysis of intra- and inter-feather variation suggested no significant changes in the types of food ingested during feather growth.
Some screech-owl species exhibit both spatial and temporal variations in their diets (e.g., Western Screech-owl M. kennicottii; Cannings and Angell Reference Cannings, Angell, Poole and Gill2001). Our study species did not show significant spatial variation; although the most depleted carbon values were from owls from cloud and humid oak forests at the Huitepec Biological Reserve and El Callejón. The region is threatened by habitat fragmentation (Ochoa-Gaona and González-Espinosa Reference Ochoa-Gaona and González-Espinosa2000) and it is not known how much prime Bearded Screech-owl habitat remains.
The Huitepec Biological Reserve is one of the last remnants of mature forest protected in the highlands of Chiapas. Forest fragmentation and habitat degradation are considered the most severe threat to conservation of raptors in the tropics (Thiollay Reference Thiollay, Newton and Chancellor1985). In the highlands of Chiapas, it has been estimated an annual deforestation rate ≥2.7% in the last 30 yrs (Cayuela et al. Reference Cayuela, Rey Benayas and Echeverría2006). These habitat modifications may affect both distribution and abundance of owls and their prey. More long-term spatial dietary and prey analysis will be necessary to gain a better understanding of how habitat conditions are determining the distribution, abundance and quality of food for the ‘Near Threatened’ Bearded Screech-owl.
Acknowledgements
We thank P. Arcese for helpful inputs about the stable-isotopes analysis. For assistance with trapping owls we would like to thank J. L. Rangel, T. Will, A. Licona, E. Pineda, E. Sántiz, J. Martínez, M. Hiron, K. Elliott, D. Bradley, J. Gómez, and R. Ruiz. R. D. Kenner from the Cowan Vertebrate Museum at the University of British Columbia provided the CITES exchange permit. G. Galzi, Laboratory Manager of Faculty of Land and Food Systems at UBC provided technical support for preparing the feather samples. D. Harris at the University of California-Davis Stable Isotope Facility made stable isotope measurements. We would like to thank M. B. Robbins, E. Morales, M. A. Altamirano, R. Corado, S. Kenney and P. Sweet who kindly provided data information and feather samples from specimens deposited in their museums. We thank the members of Pronatura-Chiapas for allowing us to conduct part of this research at Huitepec Biological Reserve. Capturing and banding owls were conducted under permit No. 5127 from Secretaría de Medioambiente y Recursos Naturales (SEMARNAT). Field and laboratory work were made possible with logistic and financial support by the Latin American Program-Canadian Wildlife Service of Environment Canada, Lincoln Park Zoo, El Colegio de la Frontera Sur, and El Consejo Nacional de Ciencia y Tecnología. S. Marsden and K. Livezey provided helpful comments to improve this paper.







