Introduction
Air quality impacts human health [Reference Tham1,Reference Brook2]; airborne contaminants include fine particulate matter (PM2.5, airborne particles with diameters less than 2.5 µm), ozone (O3), volatile organic compounds (VOCs), and biological particles (e.g., allergens and pathogens). Since individuals spend about 90% of their time indoors, indoor air quality (IAQ) is a key driver of the effect of air quality on human health [Reference Huang3,Reference Chi4]. In particular, IAQ is linked to cardiovascular [Reference Simkhovich5] and respiratory morbidity [Reference Kurmi6,Reference Perez-Padilla, Schilmann and Riojas-Rodriguez7] and mortality [Reference Pope8–Reference Schraufnagel11]. Modeling data estimated that indoor exposure to PM2.5 accounts for the vast majority of the mortality burden being attributed to total exposure to PM2.5 [Reference Azimi and Stephens10]. To evaluate the effectiveness of interventions to improve IAQ, one must study relevant outcomes. Cardiovascular and respiratory events can take a long time to accrue and be challenging to study in a randomized design. Thus, intermediate endpoints that respond to natural or intervention-induced changes in IAQ are critical to research in this field. The American Heart Association Scientific Statement on air pollution and cardiovascular disease underscored the need to “better describe the physiological relevance in humans and the fundamental details of the mechanisms” [Reference Brook2].
The goal of the present review is to address this stated need and summarize current knowledge on biomarkers associated with IAQ exposure in order to guide the design of translational research studies on indoor air quality.
Methods
Data Sources and Search Strategies
A comprehensive search was conducted from January 1, 2000 to September 17, 2019 to identify studies that reported on blood, urine, and salivary biomarkers relevant to indoor air pollution exposure and toxicology. Breath biomarkers were beyond our intended scope and are not addressed herein. The search strategy was designed and conducted by an experienced librarian (L.C.H.) with input from investigators (A.M.S. and S.M.M.) and was performed in Ovid Medline, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. Controlled vocabulary supplemented with keywords was used, the search was limited to the English language, and animal studies were excluded. The full search strategy is included in the online supplemental Appendix 1.
Study Selection
A total of 1124 papers were identified. Phase 1 involved 2 investigators (A.M.S. and S.M.M.) reviewing all titles and abstracts. We included all English language original research studies with at least 10 adult participants published over the last decade between January 1, 2010 and September 17, 2019. Only studies that measured biomarkers in blood, urine, or saliva and focused on indoor exposures were included. We excluded studies that involved only children, factory workers, or pregnant women, involved biomass, coal, or open wood-burning studies; focused only on tobacco, lead, or dust exposures. Studies with industrial settings were excluded because indoor pollutants that may be encountered in industrial settings are not representative of indoor exposures in most buildings, including homes, offices, schools, and healthcare settings. In doing so, we selected 53 full-text papers for analysis. Phase 2 involved 2 investigators (A.M.S. and S.M.M.) reviewing the full-text papers. Data reviewed included the type of biomarkers and specimen type (blood, urine, and saliva), country, setting (home, office, etc.), seasons, frequency of data collection, study length, intervention type, population type and size, air pollutant levels and types, and a summary of methods and results. Among these, 23 papers were excluded: 21 did not meet the inclusion criteria (1 article had no mention of biomarkers, 7 collected air exposure measurements off-site, 8 had no mention of IAQ exposures, 1 focused on factory workers, 3 used coal/biomass/open wood burning, 1 included participants with a disease), and 2 were inaccessible. Thirty articles were retained for the final analyses (Fig. 1).

Fig. 1. Flow diagram illustrating the methods applied to the review. aPhase 1 of the review involved reviewing the title and abstract, and excluded studies that involved only children, factory workers, or pregnant women, involved biomass, coal, or open wood-burning studies; focused only on tobacco, lead, or dust exposures. bPhase 2 involved reviewing the full-text papers and used the same exclusion criteria as Phase 1.
