Introduction
The common hippopotamus Hippopotamus amphibius is under considerable pressure from habitat loss and hunting (Lewison, Reference Lewison2007). Hippopotamus populations have declined precipitously in some African countries, falling by as much as 95%. These declines occurred during recent civil unrest (such as in the Democratic Republic of Congo and Uganda), and continent-wide declines of 7–20% have been reported over the last 10 years (Lewison, Reference Lewison2007). In West and Central Africa hippopotamuses have been extensively hunted for meat, ivory (found in the large canines) and in retaliation for crop raiding (Vega, Reference Vega1995). The high resale value of hippopotamus meat, hide and teeth, combined with a desire to reduce crop raiding, is likely to increase the hunting of hippopotamuses (De Boer & Baquete, Reference De Boer and Baquete1998). In addition, habitat loss has been accelerating as grazing grounds are converted into agricultural lands and aquatic refugia are diminished by irrigation and other human activities (Smuts & Whyte, Reference Smuts and Whyte1981; Eltringham, Reference Eltringham1999; Lemly et al., Reference Lemly, Kingsford and Thompson2000; Lewison & Oliver, 2010). Furthermore, climate change is predicted to bring major droughts in parts of Africa, reducing available habitat and increasing human–hippopotamus competition for water (Bwango et al., Reference Bwango, Wright, Elias and Burton2000).
More people are killed by hippopotamuses than by any other African animal (Durrheim & Leggat, Reference Durrheim and Leggat1999; Dunham et al., Reference Dunham, Ghiurghi, Cumbi and Urbano2010). Conflict continues to be a primary incentive for culling and hunting (Clarke, Reference Clarke1953; Mkanda & Kumchedwa, Reference Mkanda and Kumchedwa1997; Eltringham, Reference Eltringham1999; Gillingham & Lee, Reference Gillingham and Lee2003). Crop damage by wildlife in general causes large economic losses throughout Africa and can jeopardize the efficacy of protected areas by undermining local support for conservation (Haule et al., Reference Haule, Johnsen and Maganga2002; Gadd, Reference Gadd2005), leading to increases in habitat loss within protected areas (Weladji & Tchamba, Reference Weladji and Tchamba2003).
Considering the likelihood of additional stress on hippopotamus populations from water shortages, conservation of the species will depend on active management of human–hippopotamus conflict (Mkanda & Kumchedwa, Reference Mkanda and Kumchedwa1997; Eltringham, Reference Eltringham1999). Although numerous studies have considered the ecological and social aspects of human–elephant and human–primate conflict (Hill, Reference Hill2000; Gillingham & Lee, Reference Gillingham and Lee2003; Sitati et al., Reference Sitati, Walpole, Smith and Leader-Williams2003; Osborn, Reference Osborn2004; Chiyo & Cochrane, Reference Chiyo and Cochrane2005), the widespread human–hippopotamus conflict has been little studied but deserves further attention, especially given the categorization of the species as Vulnerable on the IUCN Red List (Lewison & Oliver, 2010). In this study I investigated human–hippopotamus conflict to assess the spatial, ecological and agricultural factors that increase farmers’ vulnerability to hippopotamus crop raiding. Understanding the causes of this crop raiding will aid in the development of management solutions, improving community relations with protected area authorities and reducing retaliatory killing of hippopotamuses.
