Introduction
Extubation failure after neonatal cardiac surgery has been consistently associated with increased intensive care unit length of stay and post-operative mortality. Reference Benneyworth, Mastropietro and Graham1–Reference Wilson, Gunsaulus and Owens5 Consensus guidelines for paediatric ventilator liberation have been published with three core recommendations as well as 12 systematic review recommendations. Reference Abu-Sultaneh, Iyer and Fernandez6 The core recommendations include protocolised screening to assess eligibility for extubation readiness testing, use of a protocolised extubation readiness testing bundle and inclusion of a spontaneous breathing trial as part of extubation readiness testing. The existing literature surrounding extubation readiness and extubation failure in paediatric populations, however, is discrepant due to variations in the definition of extubation failure, extubation readiness testing components, and study populations. Predictive indicators for extubation readiness have been validated in adults but studies to determine predictive indicators in paediatrics have demonstrated varying success rates. Reference Curley, Wypij and Watson7–Reference Hames, Sleeper and Bullock9 Furthermore, literature in the paediatric cardiac surgical population evaluating the ability of extubation readiness testing and its components to predict extubation success has similarly produced conflicting results. Reference Gaies, Tabbutt and Schwartz2,Reference Laudato, Gupta and Walters3,Reference Hames, Sleeper and Bullock9,Reference Ferreira, Sugo and Aragon10
Given the risks associated with neonatal extubation failure, development of a standardised method for assessing extubation readiness in paediatric cardiac surgery patients is crucial to minimise ventilator days while carefully considering the risk of extubation failure.
We hypothesised that a quality improvement project aimed at creating and implementing a peri-extubation bundle, informed by best practices at high-performing centres, would decrease the frequency of extubation failure in neonates and infants undergoing congenital heart surgery in a single tertiary care paediatric referral centre. We aimed to decrease neonatal and infant extubation failures by 20% from baseline 15.7 % to 12.6 % over a 2-year period.
Materials and methods
This quality improvement project was conducted in a single-centre 17-bed cardiac intensive care unit. The quality improvement project was approved and a waiver of informed consent granted by the University of Louisville and Norton Children’s Hospital Institutional Review Board. Prior to embarking on this quality improvement project, extubation practice in the cardiac intensive care unit was variable. There were no formal extubation readiness assessments or spontaneous breathing trials. The process for extubation was at the discretion of the cardiac intensivist caring for the patient.
Pre-implementation baseline data were collected by retrospective chart review for neonates and infants who underwent congenital heart surgery from January 2020 through November 2021. Data collected included Society of Thoracic Surgeons – European Association of Cardio-Thoracic Surgery Congenital Heart Surgery (STAT) mortality category, days of mechanical ventilation prior to extubation, and extubation failure (yes/no). Reference Jacobs, Jacobs and Thibault11 Extubation failure was defined as need for reintubation within 48 hours of extubation for anything other than a procedure. All neonates and infants less than 60 days who underwent an index cardiac surgical procedure were eligible for inclusion. Patients on extracorporeal membrane oxygenation (ECMO), high-frequency oscillatory ventilation, or with an open chest or intracranial pressure monitor were excluded from extubation readiness testing until they underwent ECMO decannulation, high-frequency oscillatory ventilation discontinuation, delayed sternal closure, or intracranial pressure monitor removal. Patients were excluded from the analysis if they had a tracheostomy, had a do not resuscitate status, or were extubated within 6 hours of arrival from the operating room. If a patient failed extubation more than twice, only the first two extubation failures were included in the analysis.
Bundle development
A key driver diagram delineated primary contributors for ventilator liberation as a standardised process for the assessment of extubation readiness, shared mental model for the definition of failure risk, and identification of patient and unit characteristics that contribute to failure (Figure 1).