Results
The thirty studies included sample sizes ranging from 20 to 200 participants (Table 1). Participants’ age ranged from 15 to 90, and originated from 11 countries (5 in the USA, 7 in China, 5 in Taiwan, 1 in South Korea, 8 in Europe, 1 in Iran, 1 in Senegal, and 2 in India). Most studies (18 out of 30) consisted of non-randomized comparisons across different settings with a few observational monitoring. Nineteen of the studies were observational and/or cross-sectional studies, while the remaining 11 studies were interventional and/or crossover trials. More details regarding study design can be found in Table 1. Almost half of the studies (n = 12) measured biomarkers at only one time point. Out of 30 studies, 3 provided an estimate of their statistical power to observe a change.
Table 1. Summary of IAR studies measuring physiological biomarkers and organic compounds in humans

aN indicates the sample size of each study.
* Denotes significant changes seen in biomarkers.
One-hundred and thirteen biomarkers were identified within the 30 articles: 83 blood biomarkers, 24 urine biomarkers, 4 found in blood or urine, and 2 were found in blood, urine, or saliva. Biomarkers are presented according to the biological pathways studied, which are centered chiefly around inflammation, coagulation, and oxidative stress (Table 1). Organic compounds are considered separately. Figure 2 shows the biomarkers listed in order of most frequently reported variations in response to IAQ exposures.
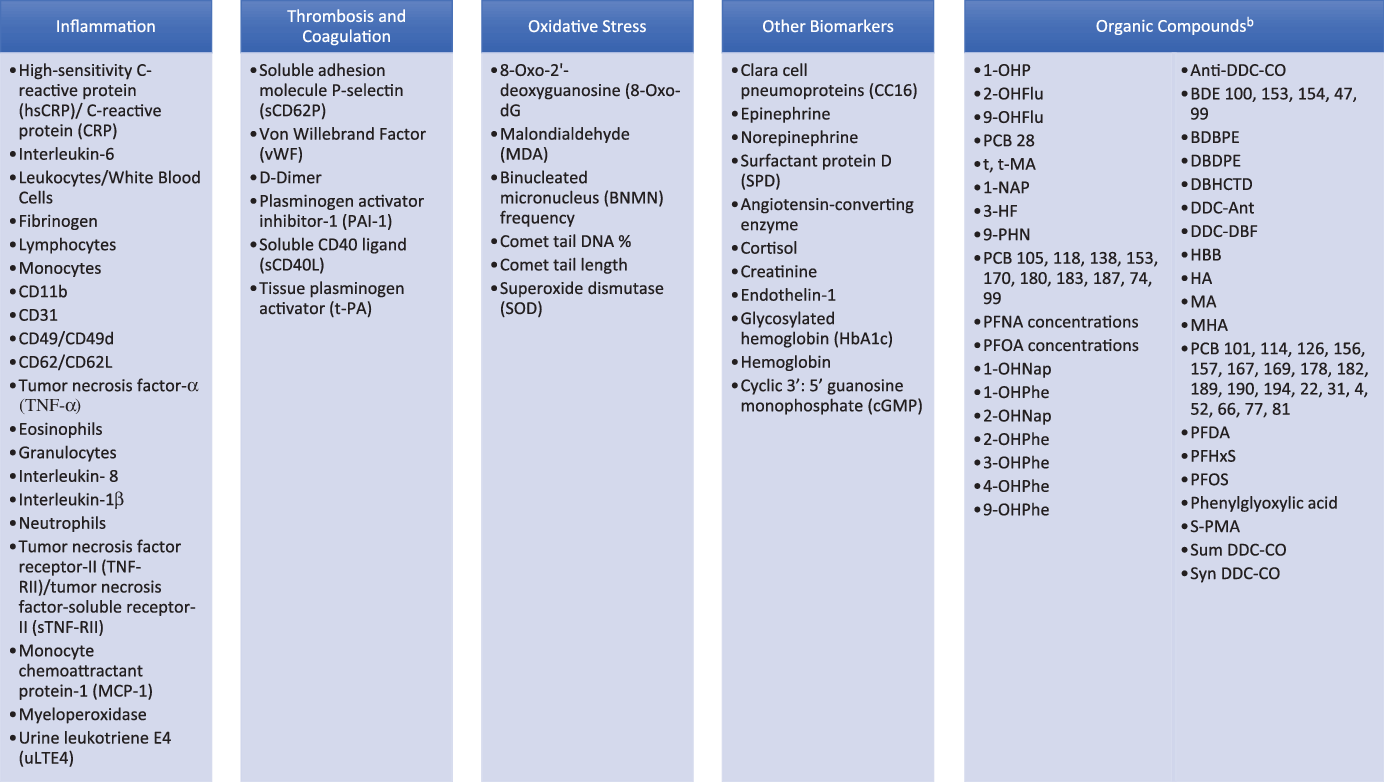
Fig. 2. Blood, urine, and saliva biomarkers identified in IAQ papers.aaBiomarkers are listed in order of most frequently reported variations in response to IAQ exposures. bAbbreviations can be found in Fig. 3.
