Advancements in medical care have increased the likelihood that children with CHD will reach adulthood. Reference Bhat and Sahn1 Thus, research and clinical care has increased focus on the emotional, behavioural, and neurodevelopmental vulnerabilities for patients with CHD and their families. Reference Soto, Olude and Hoffmann2 Although the majority of families of children with CHD are resilient and will adjust to their child’s diagnosis well, approximately 40% of families are at an elevated risk for psychosocial distress. Reference Cousino, Schumacher and Rea3,Reference Hearps, McCarthy and Muscara4 Parents report increased risk of anxiety, depression, and attachment concerns when raising a child with CHD. Reference Bright, Franich-Ray and Anderson5–Reference Wei, Roscigno, Swanson, Black, Hudson-Barr and Hanson9 Children and teenagers with CHD are at an increased risk for behavioural problems, mood conditions (depression, anxiety), and impaired health-related quality of life compared to same-aged peers. Reference Latal, Helfricht, Fischer, Bauersfeld and Landolt10–Reference Lantin-Hermoso, Berger and Bhatt18 Moreover, children with CHD also have higher rates of neurodevelopmental concerns, with children with more complex and severe CHD particularly vulnerable to varying degrees of cognitive impairment. Reference Spijkerboer, Utens, Bogers, Verhulst and Helbing12,Reference Karsdorp, Everaerd, Kindt and Mulder16,Reference Huisenga, La Bastide-Van Gemert, Van Bergen, Sweeney and Hadders-Algra19,Reference Wilson, Smith-Parrish, Marino and Kovacs20
To promote early identification and intervention for these children, multiple professional groups (American Heart Association, American Academy of Pediatrics, Cardiac Neurodevelopmental Outcome Collaborative) have published guidelines recommending youth with high-risk CHD be systematically screened and evaluated at periodic time points (i.e., 9 months, 18 months, 24–30 months, 3.5–5 years, 11–12 years). Reference Marino, Lipkin and Newburger21–Reference Ilardi, Sanz and Cassidy23 These scientific statements also have attempted to facilitate guidance in age-specific assessment tools to consider when evaluating children and adolescents with CHD, though this has not been standardised or consistently adopted across cardiac clinics. Reference Miller, Sadhwani and Sanz24
In an effort to ensure children with CHD receive neurodevelopmental interventional services, some cardiology clinics have created clinics with a focus on monitoring the neurodevelopment of cardiac patients at high-risk for impairments. Reference Soto, Olude and Hoffmann2,Reference Brosig, Butcher and Butler25,Reference Mahle26 These efforts to improve systematic surveillance of children with high-risk CHD through neurodevelopmental programmes may be responsible for a nearly threefold increase in referral rates to interventional services. Reference Fourdain, Caron-Desrochers and Simard27 However, there is considerable variability across cardiac neurodevelopmental follow-up care programmes with respect to assessment tools and standards for evaluation. Reference Miller, Sadhwani and Sanz24
Given patients with CHD are at elevated risk for psychosocial and neurodevelopmental difficulties throughout the lifespan, particular attention to transition to adult cardiac care is warranted. A recent meta-analysis estimated 34% and 25.7% of young adults with CHD in the United States and Canada, respectively, experience a lapse of continuous care during the transition from paediatric to adult care, Reference Moons, Skogby, Bratt, Zühlke, Marelli and Goossens28 which can lead to the need for more significant cardiac intervention. Reference Yeung, Kay, Roosevelt, Brandon and Yetman29 The majority of these patients have reported not appreciating the importance of follow-up care and only decided to seek out treatment in response to cardiac-related symptoms. Reference Yeung, Kay, Roosevelt, Brandon and Yetman29 Formal transition programmes designed to educate patients and families about their cardiac disease and long-term care needs may reduce lapses in care; Reference Moons, Skogby, Bratt, Zühlke, Marelli and Goossens28 however, only one-third of cardiac clinics across the United States and Europe have established such programmes. Reference Hilderson, Saidi and Van Deyk30 Guidelines for supporting adolescents as they transition to adult care have been developed by the American Heart Association, Reference Sable, Foster and Uzark31,Reference Warnes, Williams and Bashore32 though it is unclear how this has been implemented nationally.