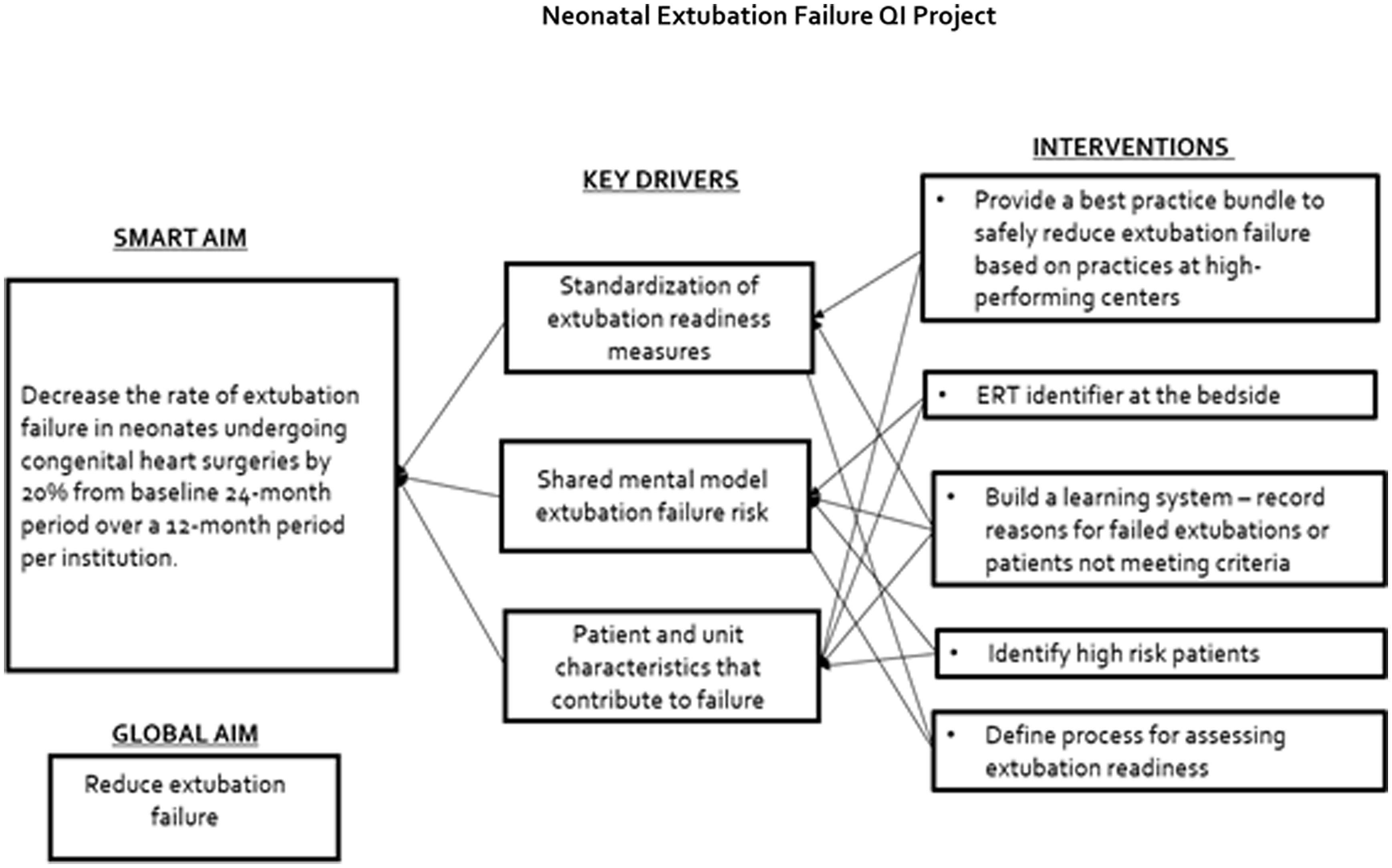
Figure 1. Neonatal extubation failure QI project.
