- AD
-
Alzheimer's disease
- REE
-
resting energy expenditure
- WL
-
weight loss
Alzheimer's disease (AD) is the most common neurodegenerative disease of the brain, representing more than 50% of all dementia cases( Reference Jönsson and Wimo 1 ). It is characterized by multiple cognitive deficits and progressive deterioration in functional performance, and leads to increasing disability and mortality. AD is frequently associated also with nutritional disorders and weight loss (WL), which is considered as one of the criteria for the clinical diagnosis of dementia( Reference McKhann, Drachman and Folstein 2 ).
The loss of body weight gives rise to loss of muscle mass and strength, and a greater risk of falls, functional dependence and worsening quality of life( Reference Buffa, Floris and Putzu 3 ). To prevent these negative consequences, it is important for clinicians to detect weight variations early and plan appropriate nutritional intervention.
The majority of studies report that AD patients lose weight especially in the intermediate and advanced stages of the disease( Reference Berkhout, Cools and van Houwelingen 4 ), but some studies suggest that WL may start even several years before dementia sets in, and others indicate that it may occur just before the cognitive symptoms become manifest( Reference Johnson, Wilkins and Morris 5 ). This variability in the onset of WL vis-à-vis the clinical signs of dementia, combined with the long latency period of AD, makes it difficult to ascertain the relationship between WL and AD. The question is not only whether WL comes before or after the onset of AD but also whether the former represents a risk factor for the latter, whether WL is an early sign of AD or a consequence of the related behavioural disturbances.
This review focuses on the link between WL and AD, and aims to define the temporal relationship between the two, and to investigate their possible aetiologic connections.
The relationship between Alzheimer's disease and weight loss: how can it best be studied?
The relationship between WL and AD is complex and has yet to be fully elucidated. To clarify their temporal and aetiologic links and to help us to identify the initial signs of cognitive symptoms of AD and weight changes with a reasonable degree of reliability, only observational studies conducted for an adequately long time with frequently scheduled cognitive and nutritional visits (e.g. every 3 or 6 months) should be considered. In the literature, however, either the studies are not long enough or the follow-up visits are scheduled at excessively long intervals, so they enable no definite conclusions to be drawn.
Given the lengthy latency period of AD, it is hard to define a maximum time interval that could elapse between the onset of WL and cognitive impairment for the WL to be considered as risk factors for dementia or one of its signs. WL starting more than a year before the onset of dementia could feasibly be seen as a risk factor for the latter.
Weight loss as a potential risk factor for Alzheimer's disease
Although overweight and obesity in middle age are notoriously associated with cerebro- and cardiovascular events, there is some evidence to suggest that WL may be a risk factor for both AD and mild cognitive impairment( Reference Gao, Nguyen and Hendrie 6 ). Dietary restrictions have been associated with a decline in vigilance, slower reaction times and worsening immediate memory( Reference Green and Rogers 7 , Reference Morrison 8 ).
WL may contribute to cognitive impairment in various ways (Fig. 1). First, it can worsen cognitive performance by causing a deficiency in several micronutrients, including vitamins and essential fatty acids( Reference Shatenstein, Kergoat and Reid 9 ). The possible mechanisms behind this effect could relate to the protective effect of vitamins against tissue oxidative damage( Reference Luchsinger and Mayeux 10 ), and the biophysical effects of essential fatty acid on neuronal membrane structure( Reference Salem and Niebylski 11 ). WL could also impair cognitive performance by raising serum cortisol and free radical levels( Reference Yen 12 ).
In recent years, leptin has also been attracting more interest because it seems to be implicated in the pathogenesis of AD. Leptin is produced in the subcutaneous and visceral adipose tissue and its levels drop when a person loses weight. This adipokine seems to improve axon growth, synaptogenesis and cell survival, protecting against glutamatergic cytotoxicity and oxidative damage, and promoting the proliferation of hippocampal progenitor cells( Reference Morrison 8 ). Data from the Framingham study indicate that people with leptin levels in the lowest quartile have a 4-fold higher risk of developing AD than those in the highest quartile after a 12-year follow-up( Reference Lieb, Beiser and Vasan 13 ).