Inflammation
C-reactive protein (CRP) is the most frequently reported biomarker. Among 11 studies, 7 measured CRP and 4 hsCRP. Five studies evaluated a filtration system in home and/or office settings [Reference Day12–Reference Karottki16] while the remaining two monitored pollutants over time in home and/or office settings [Reference Karottki17,Reference Olsen18]. Only one study detected an association between PM2.5 and CRP [Reference Day12–Reference Karottki15,Reference Karottki17]. Exposures evaluated included: mostly PM2.5 mass concentrations and/or total VOCs; [Reference Day12–Reference Lin21] particle number concentrations (PNCs), black carbon and O3 [Reference Day12,Reference Shao13,Reference Karottki16–Reference Olsen18]. Among the four hsCRP studies, two studies evaluated a filtration system [Reference Chuang19,Reference Brugge22], one evaluated an air conditioning (AC) unit [Reference Lin21], and one monitored pollutants over time [Reference Hassanvand20]. Most studies detected significant relationships between PM2.5 mass concentrations and hsCRP in a home setting. Levels of hsCRP also increased with increased total VOC exposures in a home setting [Reference Chuang19,Reference Lin21] and PM10, PM10–2.5, and PM1–2.5 mass concentrations in a retirement home setting [Reference Hassanvand20]. There were no associations between hsCRP and CO2 or CO [Reference Lin21].
Interleukins were measured in numerous studies, with IL-6 being the most reported. Of seven papers, four compared a sham filtration system with an active filtration system [Reference Shao13,Reference Chen14,Reference Brugge22,Reference Cui23] and three monitored pollutants over 1 day [Reference Jung24] or over time [Reference Hassanvand20,Reference Ndong Ba25]. With regards to exposures, five papers measured PM2.5 mass concentrations [Reference Shao13,Reference Chen14,Reference Hassanvand20,Reference Cui23,Reference Jung24]. Additional exposures were measured: CO, CO2, and TVOCs [Reference Jung24]; PM10, PM10–2.5, PM1–2.5, and PM1 [Reference Hassanvand20]; black carbon; [Reference Shao13] O3, NO2, and PNC; [Reference Cui23] PNC; [Reference Brugge22] and VOCs and PM10 [Reference Ndong Ba25]. Only two papers detected an association between IL-6 and PM10, PM10–2.5, and PM1–2.5 [Reference Hassanvand20]. A decrease in IL-6 was reported 1 day after the installation of a high-efficiency particulate air (HEPA) filtration system [Reference Cui23]. The evidence of an association between air pollution and IL-8 and IL-1β is scarce.
Four studies measured blood fibrinogen in home or dormitories: three compared a sham and active filtration system [Reference Shao13,Reference Chen14,Reference Chuang19], and one compared air quality when windows were open, closed, and when AC was on [Reference Lin21]. All four studies measured indoor PM2.5. Additional exposures measured included black carbon; [Reference Shao13] TVOCs; [Reference Chuang19] and PM10, TVOCs, CO2, and CO [Reference Lin21]. Only one [Reference Lin21] study detected an association between fibrinogen and PM2.5 and TVOCs. Fibrinogen approached statistical significance in one study where participants were exposed to relatively higher PM2.5 and TVOCs [Reference Chuang19]. The value of fibrinogen to study IAQ pollution appears marginal, calling for further research.
