Donkey milk has gained popularity in recent years due to its perceived beneficial and therapeutic effects such as anti-microbial activity (Zhang et al., Reference Zhang, Zhao, Jiang, Dong and Ren2008) and potential anti-inflammatory and anti-carcinogenic effects. There is some human consumption of donkey milk, for instance in the diet of children with cow milk protein allergy (Monti et al., Reference Monti, Viola, Baro, Cresi, Tovo, Moro, Ferrero, Conti and Bertino2012). Compared to cow, goat and sheep milk, the microbial count of donkey milk was found to be lower and foodborne pathogens were not detected in donkey milk (Ivanković et al., Reference Ivanković, Ramljak and Štulina2009; Malissiova et al., Reference Malissiova, Arsenos, Papademas, Fletouris, Manouras, Aspri, Nikolopoulou, Giannopoulou and Arvanitoyannis2016), perhaps due to the high concentration of antimicrobial whey proteins (Uniacke-Lowe et al., Reference Uniacke-Lowe, Huppertz and Fox2010; Cunsolo et al., Reference Cunsolo, Saletti, Muccilli, Gallina, Di Francesco and Foti2017). The putative functional effects of donkey milk are thought to be related to its high lactoferrin and lysozyme activities (Cunsolo et al., Reference Cunsolo, Saletti, Muccilli and Foti2007; Polidori and Vincenzetti, Reference Polidori and Vincenzetti2010; Ozturkoglu-Budak, Reference Ozturkoglu-Budak2018), as well as its nutritional similarity to human milk (at least in terms of low protein and high lactose contents: Vincenzetti et al., Reference Vincenzetti, Savini, Cecchini, Micozzi, Carpi, Vita and Polidori2011; Malissiova et al., Reference Malissiova, Arsenos, Papademas, Fletouris, Manouras, Aspri, Nikolopoulou, Giannopoulou and Arvanitoyannis2016). Lysozyme has bactericidal effects and inhibits the growth of bacteria by hydrolyzing the glycosidic bond of mucopolysaccharides in bacterial cell walls (Polidori and Vincenzetti, Reference Polidori and Vincenzetti2010). This enzyme was found at a high concentration of 1300–4000 mg/l in donkey milk (Soto Del Rio et al., Reference Soto del Rio M de los, Dalmasso, Civera and Bottero2017).
Most studies examined the effects of different processing treatments on donkey milk, such as ultra-high pressure homogenization (100, 200, 300 MPa), pasteurization treatments (Addo and Ferragut, Reference Addo and Ferragut2015), heat treatment and freezing (Ozturkoglu-Budak, Reference Ozturkoglu-Budak2018), freezing and spray drying (Polidori and Vincenzetti, Reference Polidori and Vincenzetti2010; Vincenzetti et al., Reference Vincenzetti, Cecchi, Perinelli, Pucciarelli, Polzonetti, Bonacucina, Ariani, Parrocchia, Spera and Ferretti2018) and freeze-drying (Vincenzetti et al., Reference Vincenzetti, Savini, Cecchini, Micozzi, Carpi, Vita and Polidori2011). However, due to the presence of heat-labile proteins in donkey milk, thermal treatments such as spray drying and heat treatments at high temperatures caused irreversible damage in components of donkey milk (Addo and Ferragut, Reference Addo and Ferragut2015). For this reason, the usual pasteurization temperature range (72–75°C up to 2 min) applied to milk is not suitable for maintaining the rheological, sensorial and nutritional properties of donkey milk (Ozturkoglu-Budak, Reference Ozturkoglu-Budak2018). In the high hydrostatic pressure (HHP) process, pressure values from 200 to 600 MPa were applied, mostly with water, and kept constant for a suitable time at the desired temperature (Koker et al., Reference Koker, Okur, Ozturkoglu-Budak, Alpas and Seydi2020). HHP is an alternative food processing method to heat treatment for the inactivation of vegetative cells of microorganisms. Reversible and irreversible alterations of the secondary, tertiary and quaternary structures of proteins are also observed after high-pressure application (Okur et al., Reference Okur, Sezer, Oztop and Alpas2021).
The global value of donkey milk market was estimated at $28 180 000 in 2019 and is expected to reach at $68 139 000 by 2027, registering a compound annual growth rate of 9.4% from 2021 to 2027 (Allied Market Research, 2021). However, since heat treatment leads to whey protein denaturation, phase separation and nutritional losses, it must be consumed raw, which causes health risks and limits its shelf life. This study aimed to determine the effect of HHP treatment on donkey milk with different pressure-temperature-time applications and to evaluate the changes with regard to some physico-chemical, microbiological, rheological and shelf-life properties and antimicrobial protein stability in comparison to heat-treated donkey milk.