WL could affect cognitive performance too, because preoccupation with hunger and anxiety caused by energy restriction may reduce and interfere with working memory capacity( Reference Shaw and Tiggemann 14 , Reference Green and Rogers 15 ).
Previous epidemiological studies suggested that, in middle age, people who eventually develop AD weigh the same as their peers who do not, and they begin to lose weight later on at a faster rate than subjects who remain cognitively intact. Data from the Honolulu-Asia Aging Study( Reference Stewart, Masaki and Xue 16 ) on 1890 individuals monitored for 32 years identified an accentuated WL starting 6 years prior to the diagnosis of dementia. This finding is consistent with other reports( Reference Barrett-Connor, Edelstein and Corey-Bloom 17 ) and with the results of the Indianapolis-Ibadan Dementia Project( Reference Berkhout, Cools and van Houwelingen 4 ), which reported that individuals with incident dementia had a BMI similar to that of people with normal cognition up to 9 years before dementia was diagnosed, but then lost weight between the ninth and the sixth years before any signs of cognitive impairment became apparent. The Religious Order Study( Reference Buchman, Wilson and Bienias 18 ) also showed that a loss of one point on the BMI per year coincided with a 35% higher risk of developing AD than in subjects whose BMI remained stable, and with an 80% higher risk than in people gaining 0·6 points a year on their BMI.
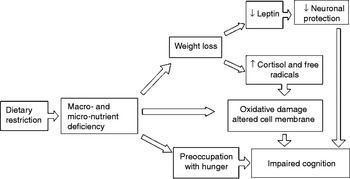
Fig. 1. Possible mechanisms explaining the relationship between weight loss and risk of Alzheimer's disease.
Involuntary weight loss as a sign of Alzheimer's disease
Involuntary WL in people with AD is not infrequent, reportedly preceding the diagnosis of AD by only a few years. As Johnson et al. reported( Reference Johnson, Wilkins and Morris 5 ), elderly AD patients seem to exaggerate the physiological WL accompanying ageing a year before their cognitive decline sets in. WL continues, however, with the progression of dementia, becoming accentuated in the advanced stages leading up to cachexia and death.
According to the laws of energy balance, WL may be caused by a lower energy intake or a higher energy expenditure due to a hypermetabolic state (with a higher resting energy expenditure (REE)) or an increased physical activity. Fig. 2 shows the various mechanisms that can lead to WL in subjects with AD.

Fig. 2. Possible mechanisms inducing weight loss in Alzheimer's disease. MTC, medial temporal cortex; ACC, anterior cingulated cortex; MTL, medial temporal lobe; IPL, inferior parietal lobe; LTN, lateral temporal neocortex; PC, prefrontal cortex; CCK, cholecystokinin.
The possible mechanisms by means of which AD could cause WL may be different in the various stages of dementia. Logically, the main cause of WL in the early stages of AD would be an increased REE or exaggerated physical activity, whereas in the advanced stages the major determinant of WL is likely to be a lower energy intake.
Involuntary weight loss due to a hypermetabolic state
A condition of hypermetabolism, defined as a more than 10% higher REE than in healthy individuals of the same sex, age and body composition, could contribute to WL in AD. It is uncertain, however, whether patients with AD have an abnormal metabolism because the condition has reportedly been associated with increases, reductions and no change in energy metabolism( Reference Donaldson, Carpenter and Toth 19 – Reference Wolf-Klein, Silverstone and Lansey 22 ); the studies involved were conducted on small samples, however, and some have been judged unreliable( Reference Vloeberghs, Van Dam and Franck 23 ).