The current study aimed to examine the practices for children with CHD regarding neurodevelopmental, psychosocial, and transitional care as part of their outpatient care across paediatric cardiology clinics in North American hospitals. Current care practices will be compared to evidence-based guidelines and statements for best practices where available. Reference Marino, Lipkin and Newburger21,Reference Sable, Foster and Uzark31
Materials and methods
Procedure
This study was approved by the appropriate institutional review board prior to data collection. A total of 114 paediatric cardiac clinics were identified in June 2019 through the American College of Cardiology’s CHD clinic directory as meeting criteria for the study: a North American, hospital-based clinic that provides outpatient care to paediatric CHD patients. Due to the different professional roles recruited for the study (e.g., cardiologist, cardiothoracic surgeon, psychologist), the term CHD provider will be used as it is inclusive of any professional providing direct care to patients with CHD as part of a cardiac team. CHD providers (n = 558) at these clinics were identified via hospital websites and the Congenital Cardiology Today’s 2018 Hospital Directory of Congenital Cardiac Care Providers in North America Offering Open Heart Surgery for Children and subsequently e-mailed a recruitment letter in June 2019 that included a link to the online study. About 2 weeks later, a second recruitment e-mail was sent to cardiac providers from clinics who had not yet participated in the study. Recruitment e-mails were also sent through the Cardiology Special Interest Group listserv, a subgroup of Society of Pediatric Psychology, Division 54 of the American Psychological Association. Members of the listserv were asked to complete the survey if they were knowledgeable of the developmental and outpatient care provided to children with CHD at their hospital, or to forward the survey to a paediatric cardiology provider who would be better qualified to respond.
The recruitment e-mail contained a link to the Pediatric Outpatient Cardiac Provisions of Care, a 31-item measure created to assess practice parameters as they relate to the psychosocial, neurodevelopmental, and transitional care provided to paediatric CHD patients and their families. Survey questions were designed following an extensive review of the CHD literature with particular attention to formal guidelines from paediatric societies’ and, in the absence of said guidelines, recommendations stemming from research in the areas of psychosocial, neurodevelopmental, and transitional care for CHD patients. Thus, this survey instrument assessed the cardiac teams’ adherence to American Heart Association and American Academy of Pediatrics conjoint developmental evaluation and management recommendations, Reference Marino, Lipkin and Newburger21 consultation/liaison patterns for mental health/psychosocial services, and procedures regarding transitional services for emerging adults. Reference Sable, Foster and Uzark31 CHD providers who endorsed cardiac neurodevelopmental programmes at their institutions were asked additional questions in accordance with recommendations for cardiac neurodevelopmental programmes by Brosig and colleagues. Reference Brosig, Butcher and Butler25 Providers were asked to list barriers and facilitators affecting their cardiac team’s ability to provide psychosocial and neurodevelopmental care for children with CHD. The Pediatric Outpatient Cardiac Provisions of Care also assessed team members’ perceptions about their clinics’ abilities to provide psychosocial care for their patients, which is not central to the current study. Participants disclosed hospital names to ensure independence in clinic data with anonymity protected to encourage veracity of responses. Demographic information related to the cardiac clinic (e.g., number of cardiac surgeries) and participating CHD provider (e.g., age, role) were also collected.
Participating providers
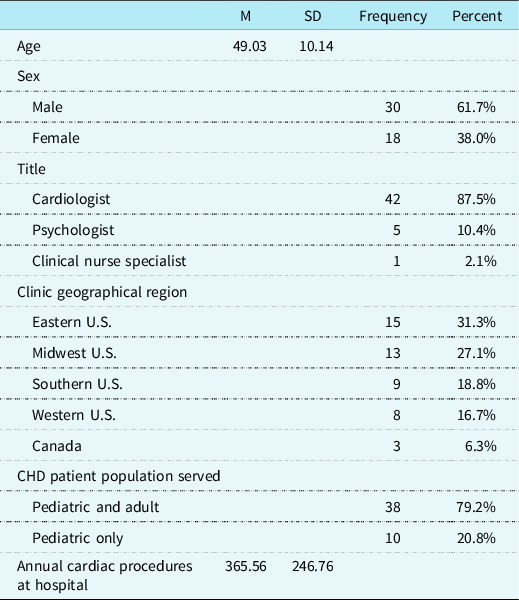
Included in the final analyses are data from CHD providers representing 48 of the originally identified 114 North American paediatric cardiac clinics (42.1%; See Fig 1 for inclusionary decisions). Participants were primarily cardiologists (n = 42; 87.5%) and reported spending an average of 50.3% (SD = 26.24) of their professional time providing outpatient clinical care to paediatric CHD patients. Most cardiac clinics serve both paediatric and adult CHD patients (n = 38; 79.2%), with an estimated average of 360.22 (SD = 246.67) paediatric CHD-related surgical procedures per year at the hospitals in this survey. See Table 1 for more demographic information.
