Introduction
Unilateral absence of intrapericardial pulmonary artery is a rare congenital malformation that can be associated with other CHD or present in isolation. Reference Bockeria, Makhachev, Khiriev and Abramyan1,Reference Bouros, Pare, Panagou, Tsintiris and Siafakas2 Untreated, it can lead to pulmonary hypertension, haemoptysis and exercise limitation with significant morbidity in adulthood. Reference Ten Harkel, Blom and Ottenkamp3 Early restoration of physiologic pulmonary flow is important Reference Welch, Hanley, Johnston, Cailes and Shah4 to facilitate postnatal development of the pulmonary vascular bed. Primary or 2-stage surgical repair has been reported with adequate results, but relatively high rates of re-intervention. Reference Welch, Hanley, Johnston, Cailes and Shah4–Reference Al-Khaldi, Tamimi and Sallam7 Transcatheter stenting of ductal dependent unilateral absence of intrapericardial pulmonary artery as an initial procedure has been rarely reported. Reference Krammoh, Bigras, Prsa, Lapierre, Miró and Dahdah8–Reference Prabhu, Maiya, Shetty, Murthy and Ramachandra11 In this report, we describe the clinical presentation, cross-sectional imaging, transcatheter recanalization, and stenting for ductal-dependent pulmonary artery branches after duct closure in two patients as an initial intervention with post procedural follow up.
Case 1
Ten-day-old term newborn was transferred to our cardiac ICU with concern for absent or aberrant left pulmonary artery. Echocardiography showed the main pulmonary artery to be in continuity with the right pulmonary artery, but the left pulmonary artery was not in continuity with the main pulmonary artery and appeared to be fed by an aortopulmonary collateral. CT scan demonstrated no proximal left pulmonary artery, but a very narrow calibre artery arising from the anterior aspect of the descending aorta supplying a small vessel located approximately 9 mm from the main pulmonary artery, a right aortic arch with mirror image branching, and a duct dimple at the base of left brachiocephalic trunk (Fig 1 a). The patient was started on Prostaglandin E1 (PGE1) at 0.1 mcg/kg/min without reappearance of a left ductus and on day of life 10, the patient was taken to cardiac catheterisation lab for potential recanalisation of left ductus arteriosus.

Figure 1. ( a ) Patient #1; reconstructed 3D CT scan showing an absent proximal left pulmonary artery, an aorto-pulmonary collateral arising from the anterior aspect of the descending aorta supplying the left lower pulmonary artery branch, and a ductus stump at the base of left brachiocephalic trunk. ( b ) Patient #2; reconstructed 3D CT scan showing an absent right pulmonary artery system, and a ductal stump arising from the aorta at the base of the right carotid artery.
Under general anaesthesia, and via right femoral arterial and venous access, a right and left heart catheterisation was performed. Pulmonary venous wedge angiography was performed showing an intrapulmonary left pulmonary artery stump measuring 4.3 mm in diameter. The left lower lobe branch measured 3.5 mm in diameter (Fig 2-b). An angiogram in the left brachiocephalic trunk showed a small duct dimple on its caudal aspect (Fig 2 a). A 3.3 Fr multipurpose catheter (Mongoose- PediaVascular, Ohio USA) was manipulated into the left brachiocephalic trunk, aiming at the duct dimple. A steerable 0.014 inch coronary guidewire (Thruway- Boston Scientific, Massachusetts USA) was inserted through the multipurpose catheter and manipulated through the obliterated left ductus into the intrapulmonary left pulmonary artery stump. The multipurpose catheter could be advanced over the guidewire into the left pulmonary artery stump. Then, two overlapping 3.5 mm x 0.9 cm Integrity coronary stents (Medtronic, Minnesota USA) were inserted and deployed by balloon inflation up to 10 atmospheres (Fig 2 c). Post stenting angiogram showed the uniformly patent stented ductus measuring 14.6 mm in length (Fig 2 d). The intrapulmonary left pulmonary artery stump had increased in diameter to 5.4 mm. After the procedure, transient unilateral pulmonary oedema developed, which resolved with diuresis on post-procedural day 3. He was anticoagulated with enoxaparin and was discharged 10 days post-procedure. He remained asymptomatic until elective surgical repair at 4.5 months post stenting when he underwent left pulmonary artery reimplantation to main pulmonary artery, stent removal, and pulmonary artery plasty. He had uncomplicated surgery and post-operative course.