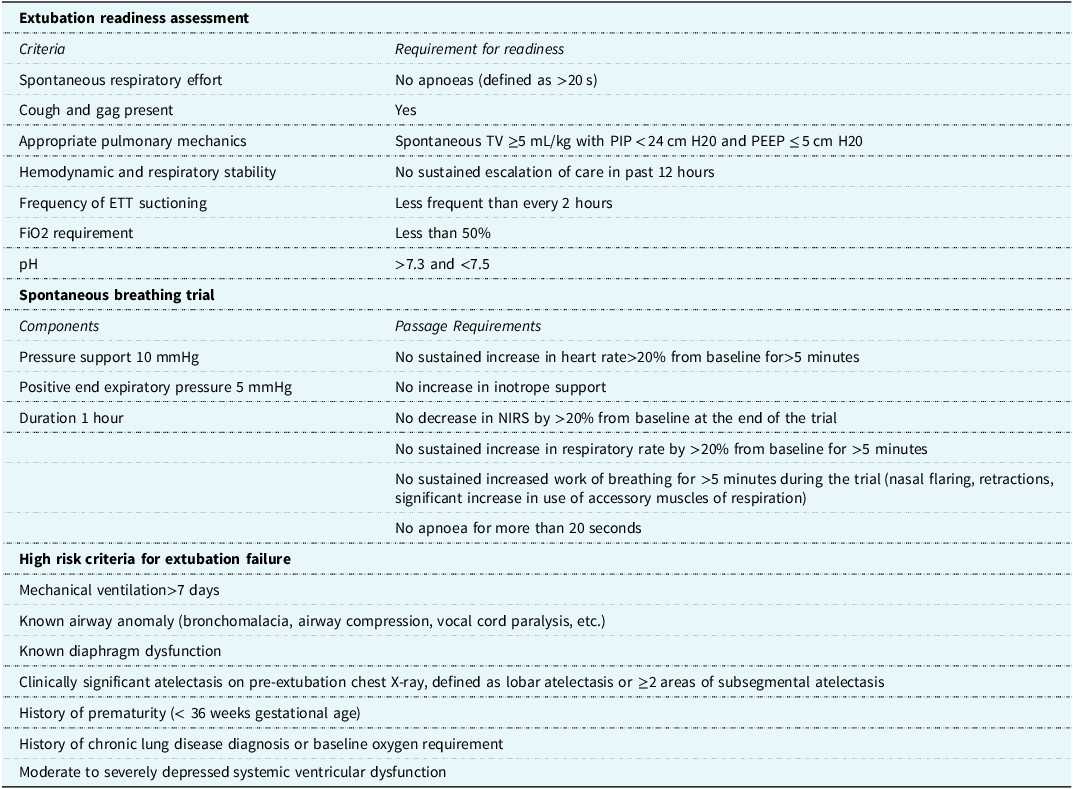
Utilising the transparency of the Pediatric Cardiac Critical Care Consortium (PC Reference Mastropietro, Cashen and Grimaldi4 ) database dashboard, five centres were identified as high-performers, having better-than-expected neonatal extubation success rates (95% confidence interval of a centre’s extubation failure rate in the target population is below the collaborative-wide mean), with the balancing metric of as-expected or better-than-expected mechanical ventilation duration (95% confidence interval includes or is entirely below the collaborative-wide median adjusted post-operative ventilation duration). Structured telephone interviews were conducted by one investigator (DKW) with cardiac intensive care unit physician leadership at the high-performing centres to determine centre specific extubation readiness assessments and practices, with 100% participation. Data from those interviews underwent qualitative content analysis (DKW, HB, DTT) which was used to develop a peri-extubation bundle. Input from physician experts at four additional centres was utilised to ensure clarity and face validity of the bundle elements. The bundle was informed solely based on best practice at high-performing centres. The bundle consists of three elements: 1) clear extubation readiness testing eligibility criteria 2) a protocolised spontaneous breathing trial, and 3) a list of high-risk criteria for extubation failure. The protocolised spontaneous breathing trial included a 1-hour trial with a positive end expiratory pressure of 5 cmH2O and a pressure support of 10 cmH2O. The neonatal and infant peri-extubation bundle components can be found in Table 1. To disperse the additional workload created by the bundle, the responsibility for performing extubation readiness testing and placing the patient in a spontaneous breathing trial was given to the respiratory therapists. Bedside nursing was responsible for monitoring the patient during the spontaneous breathing trial. At spontaneous breathing trial conclusion, both the respiratory therapist and the bedside nurse completed the spontaneous breathing trial paperwork. Physicians were then responsible for reviewing the extubation readiness testing and spontaneous breathing trial data and the high-risk criteria. If patients met high-risk criteria for extubation failure, it was suggested that the patient be extubated to non-invasive respiratory support. The ultimate decisions to extubate and type of non-invasive positive pressure respiratory support, regardless of meeting extubation readiness testing criteria or passing the spontaneous breathing trial, were left to the discretion of the attending physician.
Table 1. Neonatal peri-extubation bundle
Intervention - bundle implementation
Video education of bundle eligibility and exclusion criteria, bundle components, and bedside data collection tools was provided via the internal learning management system to all respiratory therapists and bedside nurses by the primary investigator. In-person education on the bundle was provided by the principal investigator to all cardiac intensive care unit physicians and non-physician providers. Awareness of the quality improvement project, design, and specific aims as well as quarterly updates of results were provided to the heart institute by the principal investigator at regularly scheduled heart institute-wide collaborative meetings. The bundle was implemented in December 2021.