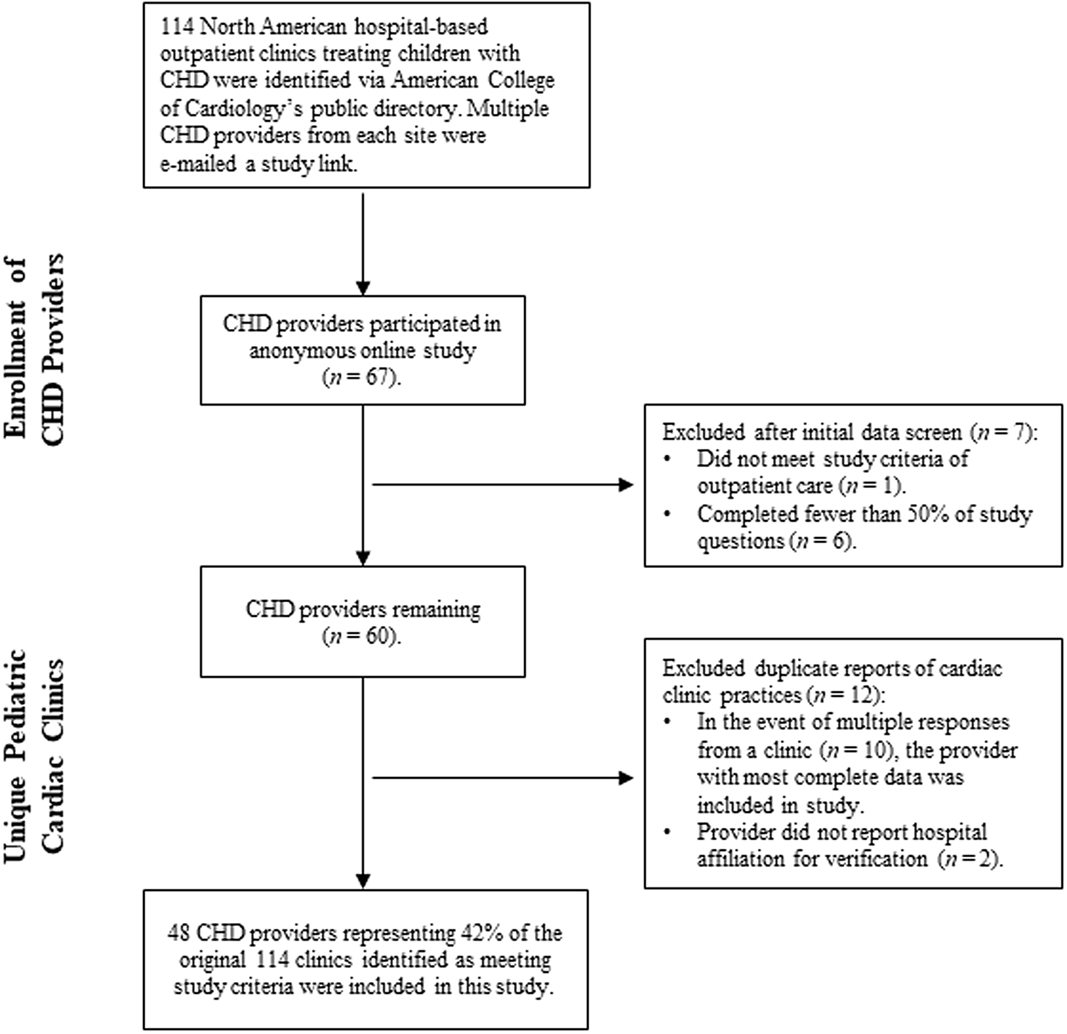
Figure 1. Identification and inclusion of CHD providers at unique cardiac clinics.