Materials and methods
Materials
Donkey milk samples were provided by a local farm (Korukoy Farm, Kirklareli, Türkiye). Fresh milk samples were transported and kept at 4°C before HHP and heat treatment.analyses. Shelf-life studies were performed during storage at 4°C. The chemical properties (protein, fat and dry matter contents) of milk samples are given in online Supplementary Table S1.
HHP treatment
HHP was performed with a laboratory-scale HHP unit (Type-760.0118, SITEC, Zurich, Switzerland) having a built-in heating–cooling system (Huber Circulation Thermostat, Offenburg, Germany) which controls the temperature rise during HHP treatment automatically via a temperature sensor. The pressure vessel has an inner diameter, length and volume of 153, 24 mm and 100 ml, respectively. The pressure transmitting medium was distilled water. Pressure vessel temperature was monitored via a type – K thermocouple. Samples were filled into 25 ml sterile high density polyethylene (HDPE) vials (LP Italiana SPA). Three pressure levels, temperatures and times were applied to donkey milk samples, namely 200, 400 and 500 MPa, at 25, 35 and 45°C and for 5, 10 or 15 min. Pressure release times were less than 20s for each pressure studied. Neither pressurization nor pressure release times were included in the treatment times. This created a total of 27 treatments.
Heat treatment
After filling the donkey milk samples into 25 mL sterile HDPE vials, they were subjected to heat treatment in a water bath (WiseCirCu, Germany) at 75°C for 1 and 2 min. A thermal probe was used to monitor the temperature of the samples. Following heat treatment, milk samples were immediately cooled in a water bath filled with cold water. Two replicates were done for both HHP and heat treatments. In total, 29 treatments were evaluated.
Compositional analyses
Total nitrogen content was assessed by Kjeldahl method, fat content by Gerber analysis and dry matter content gravimetrically. A pH meter was used to determine pH and the method reported by Bradley (Reference Bradley and Marshall1993) was used to perform titratable acidity measurements with results expressed in lactic acid percentage (LA%). Details of all analyases are given in the online Supplementary File.
Microbial analysis
Pour plate technique was used to determine the total aerobic bacteria count (TABC) of the raw donkey milk, HHP-treated and heat-treated samples. Firstly, 1 ml of milk was placed into a tube containing 9 ml sterile Ringer solution (Merck, Darmstadt, Germany). Then, the tube was sealed with a screw cap, vortexed and serially diluted. Bacterial counts were enumerated on plate count agar (Merck, Darmstadt, Germany) and incubated at 37°C for 24 h. Colonies in the Petri dishes (30–300 CFU) were counted.
Determination of lysozyme and lactoferrin content
The method described by Billakanti et al. (Reference Billakanti, Fee, Lane, Kash and Fredericks2010) was utilized to determine the lysozyme and lactoferrin activity of the samples. This is detailed in the online Supplementary File.
Rheology analysis
Kinexus Pro + rheometer (Malvern, UK) was used to perform rheological analyses. Probe was of 4° conical shape with 2-mm gap with stainless steel. Shear stress and shear rate values were recorded and described by the power-law model (Li et al., Reference Li, Joyner, Lee and Drake M2018).
Color analyses
Lightness (L*), red-green (a*) and blue-yellow (b*) of the samples were measured in CIELAB color scale by a dual-beam d/8° spectrophotometer (Datacolor 110, Lawrenceville, NJ, USA).
Shelf-life analysis
Shelf-life analyses were conducted at day 0 as raw milk, immediately after HHP and heat treatment and then at days 3, 7, 14, 21 and 28. pH, titratable acidity, TABC, rheology and color measurements of raw, HHP-treated (200, 400, 500 Mpa) and heat-treated (75°C for 1 and 2 min) milk samples were performed for samples stored at 4°C (refrigeration temperature) and 25°C (room temperature) for shelf-life evaluations.
Statistical analysis
A three-way ANOVA was used to detect significant differences between samples subjected to different HHP and heat treatment parameters during the storage time and temperature, at P-values less than 0.05. Effects of storage time on shelf-life were analyzed by one-way ANOVA. In order to identify statistical differences between the samples, Tukey's multiple range test was used. All analyses were performed via Minitab Software (16.1.1., State College, PA).