To evaluate the results, data were collected on the post-implementation cohort from December 2021 through November 2023. Data collected included STAT mortality category, days of mechanical ventilation prior to extubation, extubation attempt number; readiness criteria for extubation readiness testing met (yes/no); reason(s) for not meeting extubation readiness testing criteria if applicable; spontaneous breathing trial success (yes/no); extubated on day of spontaneous breathing trial (yes/no); high-risk criteria met (yes/no); type of extubation support; and extubation failure (yes/no). Data to evaluate compliance to the extubation readiness bundle were collected via weekly audits by the PI. Target compliance was 95%. The number of successful extubations between failures was measured.
Analysis
The number of successful extubations between failures were plotted on a control g chart created using QI Macros for Excel software. The percent decrease in extubation failure between pre- and post-implementation cohorts was also calculated. Continuous and ordinal variables were compared pre- and post-bundle implementation using the Mann–Whitney U test. Sex (male/female) and extubation failure (yes/no) were compared between the two groups using the chi square test. Statistical analysis was performed using SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.).
Results
Outcome measures
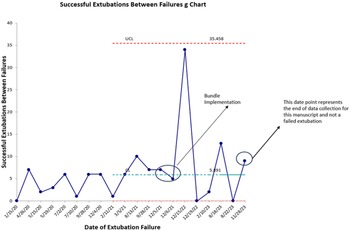
During the pre-intervention period, there were 76 extubations in 54 subjects. During the post-implementation period, there were 65 extubations in 59 subjects. There were 12 failed extubations in the pre-implementation period (15.7%) and 6 failed extubations in the post-implementation period (9.2%) resulting in a 41.4% decrease in extubation failure following bundle implementation. Prior to bundle implementation, the highest number of successful extubations between extubation failures was 10. Following bundle implementation, the highest number of successful extubations between extubation failures was 34. The control g chart of successful extubations between failures is shown in Figure 2. Granular details of the six post-bundle implementation extubation failures are provided in Table 2.