Table 1. CHD provider and cardiac clinic demographic characteristics (N = 48).
Statistical Analyses
Frequencies and descriptive statistics were analysed using SPSS version 24. Open-ended responses to the barriers and facilitators of care were manually coded by the first and second authors independently and then compared for accuracy.
Results
Psychosocial services
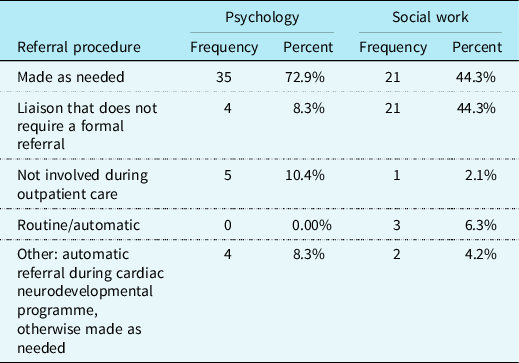
The majority of CHD providers reported that outpatient referrals for children with CHD are made “as needed” to psychology (n = 35; 72.9%). For outpatient referrals to social work, the majority of respondents reported that social work is a liaison to the cardiac team and does not require a formal referral to provide care to patients (n = 21; 43.8%), or referrals are made “as needed” to social work (n = 21; 43.8%). Please see Table 2 for a more complete explanation of referral procedures to psychology and social work.
Table 2. Psychology and social work referral procedures (N = 48).
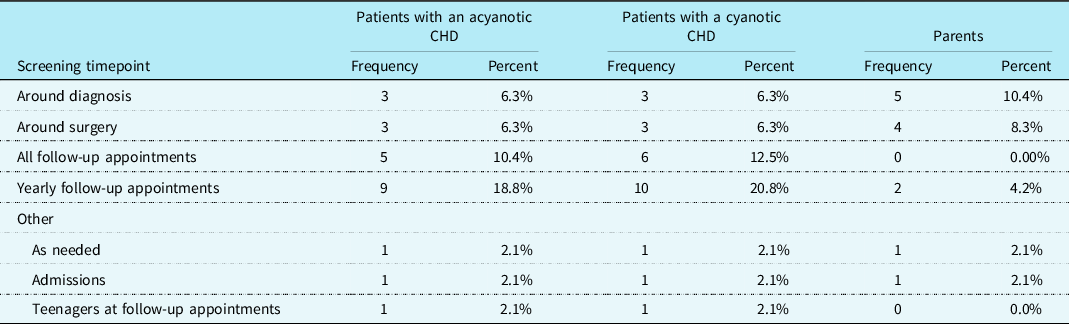
Psychosocial screening practices were assessed. It was reported that 39.6% (n = 19) of cardiac care teams screened patients with CHD for psychosocial distress, 16.7% (n = 8) screened their patients’ parents, and 10.4% (n = 5) screened their patients’ siblings. CHD providers most frequently indicated that psychosocial screenings are conducted at yearly follow-up appointments for patients with a cyanotic heart defect (n = 10; 20.8%) as well as for those with an acyanotic heart defect (n = 9; 18.8%). Additionally, parental screenings for psychosocial distress were most frequently reported to be conducted around the time the child is diagnosed with a CHD (n = 5; 10.4%). See Table 3 for additional information regarding psychosocial screening timepoints. A chi-square test for independence showed that there was not a significant association between screening patients for psychosocial distress and the presence of a cardiac neurodevelopmental programme at the cardiac clinic, χ2 (1, N = 48) = 2.13, p = 0.144.
Table 3. Psychosocial screening timepoints conducted by the outpatient cardiac team (N = 48).