Results and discussion
Microbiological analysis
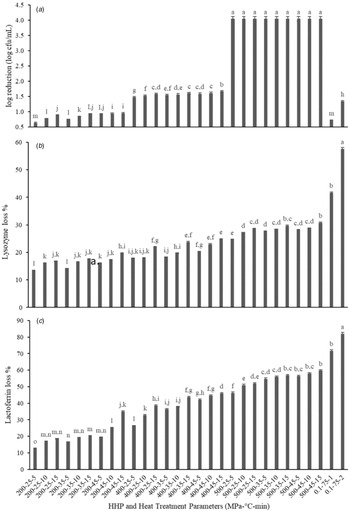
The effects of HHP treatment and heat treatment on the microbial population of donkey milk are shown in Fig. 1a. Low microbial counts found in this study are in accordance with the data reported in the literature (Zhang et al., Reference Zhang, Zhao, Jiang, Dong and Ren2008; Addo and Ferragut, Reference Addo and Ferragut2015). The initial microbial count of donkey milk was found to be less than 5 log CFU ml−1 which we ascribe to antimicrobial proteins present in the donkey milk (Zhang et al., Reference Zhang, Zhao, Jiang, Dong and Ren2008). HHP treatments of 200, 400 and 500 MPa at all temperature and time values studied caused reduction in TABC counts as 0.85, 1.58, and 4.04 log CFU ml−1, respectively. 75°C for 1 and 2 min pasteurization resulted in 0.72 and 1.34 log CFU ml−1reduction of TABC. Similarly, 3 log CFU ml−1 reduction of initial microbial load was reported in heat-treated donkey milk at 63°C for 30 min in the literature. Charfi et al. (Reference Charfi, Tidona, Makhlouf, Rezouga, Boukhari and Bornaz2019) also reported 1 and 2 log CFU/ml reductions of total bacterial count in donkey milk samples treated at 68°C for 2.5 and 75°C for 10 min, respectively.

Figure 1. Effect of HHP and heat treatments on (a) TABC count (log CFU ml−1) reduction, (b) native lysozyme loss % (w/w) and (c) native lactoferrin loss % (w/w) in donkey milk. Different lowercase letters indicate significant differences (P < 0.05). All the experiments were performed in triplicate. The initial total bacterial count of untreated donkey milk was 4.04 log CFU ml−1.
Lysozyme and lactoferrin activity
The effects of HHP and heat treatment on native form of lysozyme and lactoferrin concentration of donkey milk are given in Fig. 1b and 1c. With increasing pressure values, lysozyme and lactoferrin native form activity losses increased significantly (P < 0.05). There were no differences among the pressure holding times (P > 0.05). Lysozyme activity of samples showed significant decreases of 16.4, 21.6 and 28.1% at 200, 400 and 500 MPa, respectively (P < 0.05), similarly significant decreases in lactoferrin activity were determined (20, 36.8 and 55.5% at 200, 400 and 500 MPa, respectively, P < 0.05). No significant reduction of lysozyme activity in human milk was reported with HHP application of 500 MPa for 8 min (Pitino et al., Reference Pitino, Unger, Doyen, Pouliot, Aufreiter, Stone, Kiss and O'Connor2019). In a study performed with human milk, loss of lactoferrin stability was reported as 9, 23, 34 and 48% at pressure treatments of 300, 400, 500, and 600 MPa at 20°C for 15 min, respectively (Mayayo et al., Reference Mayayo, Montserrat, Ramos, Martínez-Lorenzo, Calvo, Sánchez and Pérez2014). Pitino et al. (Reference Pitino, Unger, Doyen, Pouliot, Aufreiter, Stone, Kiss and O'Connor2019) also reported that HHP application of 500 MPa for 8 min decreases lactoferrin stability in human milk.
Considering processing temperature, although there is a significant loss of lysozyme stability at 45°C (P < 0.05), no significant decrease was observed in lysozyme stability at lower temperatures such as 25 and 35°C (P > 0.05). HHP treatment (400 MPa) caused a significant activity loss of lactoferrin when a higher temperature (45°C) was applied. The loss of stability increased from 18% at 25°C to 25% at 45°C (P < 0.05), although there was no significant change from 25 to 35°C, nor from 35 to 45°C (P > 0.05). In terms of heat-treated samples, the native lysozyme activity of samples decreased 71.6 and 93% with application of 75°C for 1 and 2 min, respectively. Ozturkoglu-Budak (Reference Ozturkoglu-Budak2018) reported 2 min heat treatment at 75 and 85°C caused 10 and 62% native lysozyme loss in donkey milk, respectively. Furthermore, Addo and Ferragut (Reference Addo and Ferragut2015) reported no loss of native lysozyme after heating at 70 and 85°C for 1 min.