Figure 2. Patient #1 procedural steps. All angiograms in PA projection. ( a ) Angiogram at the base of left brachiocephalic trunk showing the ductal stump. ( b ) Left upper pulmonary venous wedge injection showing an intrapulmonary LPA stump and normal appearing peripheral pulmonary vasculature. ( c ) The first integrity stent deployed distally, and the second stent telescoped at the proximal end of the first stent ready for deployment. ( d ) Post stenting angiogram showing patent stented ductus, and intrapulmonary LPA segment. LPA = left pulmonary artery, PA = pulmonary arteries.
Case 2
Post-natal echocardiography of a four-day-old term twin gestation newborn prenatally diagnosed with tetralogy of Fallot, and with fetal demise of the other twin at 21weeks showed tetralogy of Fallot with left aortic arch, aberrant right subclavian artery, and discontinuous right pulmonary artery. The left pulmonary artery was continuous with the main pulmonary artery and there was a large tortuous left ductus arteriosus. The patient was maintained on PGE without reappearance of a right ductus. CT scan confirmed the anatomy showing an atretic right ductus with ductal stump arising from the base of the right common carotid artery (Fig 1 b) but did not demonstrate an intrapulmonary right pulmonary artery.
Under general anaesthesia, and via right femoral arterial and venous access, a right and left heart catheterisation was performed. A right upper pulmonary venous wedge angiogram showed complete retrograde filling of the right pulmonary artery system without significant washout from collateral inflow and long persistence of contrast in the pulmonary artery system (Fig 3 a). The balloon wedge catheter was then manipulated across the ventricular septal defect into the ascending aorta and advanced into the right carotid artery at the level of the right ductus arteriosus stump, where an angiogram was performed showing a short proximal right ductal stump measuring 2.9 mm in diameter (Fig 3 b). The length of obliterated ductus measured 1 cm. The balloon wedge catheter was exchanged for 4-French Judkins right 1.0 coronary catheter (Cook Medical, Indiana USA) that was advanced into the right carotid artery. The ductal stump was engaged and a 0.014 inch steerable coronary guidewire was manipulated across the obliterated ductus arteriosus into the right pulmonary artery. A 3.0 x 1.5 cm Integrity coronary stent was deployed by balloon inflation up to 12 atmospheres of pressure with no residual waste. The subsequent angiogram showed the uniformly patent recanalized right patent ductus arteriosus measuring 3.0 mm in diameter with excellent stent position (Fig 3 c). The patient was anticoagulated with Enoxaparin and discharged home 3 weeks after the procedure. He remained asymptomatic until elective surgical repair at 2 months post stenting when he underwent tetralogy of Fallot repair, excision of the stented right ductus, and reimplantation of the right pulmonary artery into the distal main pulmonary artery with patch augmentation of the proximal right pulmonary artery anastomosis. He had uncomplicated surgery, and post-operative course, and was discharged home 8 days post-surgery.