Study area
With an area of > 20,000 km2 the recently expanded Ruaha National Park is the largest national park in Tanzania (Fig. 1). Gazetted in 1964, the Park is surrounded by several game reserves, including Rungwa–Kisigo–Muhezi. The Park comprises several habitat types, predominantly woodlands and grasslands (Barnes, Reference Barnes1983; Weladji & Tchamba, Reference Weladji and Tchamba2003). The Great Ruaha River borders the park to the south and provides an ideal environment for hippopotamuses as it includes both large water sources and surrounding grassland vegetation (Lock, Reference Lock1972). Historically, the Great Ruaha River flowed through the Park year-long, allowing large hippopotamus populations to flourish. Recently, water flow in the river has declined and large sections of the river dry out during the dry season (Mwakalila, Reference Mwakalila2005). Although irrigation has been identified as the major pressure on water resources, current policy has limited effectiveness in managing farmers’ use of the water and is poorly enforced (Sokile & Koppen, Reference Sokile and Koppen2004). Based on preliminary data, hippopotamus population sizes and their spatial extent in the Park have declined in recent years (P. Coppolillo, pers. comm.). The decrease in water availability combined with selective shooting of hippopotamuses following crop damage are the suspected causes of these declines.

Fig. 1 The study area, including Ruaha National Park, the Great Ruaha River, and the three study villages. The shaded area on the inset shows the location of Ruaha National Park in Tanzania.
Farmers living around the Park have been plagued by crop raiding (P. Coppolillo, pers. comm.). During the rainy season (November–April), when river flow increases, hippopotamuses are able to move down the Great Ruaha River into tributaries that surround various villages to access other areas of water and grassland resources. The villages of Tungamalenga, Mapogoro and Itunundu lie to the south-east of Ruaha National Park. These three villages were selected based on reported high levels of human–wildlife conflict and proximity to the river systems, which would predispose the villages to conflict with hippopotamuses (P. Coppolillo, pers. comm.). While much of the damage has been blamed on the substantial elephant Loxodonta africana population (Barnes, Reference Barnes1983), human–hippopotamus conflict in the area was suspected but unstudied.
Methods
Interviews
A total of 151 interviews were conducted with farmers, with 50–51 individuals, only one per farm, in each of the three villages. Interviews were generally conducted near farmers’ homes. Efforts were made to minimize the number of other people present during interviews, as this can alter the interviewee’s expression of opinion (P. Coppolillo, pers. comm.). All data were collected during the dry season, during June–August 2007, after completion of the harvest, increasing the probability that farmers would have time to be interviewed (Mkanda & Kumchedwa, Reference Mkanda and Kumchedwa1997).
Efforts were made to talk with an equal number of respondents who had and had not experienced hippopotamus crop raiding to facilitate comparisons. Village chairmen and other leaders provided a list of farmers who had reported hippopotamus crop damage on their farms in recent years. Interviews were then conducted with farmers with reported hippopotamus crop damage and a control group of farmers for which there was no prior knowledge of any history of hippopotamus crop damage. A farm was defined as having suffered crop raiding from hippopotamuses if crop damage was reported by the species in the growing season of either 2006 or 2007. All results are based on reported crop raiding and farmers’ perceptions; crop-raiding reports were not independently verified.
Interviews consisted of 39 questions and lasted 30–60 minutes. All questions were asked in Swahili through a translator and recorded by CJK. Informed consent was obtained from each interviewee following guidelines set out by the Institutional Review Board of Columbia University. The survey was briefly described to the respondent as a study of crop-raiding issues in the area, and participants were asked if they would like to participate. The interviewers emphasized that no particular opinions were being sought and that the research was simply to gain insight into the experience and opinions of the farmers.
The questionnaire (Appendix) was designed based on similar questions from other human–wildlife conflict studies (Post, Reference Post2000; Haule et al., Reference Haule, Johnsen and Maganga2002; Gadd, Reference Gadd2005; Wang et al., Reference Wang, Curtis and Lassoie2006). It included questions about agricultural and spatial characteristics of the farm as well as hippopotamus crop-raiding issues. Farmers were asked to describe the stage of plant growth at which crop raiding occurred. Plants were classified as seedlings, intermediate (for those plants larger than half a metre but not yet harvestable) and mature (or harvestable). Questions were also asked about deterrent techniques and attitudes but these are not described here.