Neurodevelopmental care
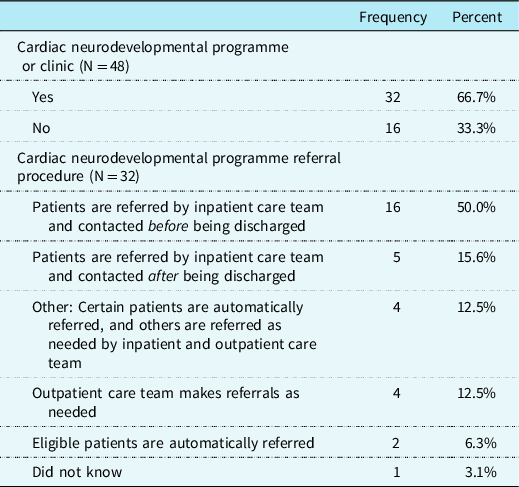
A specialised cardiac neurodevelopmental programme or clinic was reported to be available at 66.7% (n = 32) of the clinics in the sample. Of these 32 cardiac clinics, CHD providers (n = 16; 50.0%) most commonly reported that the inpatient care team refers patients with pre-identified criteria to the neurodevelopmental programme and families are contacted for enrolment before discharge (see Table 4 for more details on referral procedures).
Table 4. Cardiac neurodevelopmental programme availability and referral procedure.
CHD providers representing 43.8% (n = 21) of cardiac clinics reported their cardiac team systematically screens and evaluates children with CHD for developmental disabilities or delays. When compared to the guidelines, Reference Marino, Lipkin and Newburger21 8.3% (n = 4) of all cardiac clinics were reported to be screening and evaluating patients with cyanotic heart defects for developmental disabilities or delays at each of the recommended ages. An additional 10.4% (n = 5) of cardiac clinics reportedly screen and evaluate patients with cyanotic heart defects at each recommended age except 11–12 years of age. Patients diagnosed with acyanotic heart defects that required open heart surgery as an infant (i.e., high-risk for developmental delays or disabilities) were reported to be screened and evaluated at each recommended age at 6.3% (n = 3) of cardiac clinics. An additional 6.3% (n = 3) of cardiac clinics were reported to be screening and evaluating high-risk patients with an acyanotic CHD at each recommended age except 11–12 years of age. See Table 5 for more details on reported developmental screening procedures.
Table 5. Systematic neurodevelopmental screening and evaluation timepoints for patients with CHD at high risk for developmental delays or disabilities (N = 48).
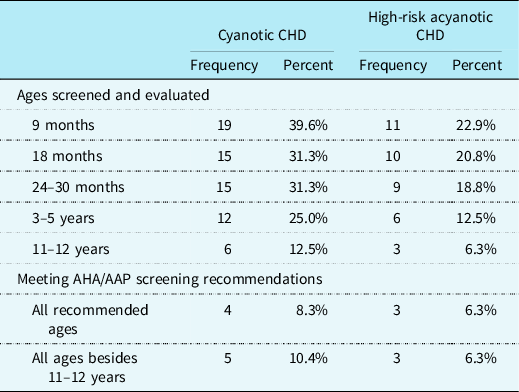
AHA = American Heart Association; AAP = American Academy of Pediatrics.
In cases where CHD providers in this sample reported that their cardiac team does not conduct developmental screenings or evaluations (n = 26; 54.2%) or were unsure (n = 1; 2.1%), communication with patients’ primary care physicians was assessed. CHD providers at 44.4% (n = 12) of this subset of cardiac clinics reported the patient’s cardiac care team and primary care physician do not typically communicate with one another regarding developmental screenings and evaluations, and 22.2% (n = 6) were unsure if communication occurs. Patients’ cardiac care team and primary care team were reported to communicate with one another at 33.3% (n = 9) of cardiac clinics, with the cardiologist being responsible for communicating between teams.
Pediatric to adult transition programme
Formal paediatric to adult CHD transition programmes were reported at 70.8% (n = 34) of cardiac clinics surveyed. Ages in which children with CHD were reported to begin preparing for the transition from paediatric to adult care at clinics with a transition programme varied: 15 years of age and younger (n = 12), 16 years of age (n = 9), between 16–18 years of age (n = 3), 18 years of age (n = 5), and over the age of 18 (n = 5). Cardiac care team members that indicated their cardiac clinic did not have an established transition programme (n = 9; 18.8%), or were unaware if a transition programme existed (n = 2; 4.2%), reported their patients with CHD begin to prepare for the transition to adult CHD care at the following ages: 16 years of age (n = 2), 16–18 years of age (n = 3), 18 years of age (n = 3), and over the age of 18 (n = 4). There was a statistically significant relationship between the presence of a transition programme and the age in which cardiac teams began preparing their patients for the transition to adult care, χ2 (1, N = 46) = 7.22, p < .01. Cardiac clinics with a transition programme were more likely to begin preparing patients aged 16 or younger for the transition to adult care than were the cardiac clinics without an established transition programme.