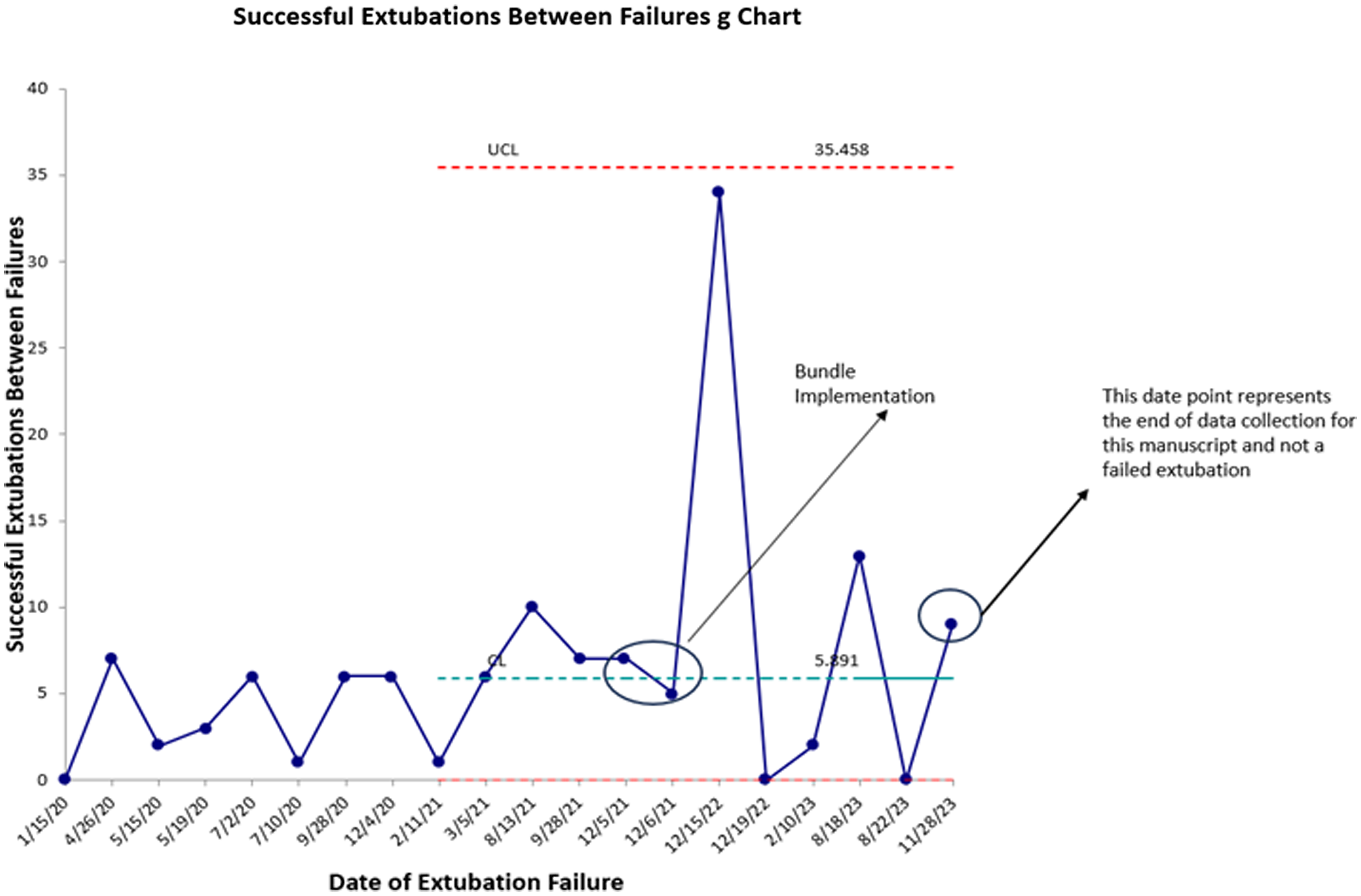
Figure 2. Sucessful extubations between failures g chart.
Table 2. Details of post-bundle implementation extubation failures
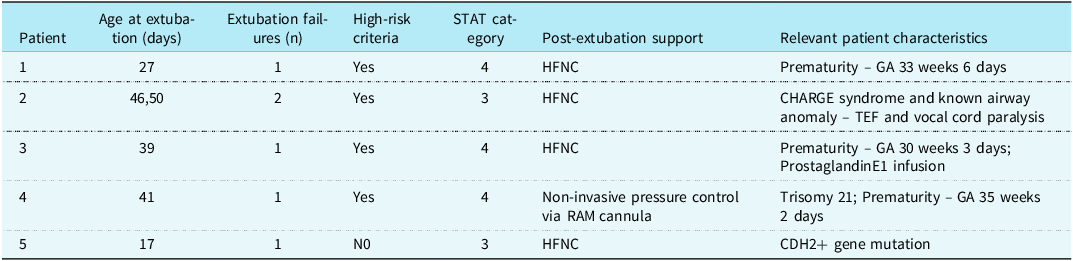
GA: gestational age; HFNC: high-flow nasal cannula; TEF: tracheoesophageal fistula.
There were no differences observed in sex (p = 0.238) and STAT mortality category (p = 0.141) for pre- and post-implementation cohorts.
Process measures
Bundle compliance, as measured by performance of extubation readiness testing and spontaneous breathing trial, was 95.4%. All extubations that met criteria for high-risk extubations (n = 28) were extubated to non-invasive support with high-flow nasal cannula being chosen most commonly (n = 23). There were nine spontaneous breathing trial failures in 8 subjects, with tachypnoea being the most common reason for failure (n = 7). In 5 instances, subjects were extubated despite having failed the spontaneous breathing trial and only one subject failed extubation.
Balance measures
There was no significant difference between post-operative ventilator days before [median = 5 (range 1–57)] and after [median = 3 (range 1–51)] implementation of the bundle (p = 0.079).
Discussion
A unique component of the PC Reference Mastropietro, Cashen and Grimaldi4 collaborative is data transparency and sharing across centres. This feature was instrumental in allowing us to identify high-performing centres in the area of neonatal extubation. Literature on strategies to prevent extubation failure in the neonatal cardiac surgery population is conflicting and incomplete, prompting us to utilise best practices from high-performing centres to inform and develop a quality improvement project aimed at reducing extubation failure following neonatal and infant cardiac surgery using a peri-extubation bundle. With this approach, we observed a 41.4% decrease in extubation failures, from 15.7% to 9.2% over a 24-month period, exceeding the goal of a 20% reduction at project implementation. Additionally, following the implementation of the bundle, maximum number of successful extubations between failures increased from 10 to 34, and extubation failures in patients who did not meet high-risk criteria were nearly eliminated.