The existence of a hypermetabolic state has nonetheless been suggested in animal models of AD overexpressing amyloid-precursor protein. Vloeberghs et al. ( Reference Morgan and Gordon 24 ) found that transgenic amyloid-precursor protein mice has a higher energy intake and a lower body weight than their wild-type litter-mates, and hypermetabolism was demonstrated in amyloid-precursor protein mice by Knight et al. ( Reference Knight, Verkhratsky and Luckman 25 ) using calorimetric cages, and it was confirmed by a higher than expected WL after energy restriction( Reference Patel, Gordon and Connor 26 ). On the other hand, these studies fail to explain whether the hypermetabolism is attributable to an increased REE or physical activity because it is impossible to create a physiological resting condition in mice. The exact reason for a higher REE is still not known, but it may have something to do with amyloid (Fig. 2), which can form membrane pores and dysfunctional ion channels, increasing proton leakage within mitochondria as a consequence( Reference Parihar and Brewer 27 ). In addition, astrocytes and microglia activated by amyloid deposits (neuro-inflammation)( Reference Rebeck, Hoe and Moussa 28 ) increase circulating cytokine levels, thus adding to the catabolic state( Reference Visser, Pahor and Taaffe 29 , Reference Patra and Arora 30 ).
Involuntary weight loss due to increased physical activity
Involuntary WL may also result from an increased energy expenditure due to an abnormal motor behaviour. Disruptive behavioural symptoms are common in AD right from the early stages and become more severe with the progression of dementia( Reference Scarmeas, Brandt and Blacker 31 ). One of the first clinical hallmarks of AD is episodic memory impairment( Reference de Toledo-Morrell, Dickerson and Sullivan 32 ), which leads to the ineffective consolidation and storage of new information( Reference Ober, Jagust and Koss 33 ). Patients frequently become restless, engage themselves in repetitive tasks( Reference Lopez, Wisniewski and Becker 34 , Reference Lyketsos, Lopez and Jones 35 ) and use a large amount of energy in trying to complete the activities of daily living. Constructional apraxia and temporo-spatial disorientation (which contribute to an intensified physical activity and excessive pacing), and anxiety due to the perception of an impaired performance, also contribute to a greater energy expenditure.
In advanced stages of AD, patients often present psychotic behavioural symptoms, such as agitation and aggressiveness( Reference Scarmeas, Brandt and Blacker 31 ), and such symptoms have been found associated with WL( Reference White, McConnell and Bales 36 , Reference Mahieux, Couderc and Fenelon 37 ), leading to a loss of 5 kg or more over a 6-month follow-up( Reference Guérin, Andrieu and Schneider 38 ).
Involuntary weight loss due to a lower energy intake
As AD progresses, patients usually also develop dietary problems, neglecting or forgetting to eat or becoming averse to some foods, and this can lead to a lower oral intake and a consequently accelerated WL( Reference Miyamoto, Higashino and Mochizuki 39 ). A decline in food consumption has been observed right from the first stage of AD( Reference Shatenstein, Kergoat and Reid 9 ), probably due to early changes in the appetite-regulating mechanisms. The exact reasons for a lower food intake have yet to be clearly defined, but changes in the brain regions involved in hunger control, a declining sense of smell and taste( Reference Morley 40 ), and an earlier satiety due to a greater sensitivity to cholecystokinin( Reference MacIntosh, Horowitz and Verhagen 41 ) are all potential contributors. In particular, some brain areas are known to have a role in regulating food intake, as medial temporal cortex, anterior cingulate cortex and olfactory epithelium, are frequently involved in amyloid deposition( Reference Grundman, Corey-Bloom and Jernigan 42 – Reference Tataranni, Gautier and Chen 44 ).
Concomitant chronic diseases, depressed mood and several types of medication could also contribute to a lower food intake by determining anorexia, constipation and an altered sense of smell and taste( Reference Plata-Salamán 45 , Reference Kishi and Elmquist 46 ). In addition, pro-inflammatory cytokines and chemokines (TNFα, IL-1 and IL-6), the levels of which are higher than normal in AD due to neuro-inflammation( Reference Khandelwal, Herman and Moussa 47 ), may mimic the action of appetite-regulating peptides and suppress feeding by taking effect directly on the glucose-sensitive neurons in the hypothalamic sites of satiety and hunger( Reference Patra and Arora 30 , Reference Espat, Moldawer and Copeland 48 ).