Barriers and facilitators to care
CHD providers were given an open-ended item to report an unrestricted number of barriers to the provision of psychosocial and developmental care to patients with CHD and their families at their respective clinics. Barriers to care were reported by 70.8% (n = 34) of the participants. Regarding barriers, CHD providers most frequently reported a lack of resources (n = 27; 79.4%) as a hindrance to providing more complete psychosocial and developmental care to their paediatric CHD patients. More specifically, a lack of personnel including psychologists and social workers (n = 9; 26.5%) or other dedicated staff (n = 8; 23.5%) who would facilitate the screening, referral, and/or provision of psychosocial and/or developmental care for their patients was noted. A lack of time (n = 5; 14.7%), money (n = 5; 14.7%), or appropriate screening tools (n = 1, 2.9%) were also reported barriers to these services. Additional barriers for optimal psychosocial and neurodevelopmental services for patients included the lack of a formal neurodevelopmental programme (n = 5; 14.7%), low levels of institutional support (n = 3; 8.8%), challenges identifying and/or communicating with appropriate healthcare providers in the patient’s community (n = 4; 11.8%), problems with reimbursement for these types of services (n = 4; 11.8%), stigma surrounding mental health (n = 2; 5.9%), parental lack of awareness or follow-through with services (n = 2; 5.9%), and limited caseload in the neurodevelopmental clinic (n = 1; 2.9%).
CHD providers were also given an open-ended item to report an unrestricted number of facilitators to the provision of psychosocial and developmental care to patients with CHD and their families at their respective clinics. Facilitators to care were reported by 53.2% (n = 25) of the participants. CHD providers reported that having a dedicated member or consultant responsible for psychosocial/neurodevelopmental testing was one of the most helpful facilitators in screening (n = 12; 48%). That said, very few institutions felt they had dedicated professionals such as social workers (n = 8; 32%), psychologists (n = 3; 12%), and nurse practitioners (n = 1; 2%). Outpatient cardiac team members also reported that the availability of an on-site neurodevelopmental clinic or programme (n = 6; 24.0%), institutional support for developing neurodevelopmental programmes (n = 4; 16.0%), CHD provider awareness of psychosocial and neurodevelopmental issues (n = 3; 12.0%), high level of collaboration with general or developmental paediatricians (n = 3; 12.0%), and electronic medical record auto-reminders (n = 1; 2.9%) were factors aiding in the provision of optimal psychosocial and neurodevelopmental services.
Discussion
The goal of the current study was to examine the psychosocial and neurodevelopmental care routinely provided to children with CHD and their families at outpatient cardiac clinics in North America. Although recommendations have been outlined, Reference Marino, Lipkin and Newburger21,Reference Sable, Foster and Uzark31 and cardiac clinics participating in the Cardiac Neurodevelopmental Outcome Collaboration recently reported aspects of their neurodevelopmental care practices elsewhere, Reference Miller, Sadhwani and Sanz24 it is not known the degree to which cardiac care teams have been able to implement these recommendations. Based off the sample of 48 CHD providers who provide outpatient care for children with CHD in North American hospitals, there is a growing number of centres with established, or interest in establishing, systematic neurodevelopmental/transition screening and intervention programmes, though considerable barriers remain.