Tumor Necrosis Factor-α (TNF-α) was measured in three studies: one study compared true air filtration with a sham system; [Reference Chen14] two studies monitored pollutants over time [Reference Jung24,Reference Ndong Ba25]. The following exposures were measured: PM2.5 [Reference Chen14,Reference Jung24], VOCs [Reference Jung24,Reference Ndong Ba25], PM10 [Reference Ndong Ba25], CO [Reference Jung24], and CO2 [Reference Jung24]. No significant association was found between TNF-α and any indoor air pollutants measured. Of note, a prior review of air pollution biomarkers that combined indoor and outdoor air studies indicated that TNF-α was a reliable indicator of inflammation [Reference Yang26]. This discrepancy underscores the importance of stratifying the review of the literature by location as performed herein.
Tumor necrosis factor-receptor II (TNF-RII) and tumor necrosis factor-soluble receptor-II (sTNF-RII) were measured in two studies: one study compared sham filtration and HEPA filtration systems [Reference Brugge22] and another study monitored pollutants over time [Reference Hassanvand20]. No association was detected between PNC and TNF-RII [Reference Brugge22]. However, an association was detected between sTNF-RII and PM2.5, PM1, and PM1–2.5 [Reference Hassanvand20]. This is another domain where more research is clearly needed.
Leukocytes including lymphocytes, monocytes, and granulocytes (neutrophils and eosinophils) were measured in five studies; lymphocytes and monocytes were measured in four; granulocytes, neutrophils, and eosinophils were measured in two. Two studies compared sham and active filtration systems [Reference Karottki15,Reference Karottki16], while three monitored pollutants over time [Reference Karottki17,Reference Olsen18,Reference Hassanvand20]. One report pertained to PM2.5 [Reference Karottki15], three measured indoor air exposures to PM2.5 and PNC [Reference Karottki16–Reference Olsen18], and one measured PM10, PM10–2.5, PM2.5, PM1–2.5, and PM1 [Reference Hassanvand20]. Significant associations were seen for the following: leukocyte counts and PNC [Reference Karottki17,Reference Olsen18] or PM10, PM10–2.5, and PM1–2.5; [Reference Hassanvand20] lymphocytes and PNC [Reference Karottki17] and PM2.5; [Reference Olsen18] increased neutrophil counts with PNC; [Reference Olsen18] and eosinophil counts with PM2.5 [Reference Karottki17,Reference Olsen18] and PNC [Reference Olsen18]. Measurements of leukocyte, lymphocyte, neutrophil, and eosinophil counts may be useful in determining relationships between indoor air pollutant exposures and inflammation.
Monocyte activation plays an important role in inflammation. CD11b, CD31, CD62/CD62L, and CD49/CD49d are different types of expressions of adhesion markers found on monocytes. Two studies evaluated the different air exposures during active filtration and sham filtration [Reference Karottki15,Reference Karottki16], while one study monitored pollutants over time [Reference Karottki17]. Three studies examined the association between these biomarkers and PM2.5 and PNC [Reference Karottki15–Reference Karottki17]. Two studies detected associations between CD11b with PM2.5 [Reference Karottki16] and PNC [Reference Karottki17]. An association with CD62L and active filtration was also detected, though biomarker concentrations were not analyzed against PM2.5 concentrations [Reference Karottki15]. No association was reported with CD49/CD49d or CD31. More research is needed to determine if there may be an association between monocyte activation and indoor air exposures.
Monocyte chemoattractant protein-1 (MCP-1) regulates migration and infiltration of monocytes/macrophages [Reference Deshmane27] while myeloperoxidase (MPO) is an enzyme released by neutrophils during inflammation [Reference Loria28]. One study measured these two biomarkers alongside PM2.5 to compare true and sham air filtrations in dormitories of college students [Reference Chen14]. An association was detected between a decrease in MCP-1 and MPO during the true filtration scenario and an increase in MCP-1 with continuous exposure to PM2.5 [Reference Chen14].
Urine leukotriene E4 (uLTE4) is used to assess changes in cysteinyl-leukotriene levels [Reference Hoffman and Rabinovitch29]. One study measured uLTE4 to evaluate VOC indoor air exposures on airway inflammation by measuring urine and indoor VOCs 7 days pre- and post-move from an old to new hospital [Reference Kwon30]. Although levels of uLTE4 significantly increased, no correlations were observed between VOCs and uLTE4 [Reference Kwon30]. While uLTE4 may play a role in environmental exposures related to asthma [Reference Hoffman and Rabinovitch29,Reference Kwon30], there is insufficient evidence to support its use in studies of indoor air exposures.