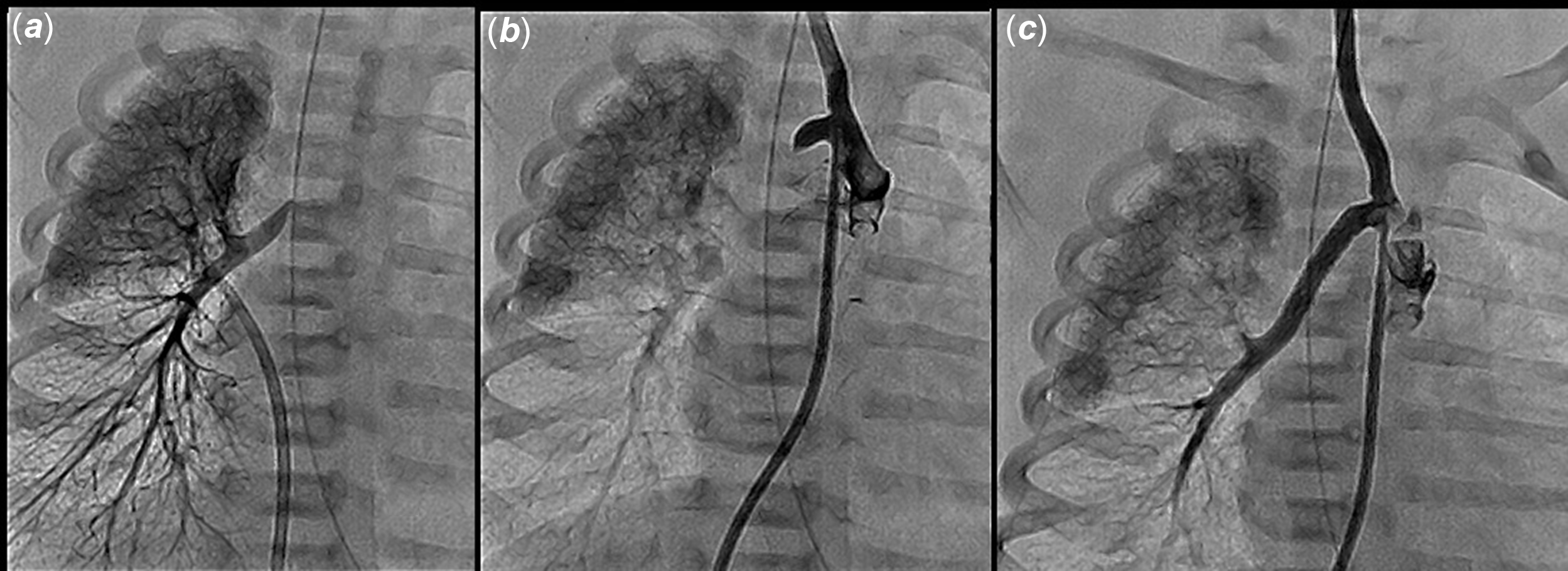
Figure 3. Patient #2 procedural steps. All angiograms in PA projection. ( a ) Right upper pulmonary venous wedge injection showing a distal RPA stump and normal appearing peripheral pulmonary vasculature. ( b ) Angiogram at the base of the right common carotid artery showing the ductal stump. ( c ) Post stenting angiogram showing patent stented ductus, and distal RPA segment. PA = pulmonary arteries, RPA = right pulmonary artery.
Discussion
Unilateral absence of intra-pericardial pulmonary artery is a rare congenital malformation that presents in association with CHD or in isolation with an estimated incidence in adults of 1:200,000. Reference Bouros, Pare, Panagou, Tsintiris and Siafakas2 About 57% Reference Bockeria, Makhachev, Khiriev and Abramyan1 of cases are associated with CHD, with tetralogy of Fallot being the most commonly associated heart defect Reference Bockeria, Makhachev, Khiriev and Abramyan1 . The right pulmonary artery is more commonly affected than the left pulmonary artery, representing 60%–63% of cases. Reference Bockeria, Makhachev, Khiriev and Abramyan1,Reference Ten Harkel, Blom and Ottenkamp3 Absent intra-pericardial left pulmonary artery is often associated with other CHDs. Reference Bockeria, Makhachev, Khiriev and Abramyan1
The proximal right and left pulmonary arteries are derived from the proximal sixth aortic arches bilaterally. Unilateral absence of intrapericardial pulmonary artery results from involution of the proximal sixth aortic arch. The persistent connection of the distal “hilar” pulmonary artery to distal sixth aortic arch becomes the ductus arteriosus (or ligamentum). Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis12 The intra-hilar lobar and segmental pulmonary arteries are derived from the primitive lung buds, hence they persist despite the absence of the “proximal” segment of the pulmonary artery. Reference Apostolopoulou, Kelekis, Brountzos, Rammos and Kelekis12 When isolated unilateral absence of intrapericardial pulmonary artery is supplied by ductus, it will lose its source of blood supply shortly after birth as the ductus closes. The affected lung is subsequently supplied from the bronchial arteries and other aorto-pulmonary collaterals. Reference Welch, Hanley, Johnston, Cailes and Shah4 In an animal model, ligation of left pulmonary artery leads to reduction in size of lobar and segmental pulmonary arteries. Reference Haworth, McKenzie and Fitzpatrick13 Failure to restore normal pulmonary circulation may result in significant hypoplasia of the associated pulmonary artery system, compensatory development of aortopulmonary collaterals, pulmonary hypertension, haemoptysis, thoracic asymmetry with secondary scoliosis, Reference Mery, Molina, Krishnamurthy, Fraser and Justino9 and limited exercise tolerance. Reported mortality was 7%. Reference Ten Harkel, Blom and Ottenkamp3 Symptoms can be unmasked by pregnancy and high altitude. Reference Welch, Hanley, Johnston, Cailes and Shah4 Early repair restores physiologic pulmonary circulation and potentially normal pulmonary vascular development. Reference Welch, Hanley, Johnston, Cailes and Shah4