Children with CHD and their caregivers are at risk for psychosocial difficulties. Reference Cousino, Schumacher and Rea3–Reference Schrøder, Boisen, Reimers, Teilmann and Brok17 Multidisciplinary paediatric cardiology clinics with mental health providers have been beneficial for improving the psychosocial health of children with CHD Reference Brosig, Yang, Hoffmann, Dasgupta and Mussatto33 and their parents. Reference Kaugars, Shields and Brosig34 Unfortunately, our results indicate it can be difficult to establish a dedicated mental health professional to be available to cardiology clinics. Although most cardiac teams reportedly refer patients as needed to psychology or social work, fewer than half routinely screen patients and caregivers for psychosocial distress. Struemph and colleagues Reference Struemph, Barhight, Thacker and Sood35 found that incorporating a psychosocial screener into outpatient cardiac follow-up visits was feasible and provided caregivers an opportunity to discretely request an appointment with mental health professionals. Cardiac teams with limited resources (e.g., no psychology, social work, or accessible cardiac neurodevelopmental programme) that are striving to meet recommendations for best practices may benefit from including psychosocial screenings into routine cardiac outpatient follow-up care. Cardiac teams could work to increase collaborations with patients’ primary care providers who might be better equipped for placing referrals to community mental health providers. Doing so may aid in the objective identification of patients and caregivers warranting referrals to psychosocial services that might otherwise go missed by healthcare providers and caregivers. Reference Struemph, Barhight, Thacker and Sood35,Reference Wang, Hay, Clarke and Menahem36
In an effort to meet the neurodevelopmental needs of patients with CHD, many cardiac clinics have developed formal follow-up programmes for children with CHD. Reference Brosig, Butcher and Butler25,Reference Mahle26 Routine neurodevelopmental screenings can lead to individualised academic and social-emotional learning care plans and earlier intervention for patients who may need additional services. Cardiac neurodevelopmental follow-up programmes were reported at 66.7% of cardiac clinics that responded. Interestingly, only 43.8% of CHD providers reported their cardiac team systematically screens and evaluates children with CHD for developmental disabilities or delays. Miller and colleagues Reference Miller, Sadhwani and Sanz24 assessed neurodevelopmental care practices at member institutions of the Cardiac Neurodevelopmental Outcome Collaborative and found fewer than half of the responding cardiac neurodevelopmental follow-up programmes had standards for routine neurodevelopmental evaluations of children older than 5 years of age. Thus, discordance between the reported number of cardiac neurodevelopmental follow-up programmes and cardiac teams conducting systematic neurodevelopmental assessments may be reflective of cardiac teams lacking established systematic neurodevelopmental protocols for managing their patients with CHD.
The American Heart Association/American Academy of Pediatrics 2012 guidelines Reference Marino, Lipkin and Newburger21 recommend developmental screenings and evaluations at 9 months, 18 months, 24–30 months, 3.5–5 years, and 11–12 years of age for children deemed high-risk of developmental disabilities or delays. Overall, our results suggest the majority of clinics are unable to provide neurodevelopmental services as often as guidelines suggest, with majority of services focusing on children under 5 years of age, similar to Miller and colleagues’ findings. Reference Miller, Sadhwani and Sanz24 The current study further contributes to the field’s understanding of neurodevelopmental care practices as it identified disparities in the services provided to children with cyanotic defects and acyanotic defects stratified as high-risk by the American Heart Association/American Academy of Pediatrics 2012 guidelines. Reference Marino, Lipkin and Newburger21 Patients with acyanotic defects stratified as high-risk for neurodevelopmental concerns may be overlooked when it comes to routine neurodevelopmental care. The small number of cardiac clinics conducting routine neurodevelopmental assessments in children older than 5 years of age and for children with acyanotic defects is concerning as minor disabilities may go unnoticed and untreated, potentially placing children with CHD at a disadvantage as they try to function in school compared to their same-aged peers. Reference Shillingford, Glanzman, Ittenbach, Clancy, Gaynor and Wernovsky11
Based on our findings, it appears that a gap remains between published neurodevelopmental evaluation recommendations and clinical practices. CHD providers reported a lack of resources as the most common barrier to implementing routine psychosocial and neurodevelopmental care practices. To address the disparities in resources across cardiac neurodevelopmental programmes, the Cardiac Neurodevelopmental Outcome Collaborative published systematic neurodevelopmental assessment protocols and developed an extended assessment battery and core assessment battery. Reference Ware, Butcher and Latal22,Reference Ilardi, Sanz and Cassidy23 This framework is very helpful for systematic, evidence-based batteries to be used to identify neurodevelopmental concerns for children with CHD; however, a barrier for programmes may be the need for qualified healthcare providers (e.g., typically developmental paediatricians, neuropsychologists, psychologists) to implement these protocols. Reference Ware, Butcher and Latal22,Reference Ilardi, Sanz and Cassidy23 Cardiac clinics without infrastructure in place to conduct systematic neurodevelopmental assessments have a concrete framework to strive towards that can reduce variability in procedures across programmes. Reference Miller, Sadhwani and Sanz24 When developing funding proposal and seeking institutional support, cardiac clinics could reference the specific resources needed to administer the core assessment battery to guide and justify their request. Reference Ware, Butcher and Latal22,Reference Ilardi, Sanz and Cassidy23 Additional research is needed to better understand methods for improving the institutional support to cardiac clinics striving to develop cardiac neurodevelopmental programmes and meet clinical recommendations.