Thrombosis and Coagulation
Three studies measured von Willebrand Factor (vWF) in office, dormitory, and home settings: [Reference Day12,Reference Hassanvand20,Reference Cui23] two compared different ventilation systems [Reference Day12,Reference Cui23] while one monitored pollutants over time [Reference Hassanvand20]. All three papers measured PM2.5, and two additionally measured O3 [Reference Day12,Reference Cui23]. Other exposures measured included: NO2 and PNC [Reference Cui23], PM10, PM10–2.5, PM1–2.5, and PM1 [Reference Hassanvand20]. All three papers showed significant associations: vWF was weakly associated with PM1–2.5, PM2.5, PM10–2.5, and PM10; [Reference Hassanvand20] true filtration significantly lowered vWF by 26.9% when compared to sham filtration; [Reference Cui23] and removal of an electrostatic precipitator (ESP) was significantly associated with an increase in vWF [Reference Day12]. This suggests PM2.5 can interfere with hemostasis by preventing the creation of the platelet plug. Of the hemostatic biomarkers reviewed, IAQ exhibited the strongest association with vWF.
Soluble adhesion molecule P-selectin (also known as sCD62P) binds vWF, acting as an anchor to the surface of endothelial cells for platelet adhesion [Reference Peyvandi, Garagiola and Baronciani31]. Three studies studied the association of PM2.5 with P-selectin in office, dormitories, and homes and compared filtration systems [Reference Day12,Reference Chen14,Reference Cui23]. O3 and PNC were also measured [Reference Day12,Reference Cui23]. A 793 ppb/hr O3 exposure increase was associated with a 16.1% increase in P-selectin [Reference Day12]. With PM2.5 exposure, no change in this biomarker was detected [Reference Chen14,Reference Cui23]. Two studies [Reference Day12,Reference Cui23] also suggested O3 exposure may impact the binding of vWF to endothelial cells, but more research is needed on PM2.5 and its possible effect on P-selectin.
Soluble CD40 ligand (sCD40L), plasminogen activator inhibitor-1 (PAI-1), tissue plasminogen activator (t-PA), and D-Dimer were measured when comparing true and sham filtration systems in dormitories over a 2-day period [Reference Chen14]. Both sCD40L and t-PA significantly increased with an increase in PM2.5, while D-Dimer and PAI-1 showed no association [Reference Chen14]. Further research is needed to better understand the relationship between the fibrinolytic system and PM2.5.
Oxidative Stress
8-hydroxy-2′-deoxyguanosine (8-OHdG) is a marker of oxidative stress that can be detected in blood or urine [Reference Jung24,Reference Lai32,Reference Pan33]. Eleven studies measured 8-OHdG; four compared functioning filtration system with a sham filtration system or control [Reference Day12,Reference Shao13,Reference Chuang19,Reference Pan33], four compared different populations based on occupation [Reference Ndong Ba25,Reference Lai32,Reference Wang34,Reference Ke35], one study monitored pollutants over time [Reference Jung24], one compared windows open, windows closed, and AC on conditions [Reference Lin21], and one report compared air exposures in different buildings [Reference Kwon30]. Indoor air exposures included PM1 [Reference Pan33], PM2.5 [Reference Day12,Reference Shao13,Reference Chuang19,Reference Lin21,Reference Jung24,Reference Pan33,Reference Ke35], PM10 [Reference Lin21,Reference Ndong Ba25,Reference Pan33,Reference Wang34], polyaromatic hydrocarbons (PAHs) [Reference Lai32,Reference Pan33,Reference Ke35], VOCs [Reference Chuang19,Reference Lin21,Reference Jung24,Reference Ndong Ba25,Reference Kwon30], O3 [Reference Day12], CO [Reference Lin21,Reference Jung24], CO2 [Reference Lin21,Reference Jung24], black carbon [Reference Shao13], and PNCs [Reference Ke35]. Seven studies detected association between 8-OHdG and the following air pollutants: PM1 [Reference Pan33], PM2.5 [Reference Chuang19,Reference Lin21,Reference Pan33], VOCs [Reference Chuang19,Reference Lin21,Reference Ndong Ba25], PAHs [Reference Lai32,Reference Pan33,Reference Ke35], UFPs [Reference Ke35], and CO2 [Reference Jung24]. 8-OHdG was frequently associated with changes in indoor air pollution, suggesting it may be of value for IAQ studies.