As the dementia progresses and the related functional impairment becomes more severe, patients' remaining abilities and the availability of adequate family/social support become more important in assuring an adequate energy intake. Hansen et al. ( Reference Hansen, Waldorff and Waldemar 49 ) showed that living alone, having a restricted social network and having lost competency in preparing meals or grocery shopping raised the risk of WL in patients with AD. Difficulties in bringing food to the mouth and chewing also correlated significantly with the loss of body weight over a 2-year follow-up( Reference Berkhout, Cools and van Houwelingen 4 ). An excessive burden on caregivers would also make them unable to invest sufficient resources in helping the patient to eat( Reference Gillette-Guyonnet, Nourhashemi and Andrieu 50 , Reference Rivière, Gillette-Guyonnet and Andrieu 51 ).
WL in advanced dementia could be likened to what happens in the terminal phase of several chronic diseases and stems from multiple causes. At this stage, patients become unwilling to eat and dysphagia often makes oral feeding difficult. Hypermetabolic states relating to newly contracted diseases, and muscle atrophy( Reference Thomas 52 ) due to prolonged immobilisation also accelerate WL, ultimately leading to cachexia.
Strategies to minimise weight loss
Given the impact of WL on cognitive performance, cognitive evaluation alone is clearly not enough in the follow-up of patients with AD; a multidimensional and multidisciplinary approach is needed. Periodic nutritional assessments should include anthropometric measures, biohumoral tests (haemocrome, albumin and prealbumin)( Reference Sergi, Coin and Enzi 53 ) and an assessment of food intake and eating behaviour( Reference Inelmen, Sergi and Coin 54 ). The possible actions that can be taken to contain WL are summarized in Table 1.
Table 1. Strategies to minimize weight loss
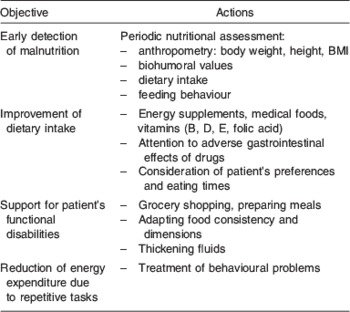
Since AD patients have multiple nutritional deficiencies, a number of energy supplements, vitamins and medical foods (i.e. foods intended to provide specific nutritional requirements for patients with certain diseases) have attracted interest for the adjuvant treatment of AD in recent years. Administering flavonoids, herbal supplements, phosphatidylserine, essential fatty acid and vitamins may improve cognition, offering some degree of neuroprotection( Reference Wollen 55 ).
As the patients’ ability to perform activities of daily living diminishes, the role of their caregivers becomes crucial. They should try to compensate for the patient's difficulties in performing tasks, be it in purchasing food, preparing meals, or changing a food's consistency to make it easier to swallow. Attention should also be paid to the patients’ preferences, and their chewing and swallowing times must be respected. Every effort should also be made to control any behavioural disorders responsible for an excessive and purposeless energy expenditure. Finally, the patients’ medication should be reviewed to avoid the use of drugs known to cause nausea, vomiting, anorexia or other gastrointestinal symptoms; in the choice between equally effective alternative drugs, those stimulating the appetite should be preferred.
Conclusions
WL not only represents a risk factor for AD, it is also so closely connected to the disease that it can be considered as one of its clinical signs. WL may start early in AD (even before the related cognitive impairment becomes overt), and it is associated with the progression of the dementia. It has numerous causes that differ in the various stages of dementia, attributable to hypermetabolism, a lower energy intake and a greater physical activity. It is consequently important to focus not only on an AD patient's cognitive performance, but also on their nutritional assessment and the prescription of appropriate dietary measures. A correct approach to the treatment and the adequate support of a caregiver are crucial to the identification and treatment of nutritional problems with a view to limiting WL and its consequences, i.e. frailty and disability.
Acknowledgements
The authors declare no conflicts of interest. This research received no specific grants from any funding agencies in the public, commercial or not-for-profit sectors. Contribution by respective authors was: G.S. and M.D.R., study concept, design and manuscript preparation; A.C., acquisition of data; E.M.I., data interpretation; E.M., revision of the manuscript.