The American Heart Association recommends cardiac clinics establish paediatric to adult transition of care programmes that provide children with CHD the skills needed to manage their healthcare needs continuously and independently (e.g., disease-related knowledge, communication skills, decision making). Reference Sable, Foster and Uzark31 Nearly three-quarters of the cardiac clinics represented within this sample were reported to have a programme in place to prepare for and guide the transition from paediatric to adult CHD care, which is an improvement from the 33% reported in a sample of 69 cardiac clinics examined a decade ago. Reference Hilderson, Saidi and Van Deyk30 Additionally, clinics with an established transition programme appear to be more in line with transition preparation recommendations than clinics without a transition programme. Specifically, patients treated at cardiac clinics with established transition programmes were more likely to begin preparing for the transition to adult care at or before the age of 16 than patients treated at clinics without an established programme. Interventions aiming to improve transition readiness in patients with mild to severe CHD appear to be promising as they have been found to increase transition readiness, decrease lapses in care, Reference Gaydos, Chowdhury, Judd and McHugh37 and improve overall psychosocial quality of life. Reference Uzark, Afton, Yu, Lowery, Smith and Norris38
Several study limitations should be noted. First, the measure used in this study was self-created, though this was necessary as no measure was available for assessing this information. Next, the sample is comprised of self-report data from one CHD provider from each cardiac clinic. No confirmatory data such as observation in the clinics or patient reports were obtained. Although much work was done to ensure good representation of cardiac care clinics, these efforts were met with some challenges. To our knowledge, there is not a publicly accessible and independently validated database inclusive of all paediatric cardiology facilities in North America. The American College of Cardiology’s CHD directory of 173 cardiac clinics located in North America was utilised as the sample of eligible clinics for this study, which narrowed to 83 as paediatric clinics. However, the information listed in this directory is self-reported by clinics and not confirmed by the American College of Cardiology. We made our best efforts to secure answers from as many institutions as we could identify as paediatric centres from this list but acknowledge that there are likely differences in practices and perspectives in centres not included on the American College of Cardiology clinic directory. Despite these obstacles, respondents were CHD providers from geographically diverse regions of the United States and Canada and represented 43.8% (n = 48) of the estimated 114 paediatric hospitals that provide outpatient services to children with CHD via a cardiac clinic.
Additional research is needed to further identify ways in which clinics can be assisted as they strive to establish care services based on the published guidelines and recommendations in the literature. Reference Marino, Lipkin and Newburger21–Reference Ilardi, Sanz and Cassidy23,Reference Sable, Foster and Uzark31 Cardiac clinics across North America that have multidisciplinary cardiac teams, cardiac neurodevelopmental programmes, and transition of care programmes in place should consider clinical publications and quality improvement projects regarding the effects of these services on patient outcomes and how to best advocate for institutional support for these programmes. Doing so may facilitate the development of these services and programmes at other cardiac clinics.
Conclusions
In summary, psychosocial, neurodevelopmental, and transitional care practices are highly varied across the 48 cardiac clinics represented in the study, which indicates inconsistent care is provided to patients with CHD and their parents in North America. While published guidelines and recommendations have aided in developing a framework for best care practices, cardiac teams are identifying many barriers to establishing these protocols in current practices. Clinics seem to be doing well in areas of neurodevelopmental management of young children with CHD and establishing paediatric to adult transition of CHD care programmes. Additional support is needed to improve the neurodevelopmental management of school-aged and adolescent children with CHD and the integration of psychosocial services during routine cardiac outpatient care.
Acknowledgements
We would like to thank the CHD providers who responded to the survey.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.