In our hands, application of heat treatment (75°C for 1 or 2 min) led to native lactoferrin loss of 35.6 and 53.4%, respectively. Lactoferrin has been reported to be a heat-labile component in donkey milk, with complete loss reported at 75°C for 2 min processing (Ozturkoglu-Budak, Reference Ozturkoglu-Budak2018). This difference probably relates to the lower initial lactoferrin concentration (0.097 mg/ml) of the donkey milk used in that study compared to the current one (0.2 mg/ml). Lactoferrin activity can vary according to lactation stage and the health status of the animals. Heat application at 75°C reported to result with 33 and 43% lactoferrin loss in bovine milk samples collected from New Zealand and China, respectively (Liu et al., Reference Liu, Boggs, Weeks, Li, Wu, Harris, Ma and Day2020). Importantly, we observed that pressure application, particulalry at lower pressures, led to higher retention of lysozyme and lactoferrin activity as compared to heat treatment (Fig. 2b and 2c).

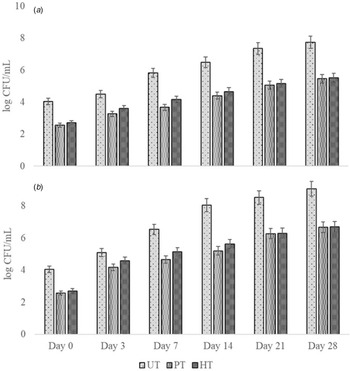
Figure 2. Microbial load values of donkey milk samples during (a) 4°C and (b) 25°C storage. UT, untreated milk; PT, HHP-treated milk (400 MPa-25°C-5 min); HT, heat-treated milk (75°C -2 min) during shelf-life analysis. All the experiments were performed in triplicate.
Shelf-life evaluation
Total aerobic bacteria counts (TABC) during the shelf-life analysis of untreated, HHP-treated, and heat-treated donkey milk samples are given in Fig. 2. Significant reduction was seen in the TABC count of HHP-treated and heat-treated samples as 1.48 and 1.34 log CFU/ml, respectively (P < 0.05). Heat-treated samples stored at 4°C showed an increase in microbial count to 5.15 log CFU/ml on day 21. At the end of storage, while the lowest TABC count was observed in pressure-treated samples stored at 4°C, the highest count was observed in untreated samples stored at 25°C.
Stratakos et al. (Reference Stratakos, Inguglia, Linton, Tollerton, Murphy, Corcionivoschi, Koidis and Tiwari2019) reported that HHP treatment at 600 MPa at 18°C for 3 min led to 3.95 log CFU/ml reduction of TABC counts in cow milk to 2.05 log CFU/ml, and after 28 d of storage at 4°C, TABC count of HHP-treated cow milk increased to 7.05 log CFU/ml. Although our values at the beginning of the shelf life have similar TABC counts as these cow milk values, counts of HHP-treated samples stored at 4°C were lower, which may be due to higher concentrations of lysozyme and lactoferrin in donkey milk compared to cow milk.
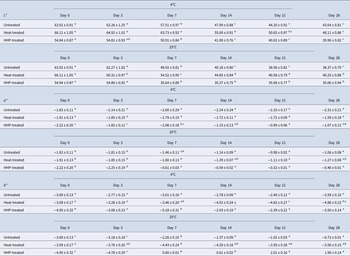
The pH and titratable acidity values of untreated, HHP-treated (400 MPa-25°C-5 min) and heat-treated (75°C-2 min) donkey milk samples during 28 d of storage are given in online Supplementary Tables S2 and S3. Values of untreated donkey milk samples did not change significantly during 7 d of storage at 4°C, while after 14 d a decrease in pH and an increase in LA% were observed. pH values of HHP-treated and heat-treated samples stored at 4°C remained substantially unchanged for 28 d of storage, however, titratable acidity values increased during 14 d of storage. A significant decrease in pH value and a significant increase in LA% were observed in HHP-treated samples during 7 and 14 d of storage at 25°C (P < 0.5). Tan et al. (Reference Tan, Chin, Tee and Chooi2020) reported that during 22 d of storage at 8°C, pH values decreased significantly and titratable acidity values increased significantly for both cow milk and goat milk. Decrease in pH and increase in titratable acidity are due to growth of lactic acid bacteria during storage.