Malondialdehyde (MDA) is a product of lipid peroxidation that can be detected in blood or urine [Reference Yang26,Reference Ke35]. Six studies measured MDA: four in a home setting [Reference Cui23,Reference Pan33–Reference Ke35], one in an office and dormitory [Reference Day12], and one in a hospital setting [Reference Kwon30]. Two studies compared different participant occupations [Reference Wang34,Reference Ke35], two studies compared HEPA with sham filtration [Reference Day12,Reference Cui23], one study compared air exposures in different buildings [Reference Kwon30], and one study compared exposures before and after installation of a cooking emissions control device [Reference Pan33]. PM1 [Reference Pan33], PM2.5 [Reference Day12,Reference Cui23,Reference Pan33,Reference Ke35], PM10 [Reference Pan33,Reference Wang34], O3 [Reference Day12], PAHs [Reference Ke35], PNCs [Reference Cui23,Reference Ke35], and VOCs [Reference Kwon30] were measured in these studies. A significant association was reported between MDA and the following indoor air exposures: PM10 [Reference Wang34] and the PAH benzo(a)pyrene (BaP) [Reference Pan33]. Additional oxidative stress biomarkers measured in one study included binucleated micronucleus (BNMN) frequency, comet tail length, comet tail DNA %, and superoxide dismutase (SOD) [Reference Wang34]. An association with PM was detected solely for comet tail length. However, there was a significant difference found in BNMNs and tail length when comparing kitchen workers and non-kitchen workers [Reference Wang34]. Both BNMNs and tail length were significantly higher in kitchen workers that were exposed to cooking oil fumes. While 8-OHdG and MDA appear to be valuable biomarkers to assess oxidative stress in indoor air exposures, more research is needed on other markers.
Other Biomarkers
Catecholamines (epinephrine and norepinephrine) and cortisol were found to be associated with CO2 concentration in office space [Reference Jung24]. Biomarkers were not measured individually, so it is unclear if CO2 was associated with epinephrine, norepinephrine, or cortisol alone. This report suggests a relationship between urinary catecholamine and CO2 exposure, but more research is clearly needed on this topic.
Clara cell pneumoproteins (CC16) and surfactant protein D (SPD) are produced in the lungs and denote epithelial damage in the lower airways. Two studies evaluated their relationship with residential filtration, compared functioning filtration systems to sham filtration systems and measured PM2.5, and PNC of particles with diameters between 10 and 280 nm [Reference Karottki15,Reference Karottki16]. No association was detected between these biomarkers and filtration systems, PM2.5 exposure, or PNC exposure [Reference Karottki15,Reference Karottki16]. While SPD and CC16 are associated with chronic obstructive pulmonary disease [Reference Sin36,Reference Lock-Johansson, Vestbo and Sorensen37], available data do not support their use in studies of indoor air exposures. Angiotensin-converting enzyme and endothelin-1 were also measured when comparing true and sham filtration systems in dormitories over a 2-day period, but showed no association with PM2.5 [Reference Chen14].
Glycosylated hemoglobin (HbA1c), was measured in urban homes of volunteers in Denmark. PM2.5 [Reference Karottki15,Reference Karottki17] and PNC [Reference Karottki17] were monitored and an association with HbA1c was detected only for PNC. Thus, while recent studies reported an association between diabetes mellitus and air pollution, available data do not support the use of HbA1c in studies of indoor air exposures.