Early surgical intervention has been reported, either primary complete repair or with initial ipsilateral systemic to pulmonary shunt. Reference Welch, Hanley, Johnston, Cailes and Shah4–Reference Al-Khaldi, Tamimi and Sallam7 Primary repair may be challenging, involves cardiopulmonary bypass, and in case of long-gap discontinuity can result in tension affecting patency. Reference Shanley, Lupinetti, Shah, Beekman, Crowley and Bove5 Using synthetic material has the limitation of growth and subsequently the need for replacement. Reference Welch, Hanley, Johnston, Cailes and Shah4,Reference Shanley, Lupinetti, Shah, Beekman, Crowley and Bove5 High rates of re-intervention in the form of surgical and catheter-based pulmonary arterioplasty have been reported. Reference Stamm, Friehs and Zurakowski6 The 2-stage repair is associated with the typical possible shunt complications. Some authors reported superior outcomes with primary unifocalizations, Reference Batlivala, McElhinney, Pigula and Marshall14–Reference Trivedi, Karamlou, Yoo, Williams, Freedom and McCrindle16 and others preferred the 2-stage repair; Reference Krammoh, Bigras, Prsa, Lapierre, Miró and Dahdah8,Reference Santoro, Capozzi and Giordano10 however, both were based on retrospective data and were confounded by selection bias in regard to the degree of pulmonary artery hypoplasia and the distance of discontinuity.
Transcatheter recanalisation and stenting of the supplying ductus is an alternative initial palliation for absent intra-pericardial pulmonary artery. Functional closure of the Patent ductus arteriosus (PDA) occurs in the first 24–48 hours, Reference Reese, Scott and Patrick17 which may not be visible on echocardiography, but a ductal stump would be present in cross-sectional imaging. The presence of a ductal stump, or “dimple,” is essential for transcatheter recanalisation after duct (Fig 1 a, b). Duct tortuosity may represent technical challenge and may require access from axillary or carotid artery but was not needed in our two cases.
Thrombosis is a concern following A Blalock–Thomas–Taussig (BT) shunt and ductal stenting. Reference Mery, Molina, Krishnamurthy, Fraser and Justino9,Reference Batlivala, McElhinney, Pigula and Marshall14 Competitive collateral flow, Reference Stamm, Friehs and Zurakowski6 low flow secondary to high pulmonary vascular resistance, and the presence of stent material were thought to be risk factors for thrombosis. Reference Krammoh, Bigras, Prsa, Lapierre, Miró and Dahdah8 Both of our patients received fractionated heparin (Enoxaparin) for anticoagulation and encountered no thrombosis.
The presence of stent material, theoretically, can impact unifocalization surgery. Reference Santoro, Capozzi and Giordano10 However, both of our patients underwent successful surgical repair with excision of the stented ductus and had an unremarkable perioperative course. Risk of pulmonary hypertension has been reported in adults with unrepaired Isolated unilateral absence of intrapericardial pulmonary artery Reference Ten Harkel, Blom and Ottenkamp3 as well as infants Reference Umezu, Harada, Sakamoto, Takigiku and Yasukochi18 . We believe early definitive repair is a key to avoiding pulmonary vascular disease.
We believe transcatheter recanalisation and stenting of the supplying ductus in the newborn period will establish adequate flow to the affected pulmonary vasculature, promote growth of the pulmonary artery system, may facilitate a larger anastomosis at later repair, and may improve long-term outcome.

Figure 4. Echocardiography. The top panel is patient#1 echocardiogram at 2 months post-cath (before surgical repair), LPA distal diameter 10 mm, and stent is patent. The lower panel is patient #2 at 5 month post surgical repair. LPA = left pulmonary artery.