Color data of untreated, HHP-treated (400 MPa-25°C-5 min) and heat-treated (75°C-2 min) samples are given in Table 1. Compared with untreated milk and heat-treated milk, HHP-treated milk stored at 25°C showed lower L* values. With increasing storage time, L* values decreased in all samples. a* and b* values mostly decreased in HHP-treated (stored at 4°C) and heat-treated milk samples. Harte et al. (Reference Harte, Gurram, Luedecke, Swanson and Barbosa-Cánovas G2007) reported that HHP-treated and HHP in combination with heat-treated cow milk loses its white color due to size reduction of casein micelles. However, when initial treatment with HHP was progressed with heat treatment subsequently, the initial color of cow milk was regained. Gervilla et al. (Reference Gervilla, Ferragut and Guamis2001) also reported a similar event in sheep milk. In this study, the lowest ΔL*, Δa*, and Δb* values were obtained in HHP-treated donkey milk samples which shows that HHP treatment affected the size of casein micelles.
Table 1. L*, a*, and b* values of untreated, HHP-treated (400 MPa-25°C-5 min), and heat-treated (75°C-2 min) donkey milk samples during 28 d of storage

*Results expressed as the mean ± standard deviation. Different lowercase letters in the same row indicate significant differences (P < 0.05). All the experiments were performed in triplicate.
Rheological analysis
Rheological analyses of untreated, HHP-treated (400 MPa-25°C-5 min) and heat-treated (75°C-2 min) donkey milk samples are shown in online Supplementary Tables S4 and S5. Xiang et al. (Reference Xiang, Simpson M, Ngadi and Simpson2011) reported that protein denaturation leads to an increase in consistency index. Consistency index values of untreated, HHP-treated, and heat-treated milk samples were analyzed during storage conditions of 4 and 25°C for 28 d. Results showed that both HHP and heat treatments increased flow consistency index values significantly (P < 0.5), however, the highest values were obtained with heat treatment.
At the beginning of the storage, flow behavior index (n) values were observed to be closer to 1 for all donkey milk samples and remained closer to 1 during 14 d, except for heat-treated samples. During 28 d of storage, significant decreases in flow behavior indexes (n) were observed in all samples and determined to be lower than 1 on day 28 which indicates shear-thinning flow behavior. Ding et al. (Reference Ding, Jiao, Chen, Song, Lu and Zhang-ji2020) reported that the flow behavior of donkey milk was shear-thinning and highly dependent on the storage temperature. Shear-thinning flow behavior depends on particle size, larger particle size leads to lower flow behavior indexes which further leads to reduced flow rates and high pressure drops (Bienvenue et al., Reference Bienvenue, Jiménez-Flores and Singh2003; Warncke and Kulozik, Reference Warncke and Kulozik2020). Debon et al. (Reference Debon, Prudêncio and Cunha Petrus2010) stated that the storage temperature of prebiotic fermented milk affects the mobility of macromolecules and intermolecular interactions. The apparent viscosity of fermented milk was reported to be dependent on storage time. pH and acidity could also have effects on the rheological properties of samples. pH values were observed to decrease and titratable acidity values were increased significantly in untreated samples both at 4 and 25°C storage. HHP-treated and heat-treated samples showed a non-significant change in pH and LA% at 4°C storage, while a significant decrease in pH and LA % was observed similar to untreated samples. The increase in K values (online Supplementary Table S1) in corresponding samples during both storage temperature could be explained by the increase in electrostatic force between casein micelles due to decreasing pH value (Köksoy and Kılıç, Reference Köksoy and Kılıç2003).
In conclusion, the results obtained in this study revealed that increasing processing pressure (200, 400, and 500 MPa) decreased microbial load, native lysozyme and native lactoferrin activities significantly (P < 0.05). Applied temperatures of 25 and 35°C during HHP treatment had no significant effect (P > 0.05) on microbial load, lysozyme and lactoferrin contents, while 45°C application reduced these values significantly (P < 0.05). Although heat treatment of donkey milk samples at 75°C for 1 and 2 min resulted in sufficient microbial inactivation, high native lysozyme and lactoferrin inactivation were also observed. HHP treatment caused a significant reduction in microbial load of donkey milk in comparison to heat treatment. A higher flow consistency index (K) and lower flow behavior index (n) were observed in heat-treated samples compared to untreated and HHP-treated samples (P < 0.05). The results suggest that HHP is a more suitable preservation and storage process than heat treatment in donkey milk due to lower loss of antimicrobial proteins and longer shelf-life obtained.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029923000572