Assessment of farm location
After interviews were completed respondents were asked to take the interviewers to their farm. If they allowed this, the location of the farm was recorded at its centre with a global positioning system unit (GPS) with an accuracy < 5 m. During these visits the interviewers characterized habitat around the farm as forest, other farms or housing. For farms that were not visited the farmer’s description was used to determine habitat around their farm. Hippopotamus access points (defined as places where hippopotamuses consistently enter and leave the river, leading to trails) were identified based on a combination of factors including identification by the farmer, evidence of distinct hippopotamus trails (such as large trenches along the river bank) or extensive hippopotamus footprints in dried mud along riverbanks, and their locations recorded with a GPS.
Data analysis
Locations of farms and hippopotamus access points were mapped in ArcGIS v. 9.0 (ESRI, Redlands, USA; Figs 2 & 3). Elevation data for the area were obtained from the Shuttle Radar Topography Mission (2009; accuracy of ± 8 m). Stream networks were then determined through a watershed analysis, using elevation data to establish the location of waterways (Tarboton et al., Reference Tarboton, Bras and Rodriguez-Irturbe1991). Distances between central points of farms and hippopotamus access points and between farms and the nearest point on a stream were calculated in ArcGIS. The elevation gradient between farms and the river was calculated as the elevation of the farm minus the elevation of the river at the nearest point to the farm divided by distance between the farm and the river (Y. Gorokovich, pers. comm.).

Fig. 2 Farms visited in Tungamalenga and Mapogoro villages near Ruaha National Park (Fig. 1) from June to August 2007. Symbols denote farms with and without reported hippopotamus crop damage and recorded hippopotamus access points.

Fig. 3 Farms visited in Itunundu village near Ruaha National Park (Fig. 1) from June to August 2007. Symbols denote farms with and without reported hippopotamus crop damage and recorded hippopotamus access points.
Survey data are generally presented as the percentage of people giving a response to a given question. Pearson’s χ2 and Mann–Whitney U tests were used to compare differences in ecological and spatial factors between farms with and without reported hippopotamus crop raiding. SPSS v. 16.0.1 was used for all statistical analyses (SPSS, Chicago, USA).
Multivariate logistic regression models were used to assess the relative importance of the factors in predicting the occurrence of hippopotamus crop-raiding. A forward likelihood ratio step approach was used; this eliminates variables with the lowest likelihood ratios from the model, thus giving a final model with the highest significance (Garson, Reference Garson1998). The overall models were tested for goodness of fit using the Hosmer & Lemeshow (Reference Hosmer and Lemeshow2000) test.
Two models were generated: Model 1 included all farms but excluded variables that were only calculated for visited farms (n = 151) and Model 2 included only farms that were visited and for which calculations of distances and gradients were available (n = 78). For both models the response variable was whether or not hippopotamus crop raiding had been reported. Reported frequency and amount of hippopotamus crop raiding were also considered as potential response variables but were found to be less sensitive measures. Ecological, spatial and agricultural variables that were significantly related to reported hippopotamus crop-raiding were used as predictor variables in the model. For Model 1 categorical predictor variables were: distance between the farm and the river (near = 1, far = 0), growing rice (yes = 1, no = 0) and use of irrigation (yes = 1, no = 0). The distances used were based on the interviews, where near is < 1.5 km and far is > 1.5 km from the river. Farm size, in acres, was also included as a predictor variable. Model 2 included use of irrigation, farm size and growing rice as for Model 1 and, in addition, log distance between the farm and the river, log distance between hippopotamus access points and the farm, and gradient. Log of distances were used because of the negative exponential relationship between crop raiding and distance. The Wald statistic was used to test for the significance of each variable. Mann–Whitney U tests provided information on possible relationships amongst variables.
Results
Characteristics of hippopotamus crop raiding
A total of 151 farmers were interviewed of which 78 farms (52% of those interviewed) were visited; 73 farmers were unwilling to go to their farms because of a lack of available time or other constraints. About half (59%) of respondents (and 63% of the farms visited) reported hippopotamus crop raiding during the past two growing seasons. Thirteen hippopotamus access points were recorded.