Cyclic 3’: 5’ guanosine monophosphate (cGMP) can increase when soluble guanylate cyclase is activated, which occurs with exposure to CO or NO [Reference Matthews38,Reference McMahon and Bryan39]. One study examined differences in levels of chronic exposure to CO across four types of residential heating (piped natural gas, coal, electricity, and liquid propane gas) and its association with cGMP; [Reference Matthews38] cGMP was higher in homes heated with liquid propane than in those heated with piped natural gas. However, CO exposures in the homes were too low to be the cause of this change, so it was hypothesized that NO may be a confounding factor [Reference Matthews38]. NO can trigger the production of cGMP, but there is not enough research to determine if CO also triggers this production [Reference McMahon and Bryan39,Reference Park, Sandner and Krieg40]. While cGMP may be a good indicator for NO exposure, more research is needed to determine if the biomarker is a good indicator of CO exposure.
Organic Compounds
Indoor exposure to organic compounds (Fig. 3) can lead to measurable concentrations of these compounds or their metabolites in the blood or urine. Two studies measured office spaces’ PFCs and blood biomarkers PFNA and PFOS [Reference Fraser41,Reference Fraser42] (Table 1). Both studies compared air exposures in new buildings, partially new buildings, and old buildings while one study [Reference Fraser41] additionally collected dust samples from participants’ offices, homes, and vehicles. Serum PFCs followed a consistent pattern with the FTOHs in the buildings’ air [Reference Fraser42]. Serum PFOA was significantly associated with 8:2FTOH and 10:2FTOH [Reference Fraser41] and positively associated with time spent in the office each week, suggesting PFOA bioaccumulation in participants [Reference Fraser42]. Blood PFDA, PFOS, and PFHxS concentrations had no significant association with air PFCs [Reference Fraser42].

Fig. 3. Glossary of organic compounds.
Thirty-three PCB compounds were measured across three studies. One study evaluated the association between residential air PCBs and serum PCB compounds in high and low PCB areas [Reference Fitzgerald43], another study evaluated PCB exposure and blood between residents of PCB-contaminated and non-contaminated flats [Reference Meyer44], and another study investigated the association between office air PCBs and office workers’ blood [Reference Kraft45]. PCB 28 was the only measured compound that was reported to have statistical significance in all three studies.
Two studies compared household air samples to residents’ PBDE blood samples [Reference Cequier46,Reference Bennett47]. BDE-47 and BDE-99 showed significant associations with air PBDE [Reference Bennett47]. Eight halogenated flame retardants were detected in participants’ serum, but none were associated with home PDBE exposures [Reference Cequier46].
Thirteen urine PAH biomarkers were measured across seven papers [Reference Ndong Ba25,Reference Lai32,Reference Wang34,Reference Ke35,Reference Singh48–Reference Li50]. Two studies [Reference Singh48,Reference Singh49] assessed PAH exposure and urinary PAH levels in kitchen and non-kitchen workers, while one study measured indoor PM2.5-bound PAH concentrations in dormitories, offices, and laboratories alongside urinary OH-PAHs [Reference Li50]. The other five studies are described above [Reference Ndong Ba25,Reference Lai32,Reference Wang34,Reference Ke35,Reference Li50]. Five papers showed significance between 1-OHP and indoor PAH exposures [Reference Lai32,Reference Ke35,Reference Singh48,Reference Singh49], and benzene, toluene, xylene in urban housemaids [Reference Ndong Ba25]. Three studies measured the remaining 12 PAH biomarkers [Reference Singh48–Reference Li50]. 2-OHFlu, 9-OHFlu, 1-NAP, 9-PHN, and 3-HF showed significant associations with air PAHs [Reference Singh48,Reference Singh49] while 1-OHNap, 2-OHNap, 9-OHFlu, 4-OHPhe, and 9-OHPhe showed significant associations with exhaled FeNO [Reference Li50]. 1-OHPhe, 2-OHPhe, and 3-OHPhe showed no associations with air exposures. The literature, alongside a 2004 review [Reference Castaño-Vinyals51], suggests 1-OHP is a reliable biomarker when measuring indoor PAHs.
Two benzene biomarkers found in the literature were t,t-MA and S-PMA; the studies were described previously [Reference Ndong Ba25,Reference Kwon30]. A significant decrease in t,t-MA was seen after moving from an old to new building [Reference Kwon30], but no significant associations were found between t,t-MA and other exposures. Significantly higher levels of S-PMA were seen in city housemaids compared to drivers, traders, and rural housemaids [Reference Ndong Ba25]. S-PMA concentration may be a better indicator of benzene exposure, and is supported in previous literature [Reference Yang26,Reference Arnold52].