Notably, 3 of 4 patients who failed extubation were born prematurely, consistent with our criteria for high-risk of extubation failure. Three of the 4 patients who failed extubation also had underlying genetic conditions, a variable that was not included as high-risk criteria for extubation failure. Indeed, for the one patient who failed extubation that did not meet high-risk criteria, an underlying genetic condition was present. Prior studies have associated underlying genetic conditions with increased risk for extubation failure after neonatal and paediatric cardiac surgery. Reference Laudato, Gupta and Walters3,Reference Harrison, Cox and Davis12,Reference Byres, Bailly and Werho13 We suspect that further research and the increasing availability of genetic testing will support the notion that children with underlying genetic abnormalities should be considered at high risk for extubation failure after cardiac surgery.
Importantly, the peri-extubation bundle included the use of a spontaneous breathing trial with pressure support of 10 mmHg and PEEP of 5 mmHg based on practice at high-performing centres. Khemai et al demonstrated that the use of pressure support in spontaneous breathing trial underestimated post-extubation work of breathing and use of pressure support during spontaneous breathing trial may still be providing too much support to allow spontaneous breathing trial with PS to be used to predict extubation readiness. Reference Khemani, Hotz and Morzov14 Indeed, 4 of the 5 extubation failures were preceded by passage of an spontaneous breathing trial. This observation could have been related to the use of pressure support during the spontaneous breathing trial, the duration of the spontaneous breathing trial, or other factors we did not evaluate. Studies evaluating pressure support versus T-piece or the use of continuous positive airway pressure (CPAP) alone with spontaneous breathing trials of varying durations have not demonstrated which of these practices are most effective in predicting extubation success. Reference Farias, Retta and Alia15,Reference Farias, Alia and Retta16 Recently published ventilator liberation guidelines suggested that performing spontaneous breathing trials in high-risk neonates without pressure support may be warranted. Reference Abu-Sultaneh, Iyer and Fernandez6 A subsequent quality improvement product implementing use of less or no pressure support for spontaneous breathing trials in high-risk cardiac neonates is a reasonable next step.
The best type of non-invasive positive pressure support for post-extubation neonates is unknown. Hassan et al did not find an association between the use of CPAP or BiPAP post-extubation and extubation failure in neonates following the Norwood procedure. Reference Hassan, Acosta and Zheng17 A recent metanalysis of high-flow nasal cannula versus other non-invasive ventilation techniques in paediatric cardiac surgery patients found that high-flow nasal cannula was superior to other modes of non-invasive ventilation in preventing extubation failure. Reference Elmitwalli, Abdelhady and Kalsotra18 All subjects in our study who failed extubation post-bundle implementation were extubated to high-flow nasal cannula except one who was extubated to non-invasive pressure control ventilation. The association between type of non-invasive support used in high-risk patients and extubation failure remains unclear. It is reasonable to consider further quality improvement projects aimed at evaluating types of non-invasive respiratory support and extubation failure rates in patients at high risk for extubation failure.
Limitations of this study include the single-centre nature and small sample size. The latter precluded the development of traditional run charts of failed extubations per ventilator days and analysis of individual bundle components and their impact on extubation success. In addition, maximal inspiratory pressure during airway occlusion (PiMax) was not included in the bundle because it was not a practice commonly used at the high-performing centres we interviewed. Assessment of PiMax has been shown to be a factor in extubation success in some studies and could be a component of the bundle for future quality improvement efforts aimed at reducing extubation failure in high-risk patients. Reference Farias, Alia and Retta16,Reference Thiagarajan, Bratton and Martin19 In addition, the additional burden the bundle implementation added to the bedside staff, including physicians, respiratory therapists, and nurses was not quantitatively measured.
The implementation of a peri-extubation quality improvement bundle reduced extubation failure in post-operative neonatal cardiac surgical patients by 41.4% in this single-centre study, with no increase in duration of ventilation. Given the morbidities associated with extubation failure in neonates following cardiac surgery, it is prudent to consider multicentre expansion of the bundle at other neonatal and paediatric cardiac centres. Multicentre expansion would also allow analysis of specific bundle components and their association with extubation failure especially in high-risk patients who, despite bundle usage, were still vulnerable to extubation failure.
Funding statement
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.