The majority of respondents (91%) who reported crop raiding by hippopotamuses used footprints to identify this species as the cause of crop damage. During farm visits I observed that farmers were able to identify hippopotamus footprints accurately. Most hippopotamus crop damage occurred at night (92%). The majority of hippopotamus crop raiding (55%) was reported to occur exclusively during the heavy rains of March–June. Most hippopotamus crop damage was reported on only seedlings (49%) or only intermediate sized plants (33%), irrespective of crop type. Most farmers grew multiple crops, including rice (52%), maize (54%), millet (5%), groundnuts (14%) and other crops (7%) such as vegetables. Based on reports by the farmers there was no clear preference of hippopotamuses for specific crop types; 44% reported a preference for rice and 39% a preference for maize.
The number of hippopotamus crop raids per year was reported to be low. Approximately 73% of interviewees with hippopotamus crop-raiding problems reported that hippopotamuses only visited their farms once or twice per year, with only 27% reporting three or more hippopotamus crop-raiding events. Of farmers who reported hippopotamus crop raiding, 38% reported losing < 5% of their farm, 24% losing < 6–15% of their farm, 26% losing < 16–25% of their farm and 12% losing > 25% of their farm. In general, farmers tended to view losses to hippopotamuses as minimal, especially in comparison to elephant crop raiding.
Factors influencing the vulnerability of farms to hippopotamus crop raiding
Three variables were significantly correlated with hippopotamus crop raiding: distance from the farm to the nearest river (based on reported distance between farm and river), farm size and distance from the farm to hippopotamus access points (Tables 1 & 2). Hippopotamus crop raiding occurred more often on farms that were closer to the river and closer to hippopotamus access points, and increased with farm size. Farm size is dependent on distance to the river, with larger farms more likely to be close to the river (n = 140, Mann-Whitney U = 1856, z = -2.514, P = 0.012).
Table 1 Pearson’s χ2 analysis of agricultural and spatial factors of farms with and without occurrence of hippopotamus Hippopotamus amphibius crop raiding based on interviews with a total of 151 farmers in three villages near Ruaha National Park (Fig. 1) from June to August 2007.
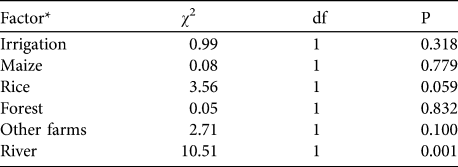
* Irrigation, rice and maize indicate the presence of these factors on the farm. Forest and other farms refer to farmers describing these factors as being around their farm. River refers to proximity of the farm to the river as recorded during surveys, with farms considered near if they were within 1.6 km of the river.
Table 2 Mann–Whitney U test of spatial factors of farms with and without occurrence of hippopotamus crop raiding based on interviews of farmers in three villages near Ruaha National Park (Fig. 1) from June to August 2007Footnote *.

* Values are means from farms with (Y) or without (N) reported hippopotamus crop-raiding problems in the last two growing seasons
In Model 1, which included all farms but only farmers’ estimates of distances, farm size (W = 5.685, df = 1, P =0.017) and distance between farm and river (W = 7.582, df = 1, P = 0.006) were the most significant variables correlated with reported crop raiding (Table 3). In Model 2, which included only visited farms but measured actual distances, distance between farm and hippopotamus access points was the most significant variable predicting reported crop raiding (W = 5.283, df = 1, P = 0.022; Table 3). The H–L statistic showed both models to have significant goodness of fit (Hosmer & Lemeshow, Reference Hosmer and Lemeshow2000; Table 4).
Table 3 Odds ratios in forward stepwise multivariate logistic regression models examining variables that contributed to occurrence of crop-raiding. by hippopotamuses.

Table 4 Model equation and Hosmer and Lemeshow (H–L) statistic for Models 1 and 2 (see text for details).