Gas-phase benzene, toluene, ethylbenzene, styrene, o-, m-, and p-xylenes were measured in one study along with their counterpart urinary biomarkers [Reference Kwon30]. Only o-, m-, and p-MHA levels significantly increased after the move from an old to new building, along with an increase in levels of TVOCs and all individual VOCs [Reference Kwon30].
Discussion
The World Health Organization (WHO) defines biomarkers as “any measurement reflecting an interaction between a biological system and a potential hazard, which may be chemical, physical, or biological” [53]. Biomarkers can serve as surrogate endpoints if they are associated with clinical outcomes [54]. The present review focused on studies of biomarkers indicative of changes in indoor air pollution exposure and of responses such as inflammation, oxidative stress, and coagulation. These biomarkers, therefore, constitute attractive intermediate endpoints for studies of IAQ. Herein, we summarize the current evidence pertaining to blood, urine, and saliva biomarkers used in IAQ research.
Indoor air exposures are a mixture of ambient air pollution brought indoors via ventilation and infiltration and indoor generated pollution emitted from combustion (i.e., candles, stove, fireplace), building materials and furnishings, and human behaviors such as smoking, cooking, and cleaning products [Reference Castro55–Reference Ni, Shi and Qu61]. Common indoor air pollutants include inorganic gases [e.g., carbon monoxide (CO), carbon dioxide (CO2)], reactive gases (e.g., O3, nitric oxides (NOX)], a wide range of VOCs and semi-volatile organic compounds (SVOCs), and particulate matter (PM), ranging from about 1 nm to 10 µm in diameter. Some compounds, such as polycyclic aromatic hydrocarbons (PAHs), perfluorinated compounds (PFCs), polychlorinated biphenyl (PCBs), and polybrominated diphenyl ethers (PBDE), are found in both the gas and particulate phases depending on partitioning behavior and emission source.
Poor air quality is associated with adverse clinical outcomes, which however take a long time to accrue and are thus challenging to use in translational research studies. Hence, the ability to rely on biomarkers as surrogate endpoints is critical to the conduct of observational studies as well as interventions. A previous review suggested that common mechanisms included inflammation and oxidative stress [Reference Yang26]. However, this study combined indoor and outdoor air pollution and its applicability to other settings or to indoor air pollution only is uncertain.
The present review extends prior knowledge by summarizing available data on the associations between biomarkers and IAQ. The mechanistic pathways associated with variations in IAQ include inflammation, coagulation, and oxidative stress. These pathways are known to be associated with chronic diseases, including cardiovascular diseases, respiratory diseases, and cancers supporting the biological plausibility of these associations.
Limitations, Strengths, and Applications
Some limitations of the reviewed studies should be mentioned. Most studies were cross-sectional and almost half of the studies measured biomarkers at only one time point during the course of the study. Methods varied considerably across studies and hence direct comparison was challenging. Randomized intervention studies measuring paired groups of individuals are recommended for future IAQ biomarker studies to reduce confounding variables and improve quality research. Additionally, power was mentioned in only 3 of the 30 reviewed papers, therefore precluding its systematic assessment. Six biomarkers were measured in more than one type of specimen (blood, urine, or saliva), however, methods of measurements were not compared across specimen type. Thus, it is unclear if one specimen is more useful in measuring a particular biomarker than the other.
Our review has a number of important strengths. We conducted a comprehensive literature review using a rigorous methodology. Our review provides the most current review of the literature over the last decade and useful guidance for the selection of biomarkers in translational studies of IAQ.
Conclusion
Herein, we summarize the current evidence on the biomarkers which most frequently responded to variations in IAQ. The biomarkers which exhibit the most consistent association with IAQ were high sensitivity CRP, vWF, 8-OHdG, and 1-hydroxypyrene (1-OHP. This summary provides a guide to select the biomarkers for translational studies evaluating the impact of indoor air pollutants on human health.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cts.2020.532.
Acknowledgments
This research was made possible by the support of the Well Living Lab, a partnership between Delos Living, LLC and the Mayo Clinic.
Disclosures
The authors have no conflicts of interest to declare.