Discussion
Identifying the spatial and agricultural risk factors for human–wildlife conflict is essential for its mitigation. Hippopotamuses are habitual animals. Studies of their behaviour have found that they leave and enter waterways each evening, following specific pathways (Eltringham, Reference Eltringham1999). Thus, it is not surprising to find that the majority of reported crop raiding by hippopotamuses occurs at night on farms that border the river. Model 1 indicates that larger farms and farms closer to the river were more likely to report hippopotamus crop raiding. The relationship between hippopotamus crop raiding and farm size could be driven by simple movement of hippos, possibly in a random walk, in which case larger farms would have a greater probability of being encountered, based on size alone. In addition, farm size and distance to the river were correlated and thus proximity to the water may be the actual factor driving the relationship between farm size and crop raiding. For every 1 acre (0.40 ha) increase in farm size, the odds of a farm being raided by hippopotamuses is 1.32 times higher, all other things equal. The odds of a farm located near a river having reported hippopotamus crop raiding are 2.78 times higher than for a farm that is far from the river. Similar trends of higher damage levels closer to streams or near areas of high activity (such as dams) have been found in other species, including beavers and bears (Clark et al., Reference Clark, Dobey, Masters, Scheick, Pelton and Sunquist2005; Martell et al., Reference Martell, Foote and Cumming2006).
Other studies have looked at location as a predictor of crop raiding in other species (Hill, Reference Hill2000; Sitati et al., Reference Sitati, Walpole and Leader-Williams2005; Arlet & Molleman, Reference Arlet and Molleman2007; Warren et al., Reference Warren, Buba and Ross2007). For crop raiding by elephants and primates, proximity to a protected area or to forest has been found to be a reliable predictor of crop-raiding occurrence or losses (Hill, Reference Hill1997, Reference Hill2000; Gillingham & Lee, Reference Gillingham and Lee2003; Wang et al., Reference Wang, Curtis and Lassoie2006). However, few studies have looked at the access points where animals enter and leave farms (Osborn, Reference Osborn2004). In Model 2, which used measured distances between farm and river and between farm and hippopotamus access points, distance between farms and hippopotamus access points was the only significant factor predicting the occurrence of reported hippopotamus crop raiding. Based on the odds ratio, a 1 m increase in logarithm of distance between a farm and the hippopotamus access points decreases the odds of crop raiding by a factor of 3.14. Identifying points of access into farms may be critical to management of crop raiding for many species, especially hippopotamuses.
For hippopotamus crop raiding around Ruaha National Park, the most significant predictor of conflict is proximity to habitat areas that hippopotamuses use most, specifically the places where they leave and enter the river. Placement of farms away from hippopotamus trails and access points may thus be a feasible and effective way to reduce levels of hippopotamus crop raiding. While it would be impossible for farmers to avoid proximity to waterways completely (because of their high dependence on water and limited access to irrigation), farmers could reduce hippopotamus crop damage by avoiding areas of high hippopotamus activity. Managers could create small buffers around waterways and larger buffers around areas of high importance to hippopotamuses, especially places where they leave and return to the river, to reduce hippopotamus crop raiding.
Hippopotamuses do not appear to favour specific crop types or irrigated crops. This is in contrast to elephants and baboons (Hill, Reference Hill1997, Reference Hill2000; Haule et al., Reference Haule, Johnsen and Maganga2002; Gillingham & Lee, Reference Gillingham and Lee2003; Weladji & Tchamba, Reference Weladji and Tchamba2003). Previous studies have found varying results in terms of selection of a given crop type by hippopotamuses. Eltringham (Reference Eltringham1999) and Clarke (Reference Clarke1953) suggested rice would be the favoured crop for hippopotamuses. However, Mkanda & Kumchedwa (Reference Mkanda and Kumchedwa1997) found a slight preference for maize. In this study, farmers’ responses regarding the favoured crop for hippopotamuses varied, primarily between rice and maize. In addition, crop types on farms with reported hippopotamus crop raiding were not significantly different from crop types on farms that did not report hippopotamus crop raiding. It appears likely that rice and irrigation may be confounding variables relating to the proximity to the river but may not actually influence a farm’s vulnerability to crop raiding.
Few farmers described major crop losses from hippopotamuses and crop raids by hippopotamuses were infrequent, generally occurring only once or twice per year. Such reported minimal losses may be due in part to the small number of hippopotamuses that frequent these areas. Many of the participants felt the amount of crop raiding and the overall hippopotamus numbers in the area were decreasing. Others have confirmed a decline in the range of the hippopotamus throughout the Ruaha River system (P. Coppolillo, pers. comm.). Thus, declines in the hippopotamus population in this area may be limiting the levels of crop damage. However, other studies have found hippopotamus crop damage to be underestimated, as farmers generally perceive a high regeneration rate of crops after trampling by hippopotamuses (Mkanda & Kumchedwa, Reference Mkanda and Kumchedwa1997). Similar opinions were expressed by farmers in this study; farmers often commented that since hippopotamus crop raiding typically occurred early in the season, when plants were only seedlings, many of the damaged crops were able to recover.
Hippopotamuses tended to raid farms during the wet season, when they are able to move outside the National Park into the smaller waterways that surround villages. In addition, they tend to come to farms while plants are seedlings, which probably resemble their normal grass-based diet (Eltringham, Reference Eltringham1999). Elephants have been found to raid crops when grass quality begins to decline below the quality of crop species and thus damage farms near harvest time (Osborn, Reference Osborn2004). Crop raiding by hippopotamuses and elephants thus has potential to create conflict with farmers throughout the growing season, with hippopotamuses coming in from rivers during initial planting and early growing periods, followed by elephants from forest areas during harvest time. These interactions between crop-raiding species may augment farmers’ losses and fuel retaliatory killing and culling of hippopotamuses because elephants are more difficult to kill and have full protection in many areas. To consider such potential interactions future research should consider conflicts with elephants and hippopotamus in conjunction.
Unlike crop raiding in other species, agricultural characteristics do not appear to affect the vulnerability of a farm to hippopotamus crop raiding. Instead, location of the farm in relation to ecologically important areas for hippopotamuses is the primary predictor of a farm’s vulnerability to hippopotamus crop raiding. This study has shown that avoidance of proximity to hippopotamus access points may be one way to reduce human–hippopotamus conflict. Continued research on hippopotamus crop raiding to understand the unique behaviours of this species will aid conservation of the species and improve our ability to mitigate future human–hippopotamus conflict and retaliatory killing of hippopotamuses. Findings from this study have been disseminated to the village chairmen in the three study villages and are being used by local staff from the Wildlife Conservation Society to mitigate future human–wildlife conflicts around Ruaha National Park.
Acknowledgements
Financial support for this study was provided by research grants from Woodland Park, Pittsburgh and Columbus Zoos. I thank Drs Joshua Ginsberg and Peter Coppolillo and Wildlife Conservation Society staff for their logistical support and advice throughout this study, Drs Rebecca Lewison, Katherine McFadden, Yuri Gorokovich and Johnny Fraser for comments, critiques and technical support, my field assistant and translator Erica Rugabandana, without whom this study would not have been possible, all the people that participated in the survey, the Tanzanian Wildlife Research Institute and the Tanzania Commission for Science and Technology for their permission to work in Ruaha National Park, Dan Rubenstein, Henry Horn, Diane Kendall and Kevin Markman for their revisions, and two anonymous reviewers for their comments. This research was conducted as part of CJK’s Master's thesis at Columbia University.
Appendix
The appendix for this article is available online at http://journals.cambridge.org
Biographical sketch
Corinne Kendall is currently studying the impacts of human activities on the avian scavenger guild in East Africa, with a focus on vultures. Her research interests include wildlife management and conservation, community ecology and landscape ecology.









